Herbal Remedies in Wound Healing: A Comprehensive Review of Plants and Non-Clinical Applications
Gauri Goyal1 , Vinay Kumar1
, Vinay Kumar1 *, Himani Tyagi1
*, Himani Tyagi1 , Priyanshi Varshney1
, Priyanshi Varshney1 , Shardendu Kumar Mishra1
, Shardendu Kumar Mishra1 and Sanjeev Chauhan2
and Sanjeev Chauhan2
1Department of Pharmacology, KIET Group of Institutions, KIET School of Pharmacy, Delhi-NCR, Ghaziabad, Uttar Pradesh, India.
2Department of Pharmaceutics, KIET Group of Institutions, KIET School of Pharmacy, Delhi-NCR, Ghaziabad, Uttar Pradesh, 201206, India.
Corresponding Author E-mail: vinaykumarpatel@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/400232
Article Received on : 24 Jan 2024
Article Accepted on :
Article Published : 04 Apr 2024
Reviewed by: Dr. Tahiri Sylla
Second Review by: Dr. Md Sanaul Moin
Final Approval by: Dr. Abdelwahab Omri
The phenomenon of wound healing encompasses a coordinated sequence of cellular and biochemical phases collaborating synergistically to promote the restoration of the injured tissue. Tissue repair is complex, posing challenges in wound management. Healing involves three phases: inflammatory, proliferative, and remodeling. Treatments include antibiotics, antiseptics, and extracts, but synthetic drugs have limitations. There is growing interest in plant-based formulations for effective wound treatment. Medicinal plants are increasingly recognized for their wound healing potential with lower side effects, particularly in diabetic, infected, or open wounds, supported by studies highlighting various identified mechanisms for improved healing. Medicinal plants such as Allium sativum, Boerhavia diffusa, Calendula officinalis, Crocus sativus, Curcuma longa L., Glycyrrhiza glabra L., Melaleuca alternifolia, Woodfordia fruticosa, etc. have demonstrated wound healing properties and have proven effective in treating wounds. This review highlights medicinal plants in wound healing, emphasizing in-vivo models, specifically examining their effectiveness in excision and incision wound healing.
KEYWORDS:Allium sativum; Boerhavia diffusa; Crocus sativus; Woodfordia fruticosa; Wound Healing
Download this article as:| Copy the following to cite this article: Goyal G, Kumar V, Tyagi H, Varshney P, Mishra S. K, Chauhan S. Herbal Remedies in Wound Healing: A Comprehensive Review of Plants and Non-Clinical Applications. Orient J Chem 2024;40(2). |
| Copy the following to cite this URL: Goyal G, Kumar V, Tyagi H, Varshney P, Mishra S. K, Chauhan S. Herbal Remedies in Wound Healing: A Comprehensive Review of Plants and Non-Clinical Applications. Orient J Chem 2024;40(2). Available from: https://bit.ly/3U1zB7k |
Introduction
The skin is essential for our survival as it functions to perceive and respond to the surrounding environment, regulating temperature and chemical balance, storing vital nutrients, offering defense mechanisms, and reacting to injuries and stress 1. To sustain these vital roles, the skin needs strong, efficient methods to shield it from harm, restore essential functions when compromised, and mend or regenerate what’s been injured or lost. Throughout history, humans have tended to their wounds for thousands of years 2. The scope of traditional wound care is often confined to locally available resources like water, soil, plants, and animal-based products. Throughout regions spanning Eastern Asia, the African continent, Southwest Asia, and Ibero-America, millions depend primarily, and sometimes exclusively, on locally sourced remedies derived from plants, animals, and natural elements for wound management 3.
The process of wound healing encompasses an intricate interplay of components, incorporating substances in solution, circulatory cells, matrix outside cells, and essential tissue cells, collaborating through a series of complex processes 4,5. The process of healing a wound consists of three separate stages: inflammation, the creation of tissue, and the alteration of tissue. Inflammation encompasses the clustering of platelets, coagulation, and the movement of leukocytes. The creation of tissue involves re-establishing the epithelial layer, angiogenesis, the formation of fibrous tissue, and the contraction of the wound. The remodeling stage, lasting approximately one month, involves the dermal response to damage, wherein fibrous protein and interstitial proteins are generated before reverting to its state before the injury, as depicted in Fig.16.
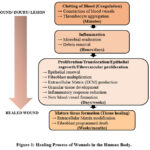 |
Figure 1: Healing Process of Wounds in the Human Body. |
Nonsteroidal anti-inflammatory drugs (NSAIDs), widely employed, are a common choice for pain relief associated with injuries such as mechanical or surgical wounds. However, continuous systemic exposure to these drugs is not advisable throughout the entire healing process. To minimize this, a more effective approach involves incorporating anti-inflammatory drugs into wound dressings or sutures. This allows for targeted drug release, providing localized pain relief. Methods like electrospinning, suture coating, and hydrogel production are frequently employed in creating these specialized wound treatments 7. Topical antibiotic delivery to wounds needs less drug compared to systemic treatment, offering concentrated and sustained drug levels at the wound site 8. By integrating antibiotics with proper nutrition, suitable dressing, and removal of dead tissues, the risk of external microbial infection can be minimized 9. However, overexposing the indigenous microbial community exposed to an abundance of antibiotics may contribute to the development of antibiotic-resistant variants 10. Moreover, managing the influence of antibiotics on the pre-existing beneficial bacterial community poses a challenge 11. In addition to resistance development, applying topical antibiotics can potentially delay hypersensitivity reactions and trigger superinfections 8. Pathogens are increasingly showing antibiotic resistance, and the decline in new antibiotic development has led to a quest for alternative approaches. Non-antibiotic elements like silver and plant-based products are gaining attention for their antimicrobial control in wound healing 12.
The diverse array of chemical compounds present in secondary metabolites is believed to contribute to the medicinal properties exhibited by plants 13. The complexity of herbal medicines presents an advantage, as their biological efficacy often stems from the combined action of multiple compounds 14. Herbal medicines have the advantage of being holistic, utilizing a variety of substances for a range of possibly synergistic health benefits 15.
Methods
In this review, articles have been included after thorough literature review using different databases like PubMed and Google Scholar etc. The literature search was performed using combination of different search keys or keywords, viz. ‘wound healing’, ‘medicinal plants used in wound healing’, ‘advantages of herbal medicines’, ‘Allium sativum in wound healing’, ‘Aloe barbadensis miller in wound healing’, ‘Boerhavia diffusa in wound healing’, ‘Calendula officinalis in wound healing’, ‘Commiphora myrrha in wound healing’, ‘Crocus sativus in wound healing’, ‘Curcuma longa L. in wound healing’, ‘Glycyrrhiza glabra L. in wound healing’, ‘Melaleuca alternifolia in wound healing’, ‘Woodfordia fruticosa in wound healing’ and ‘in-vivo wound healing model’. Our paper selection criteria involved exclusively peer-reviewed articles supported by credible references and substantial data as evidence. Specifically, the literature collection was focused on excision and incision in-vivo wound healing models.
Medicinal Plants employed in wound healing
Fig. 2 presents a compilation of the plants most frequently utilized for their wound healing properties.
 |
Figure 2: Plants used in wound healing. |
Allium sativum
Allium sativum, known widely as garlic, is a member of the Amaryllidaceae family 16. Garlic is used globally for both culinary purposes and as a traditional remedy for diverse health issues. Its distinct taste and aroma make it a popular ingredient in various cuisines worldwide. Beyond its culinary role, garlic has a rich history of medicinal use. Acknowledged for its potential health advantages, which encompass reducing blood pressure, enhancing cholesterol levels, supporting cardiovascular well-being, managing blood sugar, and possessing antimicrobial properties to counter infections. Furthermore, its antioxidants might contribute to anti-cancer and anti-aging effects 17.
The chemical compound allicin, characterized by its pungent odor and flavor, stands out as the most bioactive sulfurous compound recognized in garlic 18,19. The majority, roughly 70%, of sulfur-containing compounds in pulverized garlic bulbs originate from allicin, mainly sourced from its precursor known as alliin (S-allyl-L-cysteine sulfoxide) 20.
Allicin demonstrates its antibacterial effects by modifying sulfhydryl groups in microbial proteins, particularly in the bacteria Pseudomonas aeruginosa and Staphylococcus aureus, both known for causing infections in burn wounds21. Moreover, allicin plays a crucial role in activating fibroblasts, accelerating the alignment of the skin borders, promoting
structured collagen formation, and hastening the increase in tensile resilience within the regenerating tissue, thereby facilitating faster healing of wounds 22.
Aloe barbadensis Miller
Aloe vera, also known as Aloe barbadensis, is a member of the Lilaceae plant family. The utilization of aloe vera in crafting new food items has increased owing to its medicinal properties and functional attributes23. The aloe vera sector is expanding owing to the plant’s numerous asserted health advantages. Its applications range from the manufacturing of cosmetic products, laxatives, and nutrient-rich foods, including facial creams, hand lotions, base makeup, facial cleansers, lip color, moisturizers, hair care products, hair tonics, shaving creams, and bath essentials, as well as formulations for makeup and fragrances24. Numerous biological processes, including detoxification, constipation relief, elimination of waste products and toxins from the body, and improved digestion, have all been related to aloe plants. The bioactivities of aloe plants, attributed to their antibacterial, antiseptic, anticancerous, anti-swelling, anti-rheumatic, and joint-protective properties, contribute to their diverse range of therapeutic effects25.
Throughout history, Aloe vera has been utilized for the purpose of wound recovery. The application of Aloe vera extends to the treatment of enduring wounds, such as pressure sores, burns, surgical cuts, fissured nipples, genital herpes, and psoriasis. Studies indicate that Aloe vera hydrogel demonstrates a positive impact on inflammation, the formation of new blood vessels, and the narrowing of wounds, leading to a 29% reduction in the total healing time and the full closure of wounds within a 15-day timeframe26.
3. Boerhavia diffusa
Boerhavia diffusa, commonly recognized as “Red Spiderling” or “Punarnava,” is a perennial herbal plant belonging to the Nyctaginaceae plant family27. Boerhavia diffusa has been extensively studied for its various pharmacological properties, and several of its traditional uses have been validated through scientific research. Various studies have reported the immune system-regulating, anti-tumor, anti-diabetic, anti-blood clot dissolution, anti-swelling, fluid-reducing, liver-protective, antimicrobial, antifungal, antiseizure, and free radical-fighting properties associated with it. The plant contains various bioactive compounds that contribute to these diverse medicinal properties, making it a subject of interest in pharmacological research and herbal medicine 28,29,30,31,32,33.
Punarnavoside, a flavonoid glycoside primarily found in the roots of Boerhavia diffusa, contributes to its antifibrinolytic properties. This attribute is valuable in exploring the plant potential for aiding wound healing treatments. Fibrin plays a vital role in the healing of wounds by creating a steadfast clot at the site of injury. Plasmin, on the other hand, is involved in breaking down clots by fibrinolysis, which can potentially destabilize the clot essential for healing. Inhibiting factors XIa and XII, which are involved in activating plasmin, can help maintain the stability of fibrin within the wound, promoting better healing outcomes. This inhibition prevents premature breakdown of the clot, allowing the wound to heal more effectively34.
Calendula officinalis
Calendula officinalis, a member of the Asteraceae family, is widely cultivated as an ornamental plant and bears medicinal importance in regions including European continent, People’s Republic of China, America, and India. Commonly known by various names such as Marigold and Pot Marigold, it holds significance in traditional medicine practices. Historically, it has been applied topically to address minor wounds, burns, and various skin ailments35,36. Known for its medicinal properties, it is commonly employed as a remedy with anti-inflammatory, diaphoretic, analgesic, and antiseptic characteristics. Its applications span treating gastrointestinal and gynecological issues, oral ailments, eye conditions, skin injuries, and certain types of burns, among other therapeutic uses37.
Preparations derived from Calendula officinalis are predominantly utilized as medicinal aids in wound healing for skin inflammations, mucous membrane issues, tissue repair, scar healing, blisters, and allergic rashes. Preparations of these varieties are available in diverse forms, including infusions, tinctures, and ointments. Studies have indicated the beneficial effects of Calendula extract cream in treating burn-related swelling. Furthermore, in vitro laboratory examinations demonstrated the restraining impact of the essential oil derived from the flowers on Bacillus subtilis, Candida albicans, Escherichia coli, Pseudomonas aeruginosa, and Staphylococcus aureus38,39.
Commiphora myrrha
Commiphora myrrha, commonly referred to as “True Myrrha” or “Molmol Myrrha,” is a botanical resin acquired by scraping the bark of the Commiphora myrrha tree, which is part of the Burseraceae family. The resin is utilized for addressing various conditions, encompassing arthritis, hyperlipidemia, obesity, pain management, fractures, cancer support, wound care, and combating bacterial and parasitic infections 40.
Commiphora myrrha exhibits a diverse array of biological attributes, including antibacterial, antinociceptive, anti-inflammatory, and antiulcer effects. Additionally, studies have indicated its antioxidant and immunopotentiating properties41.
In vitro experiments demonstrated that Commiphora myrrha possesses anti-inflammatory properties by inhibiting interferon-γ, interleukin-12 (IL-12), TNF-α, IL-1β, and nitric oxide levels. These properties contribute to the promotion of wound healing42.
Crocus sativus
Part of the Iridaceae botanical family, Crocus sativus L. is widely recognized as saffron, denoting the seasoning obtained from the desiccated stigmas of the Crocus sativus blossom. Saffron holds significance in traditional medicinal practices such as Chinese, Ayurvedic, Persian, and Unani medicine, where it is utilized for various therapeutic purposes [43]. Recent pharmacological investigations have confirmed that saffron extract or its active components, comprising carotenoid derivatives, simple terpenes, flavones, phenolics, and plant sterols, they showcase a varied range of therapeutic advantages44.
Crocin and Safranal, alongside their precursor picrocrocin, form carotenoid elements found in Crocus sativus L., showcasing substantial antioxidative characteristics and the capability to counteract free radicals.Top of Form
Research indicates the potential anti-inflammatory and antitumoral characteristics associated with these compounds 45,46.
Saffron significantly enhanced blood vessel formation and improved the multiplication and mobility of fibroblasts. It diminished inflammation and remarkably accelerated wound closure rates, encompassing the regeneration of epithelial tissue and wound contraction, in comparison to wounds treated through alternative approaches. Additionally, saffron intervention reduced neutrophil numbers and alleviated the occurrence of free radicals and reactive oxygen species (ROS) in the burn setting47.
Curcuma longa L.
Curcuma longa L., recognized for its rhizomes, is a perennial leafy herb belonging to the Zingiberaceae family and the Curcuma genus. The spice, commonly referred to as “turmeric,”
extracted from the rootstock of Curcuma longa, is rich in polyphenolic antioxidant compounds48.
There is substantial evidence supporting the advantageous effects of turmeric in alleviating conditions such as breakouts, irritation, discomfort in joints, respiratory distress, dermatitis, and sudden allergic reactions. Additionally, turmeric has shown positive impacts on wound healing, mood regulation, blood sugar level maintenance, and immunomodulation49.
Curcumin, a bioactive and lipophilic compound, originates from the rootstock of the Curcuma longa Linnean botanical specimen50.
The potential anti-swelling mechanism of action attributed to curcumin involves the modulation of gene expression linked to inflammatory cytokines. This modulation contributes to the regulation of the release of heightened levels of interleukin-6 (IL-6), tumor necrosis factor (TNF), and nitric oxide (NO), which have the potential to trigger enduring inflammation51. Furthermore, its non-targeted antimicrobial mechanism against bacteria is probably accomplished by attaching to FtsZ proteins. This attachment hinders the formation of FtsZ protofilaments, thereby efficiently obstructing bacterial growth and reproduction. Additionally, its method of operation may involve the interference with mecA gene transcription, leading to a reduction in the expression of penicillin-binding protein-2α. To illustrate, the interaction of curcumin with peptidoglycan on the cell wall of S. aureus makes it inaccessible for new peptidoglycan production, thereby weakening the peptidoglycan layer and ultimately causing bacterial breakdown52.
Glycyrrhiza glabra L.
Glycyrrhiza glabra, a perennial leafy plant within the Fabaceae legume family, is recognized for generating licorice, a flavoring with a sweet and aromatic profile derived from its root. This plant is extensively utilized as a herbal remedy and frequently incorporated into skincare items 53,54.
Traditionally, licorice has been documented as a remedy for a multitude of conditions, including bronchial condition like asthma, inflammation of the tonsils, and throat discomfort, excessive thirst, bloating, seizures, pyrexia, erectile dysfunction, immobility, respiratory irritation, gastric lesions, acid reflux, abdominal spasms, swelling, musculoskeletal discomfort, dermatological conditions, gastric acidity, vaginal discharge, hemorrhage, abnormal bleeding conditions, and icterus. Furthermore, it was historically utilized as an insect repellent, substance to promote bowel movements, anti-inflammatory agent, anti-ulcer remedy, antibiotic, anti-arthritis solution, antiviral substance, and cognitive booster associated with its capacity as an MAO inhibitor, along with having anti-cholinergic properties, cough suppressant, cavity prevention, cholesterol-lowering, antimycotic, estrogen like substance, antioxidative, anticancerous, and antidiuretic agent55.
As reported by Zangeneh et al. in 2019, the aqueous extract ointment derived from Glycyrrhiza glabra demonstrated significant enhancements across different stages of wound healing. These improvements encompassed wound contraction, epithelialization, the inflammatory response, and tensile strength, surpassing the effects observed in the control group56.
Melaleuca alternifolia
Melaleuca alternifolia, commonly known as tea tree, is a small tree belonging to the Myrtaceae family and native to Australia. The essential oil derived from tea tree leaves is categorized into three primary chemovarieties: terpinen-4-ol, terpinolene, and 1,8-cineole. There are also additional chemotypes displaying diverse compositions of dominant and non-dominant constituents. Among these, the terpinen-4-ol chemotype stands out as both dominant and more medically compelling. In medicinal applications, tea tree essential oil is employed to combat acne, alleviate contact dermatitis, and enhance the process of wound healing57.
The essential oil derived from tea tree leaves possesses antimicrobial properties, which can help prevent infection in wounds. It also exhibits anti-inflammatory effects that may aid in reducing inflammation at the wound site. Furthermore, the use of tea tree oil has been suggested to promote the healing of wounds by encouraging the formation of new tissue and hastening the closure of injuries58.
Woodfordia fruticosa
Woodfordia fruticosa, commonly identified as Fire flame bush, belongs to the Lythraceae family. This plant is distinguished for its widely acknowledged pharmacological characteristics, encompassing antimicrobial, anti-swelling, ulcer-protective, liver-protective, immune system-regulating, tumor-fighting, heart-protective, pain-relieving, and wound-repairing properties59.
While all components of plants harbor medicinal attributes, flowers, particularly with reddish-brown hues, are highly sought after in both domestic and global markets. These blossoms boast pungent, acrid, cooling, and alexiteric properties, serving as a uterine sedative and anthelmintic agent. The dried flowers of Woodfordia fruticosa are employed in the treatment of various ailments such as sprue, dysentery, intestinal complaints, rheumatism, hematuria, as well as for addressing wounds, bleeding, and injuries60.
The Woodfordia fruticosa flower is chemically abundant in tannins, specifically constituents like Ellagic acid and Gallic acid, which play pivotal roles in its pharmacological effects. During the proliferative phase, marked by the proliferation of granulation tissue primarily driven by fibroblasts and the crucial angiogenesis process, the flower contributes significantly by facilitating oxygen and metabolite supply to tissues. Notably, the flower showcases antifertility properties and demonstrates in vitro antibacterial effects, effectively preventing microbial wound infections. Phytochemical analysis has confirmed the presence of polyphenolic compounds, particularly tannins, which exhibit robust wound healing activity. Tannins, recognized for their astringent properties, are likely responsible for the observed antibacterial effects as well61.
Following are the plants which have been included in this study and their description is given in the Table. 1 which includes their common names, family, wound healing animal models, formulation used in the animal models and critical findings from the research work performed on animals by using different types of herbal preparation.
Table 1: Pharmacological profile of plants used for wound healing
|
Name of the Plant |
Family |
Wound Healing Animal Model |
Formulation used in Animal Model |
Findings |
References |
|
Allium sativum (Garlic, Lahsun) |
Amaryllidaceae |
Wound excision model in male Wistar rats |
10% topical garlic extract cream |
The utilization of a topical cream containing garlic extract (Allium sativum) demonstrated superior wound recovery in comparison to the control group, as evidenced in the final phase of the healing process. This superiority was observed through the highest expression of Vascular Endothelial Growth Factor (VEGF) in comparison to the control group, an increased presence of fibroblast cells relative to the control group, and the smallest wound diameter compared to the control group. |
[62] |
|
Aloe barbadensis Miller (Aloe vera) |
Asphodelaceae (Liliaceae) |
Excision Wound Model in Male Wistar rats |
A novel multifunctional wound dressing (FWD) composed of collagen/chitosan-glucan complex hollow fibers encapsulating aloe vera (CO/CSGC@AV). |
The CO/CSGC@AV (1/2/2) wound bandage outperformed others due to its combined properties of CSGC and AV, enhancing all healing stages. It increased antioxidant enzymes, fibroblast migration, collagen formation, and wound contraction, reducing healing time. Compared to other dressings, CO/CSGC@AV-FWD showed superior efficacy, emphasizing its potential for optimal tissue hydration and accelerated healing. |
[63] |
|
Boerhavia diffusa (Punarnava, Red Spiderling) |
Nyctaginaceae |
Wound excision model in Wistar Albino rats |
A 10% (w/v) ointment was formulated using the methanolic extract of Boerhavia diffusa leaves in a saline solution containing 0.1% propylene glycol |
In a wound excision model conducted on albino Wistar rats, Boerhavia diffusa leaf methanol extract (ME) showed significant healing, achieving nearly complete wound closure (91%) within 14 days. The wounds treated with ME showed minimal scarring, indicating effective wound healing. |
[27] |
|
Calendula officinalis Linn. (Pot marigold) |
Asteraceae |
Excision Wound Model in Albino Wistar rats |
10% and 20% Herbal Ointment (HO) of Calendula officinalis Linn. |
In summary, the herbal ointment incorporating Calendula officinalis Linn. exhibited notable attributes in promoting wound healing. This was substantiated by heightened collagen synthesis, enhanced wound contraction, and modifications in essential factors such as IL-6, EGF, PDGF, and TNF-α. |
[64] |
|
Commiphora myrrha (Guggul, Guggal) |
Burseraceae |
Wound excision model in Wistar Albino rats and wound incision model in Swiss Albino mice |
Ointments containing 4% (v/w) essential oil and 5% (w/w) resin extracted from Commiphora myrrha |
The essential oil and resin obtained from the plant possess the capability to facilitate wound contraction, strengthen tensile resilience, and decrease epithelization time.Top of Form |
[65] |
|
Crocus sativus (Saffron, Kesar) |
Iridaceae |
Wound excision model in female Sprague-Dawley rats |
5% w/w topical cream containing safranal and crocin |
In the study, both safranal and crocin demonstrated wound healing properties, with safranal showing slight superiority. Nevertheless, saffron demonstrated a more pronounced efficacy in promoting wound healing when compared to safranal and crocin, which constitute its primary active components. |
[66] |
|
Curcuma longa L. (Turmeric, Haldi) |
Zingiberaceae |
Wound excision model in Wistar rats |
0.5% curcumin-gel and 0.5% curcumin NEG (Curcumin Nanoemulgel) |
The healing properties of treatments containing curcumin, combined with its integration into a novel emulsion gel (NEG) as a carrier, bestow an extra feature to curcumin, enabling more profound skin permeation and localized drug deposition. As a result, an improved efficacy in the healing process was observed for curcumin. |
[67] |
|
Glycyrrhiza glabra L. (Licorice) |
Fabaceae |
Wound excision model in male Sprague-Dawley rats |
Glycyrrhiza glabra aqueous extract ointment |
The research demonstrated that the ointment containing Glycyrrhiza glabra aqueous extract enhanced different stages of wound healing, encompassing wound shrinkage, re-epithelialization, inflammatory response, and tensile resilience, surpassing the effects of alternative treatments. These results substantiate the historical application of Glycyrrhiza glabra leaves for wound treatment. |
[56] |
|
Melaleuca alternifolia (Tea Tree) |
Myrtaceae |
Excision Wound Model in Swiss Mice (Mus musculus) |
Melaleuca alternifolia essential oil–loaded bicontinuous microemulsion |
In vivo investigations have indicated the efficacy of MEO-loaded BME in facilitating the recuperation of the skin wounds by promoting a greater percentage of wound margin contraction. Furthermore, it exhibited antimicrobial effectiveness against both Gram-positive and Gram-negative microbes. Consequently, the novel BME system incorporating MEO emerges as a favorable substitute for external application, demonstrating potential as a potent agent for promoting healing and addressing microbial concerns in skin wounds. |
[58] |
|
Woodfordia fruticosa (Fire Flame Bush, Dhataki) |
Lythraceae |
Excision Wound Model in Female Swiss Mice |
1% v/v topical gel infused with Silver Nanoparticles derived from Woodfordia fruticosa (WfAgNPs-Carbopol® 934 nanoformulation) and 1% v/v topical Woodfordia fruticosa Flower Extract Gel |
Applying the WfAgNPs-Carbopol® 934 nanoformulation topically to impaired tissue in Swiss mice led to accelerated wound healing. It prompted collagen fibril aggregation, granular tissue development, and epithelial lining revitalization, resulting in swift wound closure. This approach showed promise in preventing scar formation, as observed in in vivo testing. These findings highlight the potential of WfAgNPs-Carbopol® 934 in wound healing, offering insights for future innovative treatment approaches using novel nanoformulations. |
[68] |
Conclusion
Globally, severe lesions have a substantial effect on overall well-being for individuals. The successful management of wounds relies on the intricate interplay among specific cell varieties, receptor sites on cells, the matrix outside cells, and therapeutic interventions. The intricate composition of skin tissue presents a challenge in formulating an ideal medication capable of promoting rapid and effective healing.
Addressing wound healing, an enduring clinical challenge since ancient times, remains a complex task in effective wound treatment. The process entails various group of cells, the matrix outside cells, and the influence of soluble signaling molecules like stimulatory factors and cell-signaling proteins. Considerable research has centered on wound management, with a notable focus on innovating healing methodologies and advancing techniques for treating
both sudden and lingering wounds, particularly within the framework of traditional indian medicines (herbal) practices. Investigators are actively investigating novel formulations, bandages, and compositions incorporating healing plants to create economical, effective, and durable, and sustainable delivery systems for the treatment of wounds. The integration of nanotechnology and the availability of novel materials are enhancing the effectiveness and patient-centric nature of wound management. Emerging innovations such as 3D printing offer beneficial possibilities for creating diverse drug delivery systems to address wound management. Looking ahead, tissue engineering and regenerative medicine represent futuristic technologies in developing comprehensive wound healing systems. The implementation of improved quality assurance methods for identifying, assessment, and quantifying plant-based constituents, coupled with carefully planned preclinical and clinical investigations, holds the potential to open new avenues in wound care management.
The present direction in wound care involves pioneering treatments that merge traditional herbal remedies with contemporary products and methodologies. Advancements in nanostructures and nanoformulations have notably addressed the limitations of conventional medications, showing promise in the field. In these procedures, numerous compounds extracted from plants collaborate synergistically to achieve the desired effect. Consequently, the concentration and blending of these phytochemicals from diverse sources, at optimal levels, are anticipated in the coming years. This aims to facilitate multi-functional approaches in healing various types of wounds, leveraging the growing understanding of the properties of essential constituents and their role in the healing processes.
Acknowledgement
Authors are thankful to KIET School of Pharmacy for providing necessary facilities to carry out systematic literature search and writing of review paper.
Conflict of Interest
The authors assert that they have no conflicts of interest.
Ethical statement
This review paper does not incorporate the use of human or animal participants.
References
- Xu, R.; Luo, G.; Xia, H.; He, W.; Zhao, J.; Liu, B.; Tan, J.; Zhou, J.; Liu, D.; Wang, Y.; Yao, Z. Biomaterials. 2015, 40, 1-11.
CrossRef - Takeo, M.; Lee, W.; Ito, M. Cold Spring Harb. Perspect. Med. 2015, 5, 1.
- Shedoeva, A.; Leavesley, D.; Upton, Z.; Fan, C. J. Evid. Based Complementary Altern. Med. 2019, 2019.
CrossRef - Bullers, S.; Berry, H.; Ingham, E.; Southgate, J. J. Tissue Eng. Regen. Med. 2012, 6, 218.
- Pesce, M.; Patruno, A.; Speranza, L.; Reale, M. Eur. Cytokine Netw. 2013, 24, 1-10.
CrossRef - Farahpour, M.R. Wound Heal. Curr. Perspect. 2019, 2019, 33-47.
- Deng, X.; Gould, M.; Ali, M.A. J. Biomed. Mater. Res. 2022, 110, 2542-2573.
CrossRef - Kaiser, P.; Wächter, J.; Windbergs, M. Drug Deliv. Transl. Res. 2021, 11, 1545-1567.
CrossRef - Gámez-Herrera, E.; García-Salinas, S.; Salido, S.; Sancho-Albero, M.; Andreu, V.; Perez, M.; Lujan, L.; Irusta, S.; Arruebo, M.; Mendoza, G. Eur. J. Pharm. Biopharm. 2020, 152, 327-339.
CrossRef - Saghazadeh, S.; Rinoldi, C.; Schot, M.; Kashaf, S.S.; Sharifi, F.; Jalilian, E.; Nuutila, K.; Giatsidis, G.; Mostafalu, P.; Derakhshandeh, H.; Yue, K. Adv. Drug Deliv. Rev. 2018, 127, 138-166.
CrossRef - Ray, P.; Singh, S.; Gupta, S. Indian J. Med. Microbiol. 2019, 37, 299-308.
CrossRef - Llorens, E.; Calderon, S.; Del Valle, L.J.; Puiggali, J. Mater. Sci. Eng. C. 2015, 50, 74-84.
CrossRef - Wink, M. Curr. Drug Metab. 2008, 9, 996-1009.
CrossRef - Carmona, F.; Pereira, A.M.S. Rev. bras. farmacogn. 2013, 23, 379-385.
CrossRef - Amparo, T.R.; Seibert, J.B.; Vieira, P.M.D.A.; Teixeira, L.F.M.; Santos, O.D.H.D.; de Souza, G.H.B. Phytother. Res. 2020, 34, 94-103.
CrossRef - Londhe, V.P.; Gavasane, A.T.; Nipate, S.S.; Bandawane, D.D.; Chaudhari, P.D. Angiogenesis. 2011, 12, 129-134.
- Vitale, S.; Colanero, S.; Placidi, M.; Di Emidio, G.; Tatone, C.; Amicarelli, F.; D’Alessandro, A.M. Molecules. 2022, 27, 3566.
CrossRef - Slusarenko, A.J.; Patel, A.; Portz, D. Sustain. Dis. Manag. Eur. Context. 2008, 313-322.
CrossRef - Rahman, M.S. Int. J. Food Prop. 2007, 10, 245-268.
CrossRef - Santhosha, S. G.; Jamuna, P.; Prabhavathi, S. N. Food Biosci. 2013, 3, 59-74.
CrossRef - Alhashim, M.; Lombardo, J. Dermatol. Surg. 2018, 44, 630-634.
CrossRef - Ejaz, S.; Chekarova, I.; Cho, J.W.; Lee, S.Y.; Ashraf, S.; Lim, C.W. Drug Chem. Toxicol. 2009, 32, 191-203.
CrossRef - Sonawane, S.K.; Gokhale, J.S.; Mulla, M.Z.; Kandu, V.R.; Patil, S. J. Food Sci. Technol. 2021, 58, 1217-1226.
CrossRef - Kumara, G. U. A.; Mudiyanselage, W.; Wadimuna, D. R. W.; Saroja, W. R. Int. J. Herb. Med. 2021, 9, 32-36.
- Salehi, B.; Albayrak, S.; Antolak, H.; Kręgiel, D.; Pawlikowska, E.; Sharifi-Rad, M.; Uprety, Y.; Tsouh Fokou, P.V.; Yousef, Z.; Amiruddin Zakaria, Z.; Varoni, E.M. Int. J. Mol. Sci. 2018, 19, 2843.
CrossRef - Albahri, G.; Badran, A.; Hijazi, A.; Daou, A.; Baydoun, E.; Nasser, M.; Merah, O. Life. 2023, 13, 317.
CrossRef - Juneja, K.; Mishra, R.; Chauhan, S.; Gupta, S.; Roy, P.; Sircar, D. J. Tradit. Complement. Med. 2020, 10, 52-59.
CrossRef - Srivastava, R.; Saluja, D.; Dwarakanath, B.S.; Chopra, M. J. Evid. Based Complementary Altern. Med. 2011, 2011.
CrossRef - Pari, L.; Amarnath Satheesh, M. J. Med. Food. 2004, 7, 472-476.
CrossRef - Das, S.; Singh, P. K.; Ameeruddin, S.; Bindhani, B. K.; Obaidullah, W. J.; Obaidullah, A. J.; Mishra, S.; Mohapatra, R. K. Front. Chem. 2023, 11. 1-5.
CrossRef - Olaleye, M.T.; Akinmoladun, A.C.; Ogunboye, A.A.; Akindahunsi, A.A. Food Chem. Toxicol. 2010, 48, 2200-2205.
CrossRef - Umamaheswari, A.; Nuni, A.; Shreevidya, R. Int. J. Green Pharm. 2010, 4.
CrossRef - Kaur, M.; Goel, R.K. Evid. Based Complement. Alternat. Med. 2011, 2011.
- Patil, S.; Ganeshpurkar, A.; Shrotriya, S.; Sawant, P.; Mulgund, S. Colloids Surf. B Biointerfaces. 2023, 230, 113483.
CrossRef - Basch, E.; Bent, S.; Foppa, I.; Haskmi, S.; Kroll, D.; Mele, M.; Szapary, P.; Ulbricht, C.; Vora, M.; Yong, S. J. Herb. Pharmacother. 2006, 6, 135-159.
CrossRef - Jan, N., Andrabi, K.I.; John, R. Proc. Indian Natl. Sci. Acad. 2017, 83, 769-787.
- AshwlayanVD, K.A.; Verma, M. Pharm. Pharmacol. Int. J. 2018, 6, 149-155.
- Arora, D.; Rani, A.; Sharma, A. Pharmacogn. Rev. 2013, 7, 179.
CrossRef - Patil, K.; Sanjay, C.J.; DoggALLI, N.; Devi, K.R.; Harshitha, N. J. Clin. Diagn. Res. 2022, 16.
- Alehaideb, Z.; Alatar, G.; Nehdi, A.; Albaz, A.; Al-Eidi, H.; Almutairi, M.; Hawsa, E.; Alshuail, N.; Matou-Nasri, S. Saudi Pharm. J. 2021, 29, 361-368.
CrossRef - Ashry, K.M.; El-Sayed, Y.S.; Khamiss, R.M.; El-Ashmawy, I.M. Food Chem. Toxicol.2010, 48, 236-241.
CrossRef - Manjula, N.; Gayathri, B.; Vinaykumar, K.S.; Shankernarayanan, N.P.; Vishwakarma, R.A.; Balakrishnan, A. Int. Immunopharmacol. 2006, 6, 122-132.
CrossRef - Kumar, R.; Singh, V.; Devi, K.; Sharma, M.; Singh, M.K.; Ahuja, P.S. Food Rev. Int. 2008, 25, 44-85.
CrossRef - Abu-Izneid, T.; Rauf, A.; Khalil, A.A.; Olatunde, A.; Khalid, A.; Alhumaydhi, F.A.; Aljohani, A.S.; Sahab Uddin, M.; Heydari, M.; Khayrullin, M.; Shariati, M.A. Crit. Rev. Food Sci. Nutr. 2022, 62, 2683-2706.
CrossRef - Khorasani, G.; Hosseinimehr, S.J.; Zamani, P.; Ghasemi, M.; Ahmadi, A. Keio J. Med. 2008, 57, 190-195.
CrossRef - Alemzadeh, E.; Oryan, A. Planta Med. 2018, 84, 1191-1200.
CrossRef - Comino-Sanz, I.M.; López-Franco, M.D.; Castro, B.; Pancorbo-Hidalgo, P.L. J. Clin. Med. 2021, 10, 3558.
CrossRef - Hassan, A. Curcuma longa, turmeric: a monograph. 2006, 66-76.
- Fuloria, S.; Mehta, J.; Chandel, A.; Sekar, M.; Rani, N.N.I.M.; Begum, M.Y.; Subramaniyan, V.; Chidambaram, K.; Thangavelu, L.; Nordin, R.; Wu, Y.S. Front. Pharmacol. 2022, 13, 820806.
CrossRef - Chattopadhyay, I.; Biswas, K.; Bandyopadhyay, U.; Banerjee, R.K. Curr. Sci. 2004, 44-53.
- Esatbeyoglu, T.; Ulbrich, K.; Rehberg, C.; Rohn, S.; Rimbach, G. Food Funct. 2015, 6, 887-893.
CrossRef - Oluwole, D.O.; Coleman, L.; Buchanan, W.; Chen, T.; La Ragione, R.M.; Liu, L.X. Pharmaceutics. 2022, 14, 1021.
CrossRef - Komes, D.; Belščak-Cvitanović, A.; Jurić, S.; Bušić, A.; Vojvodić, A.; Durgo, K. Int. J. Food Sci. Nutr. 2016, 67, 53-66.
CrossRef - Jeon, J.S.; Kim, H.T.; Kim, M.G.; Oh, M.S.; Hong, S.R.; Yoon, M.H.; Shin, H.C.; Shim, J.H.; Afifi, N.A.; Hacımüftüoğlu, A.; Abd El-Aty, A.M. Chromatographia. 2016, 79, 851-860.
CrossRef - El-Saber Batiha, G.; Magdy Beshbishy, A.; El-Mleeh, A.; M. Abdel-Daim, M.; Prasad Devkota, H. Biomolecules. 2020, 10, 352.
CrossRef - Zangeneh, A.; Pooyanmehr, M.; Zangeneh, M.M.; Moradi, R.; Rasad, R.; Kazemi, N. Comp. Clin. Pathol. 2019, 28, 1507-1514.
CrossRef - Borotová, P.; Galovičová, L.; Vukovic, N.L.; Vukic, M.; Tvrdá, E.; Kačániová, M. Plants. 2022, 11, 558.
CrossRef - de Assis, K.M.A.; da Silva Leite, J.M.; de Melo, D.F.; Borges, J.C.; Santana, L.M.B.; Dos Reis, M.M.L.; Moreira, V.M.; da Rocha, W.R.V.; Catao, R.M.R.; Dos Santos, S.G.; da Silva Portela, A. Drug Deliv. Transl. Res. 2020, 10, 1748-1763.
CrossRef - Aileni, M.; Bulle, M.; Malavath, R.N.; Thurpu, S.; Bandaram, K.; Balkampeta, B.; Marri, M.; Singasani, V.S.R.; Murthy, E.N. Appl. Microbiol. Biotechnol. 2023, 107, 5855-5871.
CrossRef - Najda, A.; Bains, A.; Klepacka, J.; Chawla, P. Front. Nutr. 2022, 9, 944856.
CrossRef - Sonawane, Y.T.; Pmipare, S.S.; Chaudhari, C.A.; Jain, N.P.; Pal, S.C.; Gadgoli, C.H.; Bairagi, V.A.; Govilkar, S.A.; Bhaskar, A. Int. J. Pharmacogn. Life Sci. 2020, 1, 06-13
CrossRef - Hemmatpor, Z.; Kamali, J.; Mehrabani, M.; Hashemi, S.A.; Marashi, S.M.A.; Pahlevan, S.; Tavakoli-Far, B. Egypt. J. Vet. Sci. 2020, 51, 181-189.
CrossRef - Abdel-Mohsen, A.M.; Frankova, J.; Abdel-Rahman, R.M.; Salem, A.A.; Sahffie, N.M.; Kubena, I.; Jancar, J. Int. J. Pharm. 2020, 582, 119349.
CrossRef - Gunasekaran, S.; Nayagam, A.A.J.; Natarajan, R. Clin. Phytosci. 2020, 6, 1-8.
CrossRef - Gebrehiwot, M.; Asres, K.; Bisrat, D.; Mazumder, A.; Lindemann, P.; Bucar, F. Ethiop. Pharm. J. 2016, 32, 85-100.
CrossRef - Deldar, N.; Monsefi, M.; Salmanpour, M.; Ostovar, M.; Heydari, M. Galen Med. J. 2021, 10, e1900.
CrossRef - Algahtani, M.S.; Ahmad, M.Z.; Nourein, I.H.; Albarqi, H.A.; Alyami, H.S.; Alyami, M.H.; Alqahtani, A.A.; Alasiri, A.; Algahtani, T.S.; Mohammed, A.A.; Ahmad, J. Gels. 2021, 7, 213.
CrossRef - Maheshwari, S. Intell. Pharm. 2024, 2, 17-27.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









