Green Mechanochemistry: Synthesis of Highly Efficient Meso-Substituted Tetra Phenyl Porphyrin Sensitizers for Emerging Organic Photovoltaics Technology
Dhote Bandhu Science College, Gondia, M.S, India.
Corresponding Author E-mail: 2manojpatle14@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/400220
Article Received on : 23 Jan 2024
Article Accepted on :
Article Published : 15 Apr 2024
Reviewed by: Dr. Farzaneh Mohamadpour
Second Review by: Dr. Chinu Sharma
Final Approval by: Dr. Dinesh Chand Sharma
The present work deals with the preparation of porphyrin derivatives using the green synthesis method. Here the focus is on reducing the steps of the reaction scheme and using a mechanochemical C-C bonding method that minimizes the use of a solvent and makes the reaction environmentally friendly. This work is about the synthesis of meso-substituted tetraphenylporphyrin (TPP). Step I of the scheme deals with the mechanochemical synthesis of oxidizable porphyrin intermediates. In Step II, these oxidizable porphyrin intermediates are treated with effective oxidizing agents to convert them into the product. Product purity is checked primarily by UV-visible spectrophotometry and confirmed by IR, H1-NMR, C13, and MALDI-TOF instrumentation techniques. The synthesized porphyrins sensitizers can also generate current without any external load. Hence, they have photovoltaic importance in emerging organic photovoltaic technology-based Solar Cells.
KEYWORDS:Green chemistry; Mechanochemistry; Photovoltaics; Tetra-phenyl porphyrin; JV plot
Download this article as:| Copy the following to cite this article: Bhagat Y. G, Patle M. R. Green Mechanochemistry: Synthesis of Highly Efficient Meso-Substituted Tetra Phenyl Porphyrin Sensitizers for Emerging Organic Photovoltaics Technology. Orient J Chem 2024;40(2). |
| Copy the following to cite this URL: Bhagat Y. G, Patle M. R. Green Mechanochemistry: Synthesis of Highly Efficient Meso-Substituted Tetra Phenyl Porphyrin Sensitizers for Emerging Organic Photovoltaics Technology. Orient J Chem 2024;40(2). Available from: https://bit.ly/43Yjlal |
Introduction
Organic photovoltaic technology has recently emerged as an optional energy extractor for solar cell applications [1]. Sensitizers are at the heart of organic photovoltaic cells. There are hundreds of sensitizers on the market [2], but the effectiveness of the current generation has its limits. Therefore, there is a need for a special architectural design of sensitizers with updated effectiveness. Porphyrins are an essential molecular system in nature, known for photosynthesis in plants and oxygen transport in the blood. Novel porphyrin sensitizers in which the aryl groups act as electron donors and the malonic acid binding group as an acceptor demonstrate the exciting potential of porphyrins as light-harvesting green dyes. The modified acceptor units of the new dyes rendered stronger electron-withdrawing ability which resulted in better charge transfer and light harvesting properties [3][4]. Although the natural occurrence of porphyrin is found everywhere, its practical synthesis in the laboratory has its limitations [5].
Different binding sites at the periphery of the ring increased the synthetic importance of porphyrins. The porphyrin ring has an active cavity in the center of the structure that can accommodate metal ions, improving its practical applicability in synthesis [6][7].
The synthesis of porphyrins has been reported using various in-depth methods, which, however, are usually very expensive and involve the use of solvents [8]. The temperature conditions for such reactions also reach drastic proportions. Even after this, the tar formed after the reaction requires tedious chromatographic exercises to clean the samples [9].
In general, mechanical-chemical reactions are carried out in a mortar and pestle or with a ball mill. In most cases, good results were achieved with a mortar and pestle compared to a ball mill [10]. Thus, mechanochemistry approach helps to reduce the excessive use of solvents.
Ullmann and J. Mannasen synthesized the heteroatomic tetraphenylporphyrin (TPP) and successfully replaced the nitrogen atom of the pyrrole ring with other heteroatoms. Their experiment triggered an initial spark in this field, but the solvent used, for example, the use of propionic acid, benzene, toluene, and acetic acid, violates the rules of green chemistry. This conversion also requires refluxing at high temperatures [11]. Zhicheng Sun & et.al. modified the method of TPP synthesis by using the mixed solvent method. Under the inert atmosphere of nitrogen gas, they achieved the final TPP product with a yield of up to ~38% from the nominally pure to fairly pure form. The mixed solvent used for this was m-nitrotoulene, DMSO, and nitrobenzene as oxidants along with the acid catalyst [12] [13]. Diane F. Marsh & et al. extended this study and prepared metalloporphyrins, e.g. H2-TPP, Zn-TPP, Ni-TPP with DMF as solvent under reflux conditions [1]. T. Phromsatit & et al. synthesized TOMPP, CuTOMPP, and CuTOMPP-NO2 and investigated the solvent effect and thermal behavior on the product yield. They achieved percentage yields of TOMPP, CuTOMPP, and CuTOMPP-NO2 of 26%, 85%, and 0.03%, respectively [6]. However, this is not enough in the current scenario concerning efficiency and the environment as current research must follow the principles of green chemistry to protect the environment from dangerous chemicals.
Experimental
The present research work is about the production of meso-substituted tetraphenylporphyrin by condensation of various benzaldehydes with the pyrrole, catalyzed by the organic catalyst PTSA (p-toulylsulfonic acid), through simple manual grinding in a mortar and pestle, followed by air oxidation along with the appropriate Oxidation agent. This method can eliminate both solvents and high-temperature conditions in porphyrin synthesis.
H. Shy et.al. reported that the band at λmax~ 417 nm confirms the synthesis of porphyrins. After fifteen days the same powder turns black due to air oxidation, again the UV analysis was performed for the same samples, and it was found that at λmax~ 417 nm some soret bands were observed. This indicates the conversion of intermediates to porphyrin [19].
Materials and Methods
All chemicals were purchased from Sigma Aldrich and used without further purification unless undesirable changes were observed. Benzaldehyde and Pyrrole were purified by simple distillation if coloration develops due to oxidation. Mortar and Pestle are utilized for grinding purposes for better mixing of reactants. Electronic Spectra were recorded on UV-1800 SHIMADZU UV spectrophotometers with a scanning range of 200-800nm. The sample was placed in quartz cuvettes of 1cm path length. H1-NMR, as well as C13 NMR Spectra, were taken with Bruker advanced Neo 500MHZ NMR Spectrophotometer at SAIF Chandigarh. MALDI-TOF mass spectrum was collected on Synapt-XS#DBA064 mass spectrophotometer at SAIF Chandigarh. IR Spectra were collected with a SHIMADZU infrared spectrophotometer.
Synthesis of Tetra-phenyl Porphyrins by Simple Manual Grinding Method
Different oxidants can also be incorporated to facilitate the reaction and increase the oxidation capacity according to the reactant used for porphyrin synthesis. In addition to molecular iodine, (2,3-dichloro-5,6-bicyanobenzoquinone) DDQ also has excellent conversion capacity. Nitric acid is a better substitute as an oxidizer, but due to the highly corrosive nature of nitric acid, it is hardly suitable for solving this purpose. Sodium perborate, sodium ethoxide, and oxone also have sufficient capacity to remove the hydrogen atoms from porphyrin intermediates and convert them into the product.
General Procedure
Step 1
The 1:1 molar ratio of pyrrole (0.259 g, 3.73 mmol) and benzaldehyde (0.380 g, 3.73 mmol) combined in the presence of an organic catalyst (P-tolylsulfonic acid) PTSA (0.026 g, 0.151 mmol) is placed in the mortar and grind together with the pestle to obtain a dry, solid, light pink powder. Mixing order must be followed as,
Pyrrole + PTSA (grinding time approximately 1-5 minutes).
Reaction mixture + Benzaldehyde
Grinding or drying agents such as sodium chloride and magnesium sulfate have also been used in experiments to improve the mixing of the components and to absorb water molecules formed during the reaction [14].
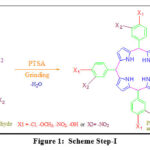 |
Figure 1: Scheme Step-I |
 |
Figure 2: Step-I Product (Precursors). |
Step 2
The pink solid was dissolved in 50 ml chloroform by catalyzing the reaction with Iodine crystals which act as an oxidizer (Ratio-Benzaldehyde/ oxidizer 1:1.5 mole percent respectively). To extract mixtures of porphyrins into CHCl3, resulted in pink solid powder was continued to stir for 2 hours with constant stirring on a magnetic stirrer at 60-800C temperature.
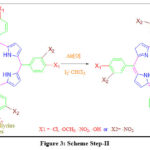 |
Figure 3: Scheme Step-II. |
 |
Figure 4: Step-II Products: TPP Sensitizers |
The liquid reaction mixture was purified by solvent extraction using a 1:3 ratio of chloroform and water three times to remove water-soluble impurities. Traces of residual water were removed by adding anhydrous sodium sulfate (Na2SO4). The resulting fraction was sent to the rotary evaporator to obtain the dry powder of the product. The obtained products were purified by further processing the recrystallization technique using ethyl alcohol. Purple solid confirms porphyrin formation on further analysis with UV, IR, NMR, and mass spectroscopy.
Synthesis of TTP Sensitizers
TPP CHO
Benzaldehyde (0.39g,3.73 mmol) was mixed with pyrrole (0.259g,3.73 mmol) in the presence of PTSA (0.026g,0.151 mmol) acid catalyst. The percentage yield of (25.51%, 0.144g) was isolated as the pure product at the end of the experiment.
TPP m-NO2
m-Nitrobenzaldehyde (0.56g, 3.73 mmol) was mixed with pyrrole (0.259g,3.73 mmol) in the presence of PTSA (0.026g,0.151 mmol) acid catalyst. The percentage yield of (22.63%, 0.167g) was isolated as the pure product at the end of the experiment.
TPP-P-NO2
To synthesize the title product involves, p-Nitrobenzaldehyde (0.56g,3.73mmol) was mixed with pyrrole (0.259g,3.73 mmol) in the presence of PTSA (0.026g,0.151 mmol) acid catalyst. The percentage yield of (23.54%, 0.173g) was isolated as the pure product at the end of the experiment.
TPP P-OCH3
To synthesize the title product, p-anisaldehyde (0.5g, 3.73 mmol) was mixed with pyrrole (0.259g,3.73 mmol) in the presence of PTSA (0.026g,0.151 mmol) acid catalyst. The percentage yield of (22.26%, 0.15g) was isolated as the pure product at the end of the experiment.
TPP P-Cl
To synthesize the title product, p-chlorobenzaldehyde (0.52g,3.73mmol) was mixed with pyrrole (0.259g,3.73 mmol) in the presence of PTSA (0.026g,0.151 mmol) acid catalyst. The percentage yield of (18.05%, 0.125g) was isolated as the pure product at the end of the experiment.
TPP P-OH
To synthesize the title product involves, p-Hydroxybenzaldehyde (0.45g,3.73mmol) was mixed with pyrrole (0.259g,3.73 mmol) in the presence of PTSA (0.026g,0.151 mmol) acid catalyst. The percentage yield of (19.72%, 0.123g) was isolated as the pure product at the end of the experiment.
Table 1 shows the molecular weight and weight taken of different aldehydes along with respective product IDs, molecular formula, and yield of TPP derivatives.
Table 1: Mechanochemical Synthesis of Meso-Porphyrin’s
|
Aldehyde ID |
Mol. wt. of Aldehyde (g/mol) |
Wt. of Aldehyde (g) |
Product ID |
Molecular Formula |
Molecular weight |
Actual Yield (g) |
% Yield |
|
Ar-CHO |
106.1 |
0.39 |
TPP CHO |
C44H32N4 |
616.2627 |
0.144 |
25.51 |
|
Ar-m-NO2 |
151.12 |
0.56 |
TPP m-NO2 |
C44H28N8O8 |
796.203 |
0.167 |
22.63 |
|
Ar-P-NO2 |
151.12 |
0.56 |
TPP P-NO2 |
C44H22N8O8 |
796.203 |
0.173 |
23.54 |
|
Ar-P-OCH3 |
136.15 |
0.5 |
TPP P-OCH3 |
C48H40N4O4 |
736.305 |
0.15 |
22.26 |
|
Ar-P-Cl |
140.57 |
0.52 |
TPP P-Cl |
C44H28Cl4N4 |
754.1039 |
0.125 |
18.05 |
|
Ar-P-OH |
122.12 |
0.45 |
TPP P-OH |
C44H32N4O4 |
680.2424 |
0.123 |
19.72 |
Results and Discussions
Spectral analysis of absorption spectra of TPP Dyes
TPP-CHO
Meso-Tetraphenyl Porphyrin (TPP CHO) gives a maximum absorbance of 0.86 a.u at 504.66nm, which indicates π-π* transition having Q-band spectra. (figure 5a)
TPP m-NO2
Meso-3-Nitro-Tetraphenyl Porphyrin (TPP m-NO2) gives maximum absorbances of 0.68 a.u at 426.31 nm wavelength and 0.36 a.u at wavelength 387.73 nm. The absorbance at 426.31 nm represents π-π* transition having Q-band spectra while absorbance at 387.73 nm represents n-π* transition showing S-band spectra. .(figure 5b)
TPP P-NO2
Meso-4-Nitro Tetra Phenyl Porphyrin (TPP p-NO2) gives maximum absorbances of 0.24 a.u at 591.70 nm. Along with this few more peaks were observed with absorbance 0.18 a.u at 556.86 nm with π-π* transition having Q-band spectra and for absorbance 0.15 a.u at 393.70 for n-π* transition showing S-band spectra. .(figure 5c)
TPP P-OCH3
Meso-4-Methoxy Tetra Phenyl Porphyrin (TPP p-OCH3) gives maximum absorbances of 0.65 a.u at 465.06 nm with π-π* transition having Q-band spectra. Similarly, for absorbance 0.61 a.u at 412.23 nm with n-π* transition showing S-band spectra. (figure 5d)
TPP P-Cl
Meso-4-Chloro Tetra Phenyl Porphyrin (TPP p-Cl) gives maximum absorbances of 1.05 a.u at 630.05 nm indicating the π-π* transition with Q-band spectra. Other than this, a few more absorbance peaks were observed in this plot at 0.97 and 0.59 a.u for wavelength 642.06, and 689.98 nm respectively indicating S and Q bands transitional energy levels for the sample. (figure 5e)
TPP P-OH
Meso-4-Hydroxy Tetra Phenyl Porphyrin (TPP p-OH) gives maximum absorbance 0.87 at 586.25 nm showing π-π* transition with Q-band spectra as well as absorbance 0.64 a.u at 646.24 nm represents π-π* transition with Q-band spectra. .(figure 5f)
Table 2 shows the name, structure, miling time, color, and absorption peaks in UV-visible spectra of synthesized TPP derivatives.
The purification of products is a hectic process and results in the loss of product and hence shows low yield.
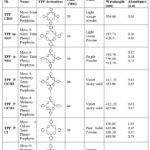 |
Table 2: Efficacy of Mechanochemical synthesis of TPP derivatives, with I2 oxidizing agent. |
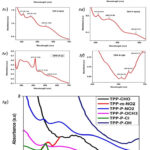 |
Figure 5(a, b, c, d, e, f, g): shows the UV-visible peaks for TPP derivatives in Alcohol. |
H1NMR, IR, C13 NMR, HRMS spectral analysis of TPP Dyes
TPP CHO
Synthesized TPP-CHO (Meso-tetraphenyl porphyrin) derivative was characterized by various characterization techniques for the exact structural elucidation of the product,
1HNMR (500 MHz, CdCl3): Ar-CH- δ7.14, P, 4H, δ7.21, m, 8H, δ7.30, o, 8H, Pyr-β-CH- δ2.2645,4H, δ5.5, 2H, δ6.9, 4H, Pyr-NH- δ2.0278, 2H. IR: Pyr-NH 3069.75cm–1(s), Ar-C-N (s) 1289.95 cm–1,1325.70cm–1, -C=C- 1688.96 cm–1, =C-H (bend) 662.81 cm–1, 707.61 cm–1. C13 (500 MHz, CdCl3): Ar-C δ128.5, p,4C, δ128.7, m,8C, δ130.19, o,8C, 132.00, I,4C, Pyr-ᾳ–C δ175.2, 2C, δ153.2,2C, δ139.9,2C, δ146.9,2C, δ144.2,2C Pyr-β–C, δ122.3, 2C, δ113.00, 2C, δ29.75, 2C, R3C- 99.0,2C, 133.5,2C. Mass m/z (MALDI/TOF): 685 [616.2661(M+) +3Na]. CHN Data: C-85.69; H-5.23; N-9.08.
 |
Figure 6 (a, b, c, d): shows the IR, H1NMR, C13NMR, and Mass spectra of TPP-CHO. |
TPP m-NO2
Synthesized TPP m-NO2 (Meso-3-Nitro-tetraphenyl porphyrin) derivative was characterized by various characterization techniques for the exact structural elucidation of the product,
1HNMR (500 MHz, CdCl3): Ar-CH- δ8.0795(P),4H, δ7.5979 (m),4H, δ7.6763 (o),4H, δ8.23, (o),4H, Pyr- β-CH δ5.9,2H, δ6.1,4H, 2.1666,4H, Pyr-NH δ2.0394,2H. IR: Ar-C-C-(s)1531.09cm–1,1617.59cm–1,1636.79 cm–1, 1531.09 cm–1, —C-N (s)1349.33,1261.81cm–1 -N-O(s) 3484.83cm–1, Ar-N-H(s)- 3413.78 cm–1. C13 (500 MHz, CdCl3): Ar-C δ149.55, o,4C, δ123.54, o,4C δ120.68 p,4C, δ129.18 m,4C δ129.2 o,4C, δ130.91 I,4C, Pyr-ᾳ-C δ141.25,2C, δ140.75,2C, 144.2,2C, 164.6,2C, Pyr-β-C δ140.55,2C, δ146.80,4C, δ28.30,2C, R3C: δ108.80,2C, δ130.91,2C. Mass m/z (MALDI/TOF): 796.2795 [M+= 531.1895+263.1242+2H]. CHN Data: C-66.33; H-3.54; N-14.06; O-16.06.
 |
Figure 7 (a, b, c, d): shows the IR, H1NMR, C13NMR, and Mass spectra of TPP-m-NO2. |
TPP-P-NO2
Synthesized TPP P-NO2 (Meso-4-Nitro-tetraphenyl porphyrin) derivative was characterized by various characterization techniques for the exact structural elucidation of the product,
1HNMR (500 MHz, CdCl3): Ar-CH δ8.14(o)(8H), δ7.2838(m)(8H), Pyr-β-CH δ4.1297(1H) (out), δ4.1443(1H), δ2.0,4H, δ6,4H Pyr–NH δ2.0,2H. IR: Ar-C-C – (s)1517.72 cm–1, 1617.28 cm–1,1635.18 cm–1, —C-N (s)1344.98 cm–1, 1262.76cm–1 -N-O(s) 3470.41cm–1, Ar-N-H(s)- 3413.23 cm–1. C13 (500 MHz, CdCl3): Ar-C δ141.28 (I),4C δ129.41 (o),8C δ120.66 (m),8C δ148.42(p),4C Pyr-ᾳ-C δ 144.6 2C, 138.58 2C, 164.2 2C, 146.9 2C, Pyr-β-C δ131.12 4C, δ28.29 2C, δ166.4 2C, R3C δ 131.75 2C, 108.03 2C, Mass m/z (MALDI/TOF): 796.1704 [M+= 531.1895+263.1242+2H]. CHN Data: C-66.33; H-3.54; N-14.06; O-16.06.
 |
Figure 8 (a, b, c, d): shows the IR, H1NMR, C13NMR, and Mass spectra of TPP-P-NO2 |
TPP P-OCH3
Synthesized TPP P-OCH3 (Meso-4-Methoxy-tetraphenyl porphyrin) derivative was characterized by various characterization techniques for the exact structural elucidation of the product.
1HNMR (500 MHz, CdCl3): Ar-OCH3 δ3.73 (12H), Ar CH- δ7.2 m,8H δ 7.4 o,8H, Pyr-β-CH δ7.0 4H, δ2.0075 4H, 5.7 2H, Pyr-NH δ4.0936(s)(2H), IR: Pyr-NH δ3414.45 cm–1 (s), -C=C-1636.59 cm–1(s), 768.46 cm–1(b), 1689.27cm– 1, -C-O 1259.08 cm–1, —C-N (s)1383.61, 1259.08cm–1. C13 (500 MHz, CdCl3): Ar-C δ159.9 (p), δ114.80(m), δ124.9(o), 129.57(I), Pyr-ᾳ-CH δ144.2 2C, δ139.9 2C, δ146.9 2C, 164.06 2C, Pyr-β-CH δ29.70 2C, 116.4 2C, 131.0 4C, R3C-C δ132.25 2C, δ108.16 2C, Mass m/z (MALDI/TOF): 736.3050 [711.2818(M+) +1Na+2H], CHN Data: C-78.24; H-5.47; N-7.60; O-8.69.
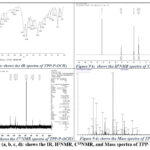 |
Figure 9 (a, b, c, d): shows the IR, H1NMR, C13NMR, and Mass spectra of TPP-P-OCH3 |
TPP P-Cl
Synthesized TPP P-Cl (Meso-4-Chloro-tetraphenyl porphyrin) derivative was characterized by various characterization techniques for the exact structural elucidation of the product,
1HNMR (500 MHz, CdCl3): Ar-H δ7.2596 m,8H δ7.5 o,8H, Pyr-CH δ2.0386 4H, δ5.7 2H, δ7.0 4H, Pyr-NH δ4.095 (s)(1H), δ4.1379(s)(1H), IR: Ar-C-Cl 801.92 cm–1, -C=C-1635.93 cm–1, Pyr-NH 3414.36 cm–1(s), -C-C- 1617.25 cm–1(s in Ar-ring), —C-N (s)1385.14, 1262.76cm–1. C13 (500 MHz, CdCl3): Ar-C δ131.5 p,4C, δ128.8 m,8C, δ127.8 o,8C, δ130.7 I,4C, Pyr-ᾳ-C 144.2 2C, 164.2 2C, 139.9 2C, 146.9 2C Pyr-β-C δ29.69 2C, 116.4 2C, 131.0 2C, R3C: δ133.5 2C, δ108.00 2C, Mass m/z (MALDI/TOF): 754.1039 [685.4374(M+) +3Na]. CHN Data: C-70.04; H-3.74; Cl-18.79; N-7.43.
 |
Figure 10 (a, b, c, d): shows the IR, H1NMR, C13NMR, and Mass spectra of TPP-P-Cl |
TPP P-OH
Synthesized TPP P-OH (Meso-4-Hydroxy-tetraphenyl porphyrin) derivative was characterized by various characterization techniques for the exact structural elucidation of the product,
1HNMR (500 MHz, CdCl3): Ar-H δ7.4618 8H, δ7.6273 8H, Pyr-NH- δ4.1228 2H, Pyr-β-CH- δ2.0434 2H, δ2.1705 2H, δ5.7 2H, δ7.2597 4H, Ar-OH-δ10.00 s,4H. IR: PyrNH (s)3413.88 cm–1 -C=C-1635.67 cm–1, 756.46 cm–1, Ar-OH- 2025.61 cm–1(s), 944.78(b), -C-C- 1617.69 cm–1(s, in Ar-ring), —C-N (s)1386.97, 1251.46cm–1. C13 (500 MHz, CdCl3): Ar-C δ129.97 m,8C, δ152.23 P,4C, δ132.55 o,8C, 125.2 I,4C, Pyr-ᾳ-C δ139.9 2C, δ146.9 2C, δ144.32 2C, δ172.70 2C, Pyr-β-C δ116.4 2C, δ26.5 2C, δ131.0 4C, R3-C- δ132.53 2C, 108.00 2C. Pyr-CH δ29.24(2C), δ 144.52(4C), δ172.70(2C), Mass m/z (MALDI/TOF): 680.2424 [685.4374 (M+-5H)]. CHN Data: C-77.63; H-4.74; N-8.23; O-9.40
 |
Figure 11: (a, b, c, d): shows the IR, H1NMR, C13NMR, and Mass spectra of TPP-P-OH |
Current-Voltage Characteristic
Dye-sensitized solar cells (DSSC) are fabricated by using a photoanode, standard electrolyte, and platinum counter electrode, and this assembly is sensitized by synthesized TPP derivatives, and I-V (current-voltage) characteristics were recorded by using a solar simulator 1.5 AG (100 mA/cm2).
 |
Figure 12 (a. b, c, d, e, f): Shows the I-V characteristics of the dyes TPP-CHO, TPP-m-NO2 , TPP-P-NO2, TPP-P-OH, TPP-P-OCH3 and TPP-P-Cl respectively. |
From the open circuit bias characteristics, the I-V Plot (Current vs. voltage) is depicted in Figure 14 (a, b, c, d, e, f). It is seen that Dark current (D) and Light current (L) have been found completely separated with a perfect diode nature. It confirms that under Dark conditions and light illumination of Solar Simulator AM 1.5G, (100 mW/cm2). The dyes are efficient to generate current in the circuit[15][16][17].
As the basic structure of TPP is the Porphyrin ring there are a lot of possibilities to indulge in donor and acceptor groups at the periphery of to ring. High efficiency can be achieved by replacing the donor-acceptor groups at the geometrically opposite positions and by increasing the conjugation by spacers between these active groups[18].
Conclusion
In the current work, we successfully reported the synthesis of TPP derivatives with a two-step mechanism. In Step I easily reducible species are formed that upon oxidation in Step II, yield the resulting porphyrin derivatives. The exact percentage yield for the synthesized TPP derivatives such as TPP-CHO, TPP-m-NO2, TPP-P-NO2, TPP-P-OCH3, TPP-P-Cl, TPP-P-OH is 25.51%, 22.63%, 23.54%, 22.26%, 18.05%, 19.72%. Of all these TPP derivatives, the maximum yield for TPP-CHO is 25.51%. The purification of products is a hectic process and results in the loss of product and hence shows low yield.
Absorbance was recorded for all dyes using a UV-visible spectrophotometer, which shows a wide absorption range of 300–700 nm. Of all these dyes, most show significant red shifts in the S-band and Q-band. An n-π and π-π* transition was observed for all derivatives. A maximum absorption of 1.05 a.u. was observed for TPP-P-Cl. recorded at 630.05 nm. The highest absorption peaks for TPP-CHO, TPP-m-NO2, TPP-P-NO2, TPP-P-OCH3, TPP-P-Cl and TPP-P-OH were found to be 0.86, 0.68, 0.24 and 0.65, 1.05, 0.87 a.u recorded at wavelengths 504.66, 426.31, 591.70, 465.06, 630.05 and 586.25 nm, respectively. Confirmation of the synthesized products is carried out by various Characterization techniques such as IR, H1NMR, C13NMR, and (MALDI-TOF) mass spectrophotometry.
The prepared DSSC was tested with the standard electrolyte/dye/TCO (Transparent Conductive Oxide) under no-load bias voltage on solar simulator 1.5 AG (100 mA/cm2). The synthesized porphyrin sensitizers are also capable of generating current without external loading, which is confirmed by the characteristic of the I-V diagrams in a positive negative quadrant with the clear division of dark and light current.
The synthesized porphyrins sensitizers are also able to generate current without any external load. Hence, they have photovoltaic importance in Emerging Organic Photovoltaic Technology based Solar Cells.
Acknowledgment
The authors are thankful to D. B. Science College, Gondia, India for facilitating our research work.
Conflict of Interest
The authors declare no competing interests.
Funding Sources
There is no funding Sources
References
- Marsh, D. F. and Mink, L. M. Microscale synthesis and electronic absorption spectroscopy of tetraphenylporphyrin H2(TPP) and metalloporphyrins ZnII(TPP) and NiII(TPP),J. Chem. Educ., vol. 73, no. 12, pp. 1188–1190, 1996, doi: 10.1021/ed073p1188.
CrossRef - Obotowo, I. B. Obot, and Ekpe, U. J. Organic sensitizers for dye-sensitized solar cell (DSSC): Properties from computation, progress, and future perspectives, J. Mol. Struct., vol. 1122, pp. 80–87, 2016, doi: 10.1016/j.molstruc.2016.05.080.
CrossRef - Campbell, W. M., Kenneth, W., Wagner, J. P., Wagner, K., Walsh, R. J., Gordon, K. C., Schmidt-Mende, L., Nazeeruddin, M. K., Wang, Q., Gratzel, M and David, L., Highly efficient porphyrin sensitizers for dye-sensitized solar cells, J. Phys. Chem. C, 111 (32), 11760-11762, 2007, doi: 10.1021/jp0750598.
CrossRef - Estrella, L. L. and Kim, D. H. Theoretical design and characterization of NIR porphyrin-based sensitizers for applications in dye-sensitized solar cells, Sol. Energy, vol. 188, no. June, pp. 1031–1040, 2019, doi: 10.1016/j.solener.2019.06.043.
CrossRef - Louda, J. W. “Porphyrins,” Encycl. Earth Sci. Ser., no. Scheme 1, pp. 1247–1253, 2018, doi: 10.1142/9789812385376_0021.
CrossRef - Phromsatit, T. Jantayot, W. Pinsuwan, K. Nuchthanom, A. and Boonyuen, S. Thermal behavior and the solvent effects of ρ-Methoxy Tetraphenylporphyrin (TOMPP), Copper Porphyrin (CuTOMPP), and Nitroporphyrin (CuTOMPP-NO2), MATEC Web Conf., vol. 69, 2016, doi: 10.1051/matecconf/20166906002.
CrossRef - Vicente, M.D. Smith K. M. Syntheses and Functionalizations of Porphyrin Macrocycles. Curr Org Synth. 2014 Feb;11(1):3-28. doi: 10.2174/15701794113106660083. PMID: 25484638; PMCID: PMC4251786.
CrossRef - Brabec, C. J. Organic photovoltaics: Technology and market, Sol. Energy Mater. Sol. Cells, vol. 83, no. 2–3, pp. 273–292, 2004, doi: 10.1016/j.solmat.2004.02.030.
CrossRef - Cheng-Hua Wu, Tsung-Yu Pan, Shang-Hao Hong, Chin-Li Wang, Hshin-Hui Kuo, Yang-Yun Chu, Eric Wei-Guang Diau, and Ching-Yao Lin, Fluorene-Modified Porphyrin for Efficient Dye-Sensitized Solar Cell, Royal Society of Chemistry, 3–7, 2012.
- Ostapowicz J, Maćkowiak B, Ostrowska K, Kaczmarek B, Pietras N, Frąckowiak D, Fundowicz M, Golusiński W, Suchorska W. Comparison of tumour tissue homogenisation methods: mortar and pestle versus ball mill. Research Square; 2023. doi:10.21203/rs.3.rs-2510226/v1.
CrossRef - Ulman, A. Manassen, J. Frolow, F. and Rabinovich, D. Synthesis of new tetraphenyl porphyrin molecules containing heteroatoms other than nitrogen. III. Tetraphenyl-21-tellura-23-thiaporphyrin: an internally-bridged porphyrin, Tetrahedron Lett., vol. 19, no. 21, 1885–1886, 1978, doi: 10.1016/S0040-4039(01)94699-4.
CrossRef - Sun, Z. She, Y. Cao, M. Zhou, Q. Lu, X. and Zhang, S. Synthesis of substituted meso-tetraphenyl porphyrins in mixed solvent systems, Arkivoc, vol. 2013, no. 3, 389–400, 2013, doi: https://doi.org/10.3998/ark.5550190.p008.097
CrossRef - Sun, Z & Jiao, S & Li, F & Wen, J & Yu, Y & Liu, Y & Cao, M & Li, L & Zhou, Y & She, Y. Acid Activation and Chemical Oxidation in the Synthesis of meso‐Tetraphenylporphyrin using a Mixed‐Solvent System, Asian Journal of Organic Chemistry, 2019, 8. 10.1002/ajoc.201900186.
CrossRef - Williams, D. B. G. and Lawton, M. Drying of organic solvents: Quantitative evaluation of the efficiency of several desiccants, J. Org. Chem., vol. 75, no. 24, 8351–8354, 2010, doi: 10.1021/jo101589h.
CrossRef - Bayannavar, P. & Mendhe, A. C. & Sankapal, B. & Sannaikar, M. S. & Shaikh, S. K. & Inamdar, S. & Kamble, R. Synthesis of metal free organic dyes: Experimental and theoretical approach to sensitize one-dimensional cadmium sulphide nanowires for solar cell application. Journal of Molecular Liquids. 2021, 336. 116862. 10.1016/j.molliq.2021.116862.
CrossRef - Mithari, P. A. Mendhe, A. C. Sankapal, B. R. and Patrikar, S. R. Process optimization of dip-coated MWCNTs thin-films: Counter electrode in dye-sensitized solar cells, J. Indian Chem. Soc., vol. 98, no. 11, 100195, 2021, doi 10.1016/j.jics.2021.100195.
CrossRef - Wagalgave, S. & Mendhe, A. C. & Nadimetla, D. & Al Kobaisi, M. & Sankapal, B. & Bhosale, S. & Bhosale, S. Aggregation induced emission (AIE) materials based on diketopyrrolopyrrole chromophore for CdS nanowire solar cell applications, Journal of Electroanalytical Chemistry, 2021, 895, 115451. 10.1016/j.jelechem.2021.115451.
CrossRef - Gangadhar, P.S. Jagadeesh, A. Rajesh, M.N. George, A.S. Prasanthkumar, S. Soman, S. Giribabu, L. Role of π-Spacer in Regulating the Photovoltaic Performance of Copper Electrolyte Dye-Sensitized Solar Cells Using Triphenylimidazole Dyes, Mater. Adv. 2022, 3, 1231–1239.
CrossRef - Shy, H. Mackin, P. Orvieto, A. S. Gharbharan, D. Peterson, G. R. and Hamilton, T. D. The two-step mechanochemical synthesis of porphyrins, Faraday Discuss., vol. 170, pp. 59–69, 2014, doi: 10.1039/C3FD00140G.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.










