Exertion of Modified Mineral and Plant – based Powders in the Sorption of Pb(II) Ions
Department of Chemistry, PSGR Krishnammal College for Women, Peelamedu, Coimbatore, Tamil Nadu, India.
Corresponding Author E-mail: muthulakshmiandal@psgrkcw.ac.in
DOI : http://dx.doi.org/10.13005/ojc/400234
Article Received on : 23 Jan 2024
Article Accepted on :
Article Published : 04 Apr 2024
Reviewed by: Dr. Supriya
Second Review by: Dr. Aruna C
Final Approval by: Dr. Tanay Pramanik
Heavy metal rich industrial discharges solemnly threaten the condition of ecosystem and human haleness. Lead pollution as a result of various anthropogenic activities is a major concern due to its high toxic nature. Chelating capacities of treated Magnolia champaca Barks (TMCB) and treated Attapulgite Clay Powder (TACP) are investigated in the process of sequestration under lab scale conditions. The sorbent matrices are subjected to microscopic, SEM / EDAX and FTIR analyses to study the variations in the adsorbents surfaces, alterations in the surface morphological characteristics, specific involvement of metal ions and functional group peaks, with respect to the sorption process. Dimensions and masses of sorbents, metal ion concentration, agitating periods, pH and temperature of the analyzed systems are optimized under Batch Equilibration studies. The experimentally verified samples are analyzed using Atomic Absorption Spectrophotometer to determine the Pb(II) ions concentrations. The derived Freundlich and Langmuir isothermal plots based on the experimental results obtained for TMCB – Pb(II) / TACP – Pb(II) exhibited a better linearity for Freundlich model, thereby, supporting multilayer sorption. A judicious comparison made between TACP and TMCB reveals a marginal sorption performance by the former.
KEYWORDS:Heavy Metals; Isotherm; Sorption; Sorbents; Sequestration
Download this article as:| Copy the following to cite this article: Indhumathy P, Andal N. M. Exertion of Modified Mineral and Plant – based Powders in the Sorption of Pb(II) Ions. Orient J Chem 2024;40(2). |
| Copy the following to cite this URL: Indhumathy P, Andal N. M. Exertion of Modified Mineral and Plant – based Powders in the Sorption of Pb(II) Ions. Orient J Chem 2024;40(2). Available from: https://bit.ly/3xjURvX |
Introduction
Environmental pollution remains the world’s most serious problem and is considered as one of the leading causes for sickness and mortality. It is not only triggered by industrialization, exploration, urbanization, population growth, but also by trans boundary movement of pollutants from one region to the other 1,2. Degradation of water ecosystem is reasoned, mainly by agricultural runoffs, industrial / municipal let-outs which comprises of various organic / inorganic pollutants.Amidst various toxicants, heavy metals with specific gravity > 5 g/cm3 are non- biodegradable, persistent and hazardous. These toxicants enter the food chain through bioaccumulation and induce damage even in trace concentrations 3,4,5.
Lead is the second most toxic metal, accounting for 0.002% of the Earth’s crust. It is naturally found in a very limited amount, but enters the environs, due to anthropogenic activities and contributions from industries manufacturing automobile parts and batteries. Further common sources of exposure include paint contamination (toys, containers, jewellery, etc.), food packaging, and water pipes 6,7.In humans, acute lead poisoning produces significant dysfunction in the kidneys, reproductive systems, liver, brain, and central nervous system. Thenceforth, the removal of this noxious element from the water bodies is vital, which has provoked the need for various treatment methodologies 8,9.
Several treatment approaches for removal of toxic substances have been documented. Biological substrates, membrane filtration, reverse osmosis, oxidation, electrochemical oxidation, coagulation/flocculation, chemical precipitation, and adsorption lay the foundations for these technologies 10,11. Factors such as cost, efficiency, dependability, feasibility, environmental impact, practicability and operational challenges influence the utilization of the aforesaid methods 12,13, 14.
Adsorption technique stands out due to its flexibility in operation and design procedures and in addition appears to have a considerable impact on the toxicity, biological availability, and transit of heavy metals in wastewater 15, 16, 17, 18. Adsorbents used, such as biomass materials, charcoal, sludge ash, microbes, clays and so on, have a large number of active binding sites on their surface via which heavy metals can be successfully retained under certain conditions 19, 20, 21.Thus the background of the present study involves in assessing the effectiveness of a biomaterial and a clay mineral in the trapping lead ions from aqueous media through batch mode and deriving out of its isothermal fit for the systems.
Materials and Methods
Collection and preparation of adsorbents
Twoeco-friendly materials (tree bark and mineral) have been identified for the present study. Magnolia champaca tree barks collected from the locales of Coimbatore, were washed with double distilled water to remove the scums and then dried thoroughly. The dried barks were crushed and pulverized using an electrical mixer. The second raw material, Attapulgite a fine clay powder, was purchased from Raisha Enterprise, Gujarat, India. It is composed of magnesium aluminium phyllosilicate (chemical formula – (Mg,Al)2Si4O10(OH)·4(H2O)). Both the native materials were categorized into different mesh sizes using Scientific Test Molecular Sieves.
Modification of the sorbent material
The categorized Magnolia champaca Barks (MCB) and Attapulgite Clay Powder (ACP) were treated with 0.1 N HCl to modify their surface nature. The modified materials are referred to as Treated Magnolia champaca Barks (TMCB) and Treated Attapulgite Clay Powder (TACP). The treated materials were washed several times using doubly distilled water to maintain neutral pH. The chemically modified sorbents were employed for the laboratory experiments. Figs 1 and 2 (a – c) represent the cleaned materials, sieved powders and their treated counterparts.
 |
Figure 1(a): MCB |
 |
Figure 1(b): Raw MCB |
 |
Figure 1(c): TMCB |
 |
Figure 2(a): ACP |
 |
Figure 2(b): Raw ACP |
 |
Figure 2(c): TACP |
Microscopic Analysis
Pulverized TMCB and TACP were analysed using Optical Microscope (Magnus Microscope CH20ILED) to assess their particle sizes. Mesh size ( 85BSS, 72BSS, 52BSS, 36BSS and 22BSS ) correspond to 0.18 mm, 0.21 mm, 0.30 mm, 0.42 mm and 0.71 mm particle sizes respectively. Microscopic images of native / treated MCB and ACP (0.18 mm) are depicted in Fig 3 (a,b) and Fig 4 (a,b).
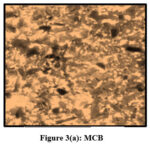 |
Figure 3(a): MCB |
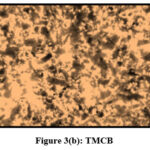 |
Figure 3(b): TMCB |
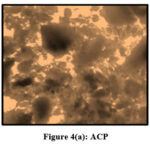 |
Figure 4(a): ACP |
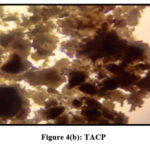 |
Figure 4(b): TACP |
Adsorbate (stock solution preparation)
A stock solution of 1000 mg/L was prepared by dissolving 1.5985 g lead nitrate (analytical grade) in doubly distilled water. A standard solution (100 mg/L) was obtained by diluting the stock solution. Further, working aliquots were prepared as appropriate dilutions of the standard solution.
Batch Optimization Studies
Experimental setup in batch mode was planned in such a way that the fixation of varied influential factors are ensured. The operating parameters were contact time period, size and amounts of the sorbent materials, required concentration of the sorbate ions, pH and temperature conditions, presence of cations and anions, to study the sorption efficiencies of TMCB and TACP. 50 mL Pb(II) solutions of desired concentrations (5 to 20 mg/L – 5 mg/L interval) were transferred into 250 mL agitation flasks and the varied sorbent doses (TMCB – 50 to 250 mg – 50 mg interval / TACP – 50 to 200 mg – 50 mg interval) were added to the solutions. The contents of the flasks were stirred (140 rpm) in a mechanical shaker under the preset conditions (3 to 30 mins – 3 mins interval). The concentrations of Pb(II) ions were determined using Atomic Absorption Spectrophotometer (Shimadzu (AA 6200) model). Pb(II) removal from the aqueous solutions, both at pre and post experimental setup were calculated (%) as follows:
% Removal = (Ci – Ce) / Ci x 100
Characterization Studies
Involvement of functional groups present in TMCB and TACP sorbents were studied using Shimadzu Fourier Transformation Infra-red Spectrophotometer (4500-500 cm-1 range). Peak variations pertaining to these groups were recorded. Assessment of the deviations in surface morphology and elemental constitutions of the processed metal loaded matrices were done using Scanning Electron Microscope (SEM) and Energy Dispersive X-ray Analysis (EDAX) (TESCAN -MIRA3 XMU). The results of these assays are discoursed further.
Results and Discussion
Fourier-Transform Infrared Spectroscopic Analysis
Fourier-transform infrared spectroscopy (FTIR) is declared as an effectual analytical method to study the vibrational modes of molecules in a sample. Figures 5(a) and 5(b) correspond to the FTIR spectra of the studied sorbents. The involvement of groups during metal adsorption is inferred from the disappearing peaks in the metal-laden TMCB spectrum with reference to hydroxyl groups (3251 cm-1) and alkoxy groups (1090 cm-1). Narrow peak of C=O stretching at 1629 cm-1 recorded a change in its counterpart spectra. Appearance of additional peaks at 2342 cm-1 and 2963 cm-1 in the post-run spectra suggests the stretching of C-H bonds in the precursor sample. Peaks with noticeable shifts less than 900 cm-1 signify variations in C-H bending vibrations which shall be due to the binding property of the metal ion.
An decrease in the intensity of the broad band at 3562 cm-1 corresponding to the O-H stretching vibrations is envisaged in the metal laden spectra (fig 5(b)) indicating its participation during metal binding on the surface of the sample. Further, the disappearance of peaks (1651 cm-1 and 1020 cm-1) characteristic of water molecules present in the silica matrix suffices its metal quenching property. Similar observations were recorded by Song Jingyan etal 22. Appearance of new peaks at 2972 cm-1 and 1921 cm-1 relating to C – H group and shift of peaks to wave numbers 1166 cm-1 and 736 cm-1 imply their involvement in the sorption process.
 |
Figure 5(a): FTIR Spectra – TMCB and TMCB – Pb(II) |
 |
Figure 5(b): FTIR Spectra – TACP and TACP – Pb(II) |
Scanning Electron Microscopic Analysis
Scanning Electron Microscopic (SEM) analysis assists high resolution imaging and overtures imperative information on the size, shape and surface structure of the studied materials. Native and metal laden TMCB and TACP materials were subjected to analysis and their corresponding micrographs are pictured in figs 6 (a and b) and 7 (a and b). Disappearance of pores and coarse from native samples evidence the covering of pores by Pb(II) ions.
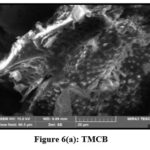 |
Figure 6(a): TMCB |
 |
Figure 6(b): TMCB-Pb(II) |
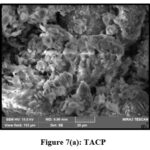 |
Figure 7(a): TACP |
 |
Figure 7(b): TACP-Pb(II) |
EDAX Analysis
The elemental compositions of the studied sorbents were dogged using Energy-Dispersive X-ray Spectroscopy. Quantitative and qualitative data regarding the specified elements were derived for the samples from the obtained spectra. Pronouncement of new peaks around 2 keV [figs 8 (a and b) and 9 (a and b)] confirm the adherence of Pb(II) ions onto the surfaces of TMCB and TACP.
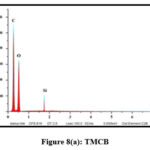 |
Figure 8(a): TMCB |
 |
Figure 8(b): TMCB-Pb(II) |
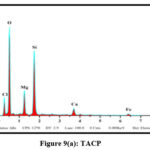 |
Figure 9(a): TACP |
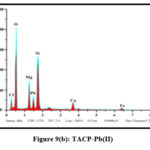 |
Figure 9(b): TACP-Pb(II) |
Batch Equilibration Studies
Impact of Particle Size
Adsorption kinetics varies significantly under the influence of particle size of samples. Smaller particles possess greater surface area per unit mass compared to larger particles. This in turn enhances the number of adsorption sites, thereby greater adsorption capacity of the former. A plot of percentage removal vs particle size comprising the two systems viz., TMCB – Pb(II) / TACP – Pb(II) is shown in Fig 10. As per the made statement, Pb(II) ion had been better sorbed by least particle size, i.e, 0.18 mm, further decline, as obvious from the curve. The decrease in percentage removal can also be attributed to the resistance in mass transport property. 0.18 mm particle size was fixed for further experiments.
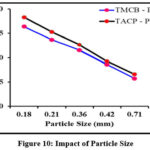 |
Figure 10: Impact of Particle Size |
Impact of Initial Concentration and Contact Time
Initial ion concentration and contact time are critical parameters in determining a system’s sorption efficiency. The sorbing ability of TMCB / TACP at varying initial concentrations (5 – 20 mg/L; 5 mg/L interval) at distributed time frames (3 to 30 mins; 3 mins interval) is displayed in fig 11 (a) and (b) respectively. Rapid sorption occurred at the initial stage shall be due to availability of extended active sites. However, a steep decrease is observed beyond the maximum Pb(II) sorption (96% by TMCB at 15 mins and 98% by TACP at 9 mins) exhibited by 20 mg/L initial sorbate concentration.
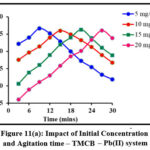 |
Figure 11(a): Impact of Initial Concentration and Agitation time – TMCB – Pb(II) system |
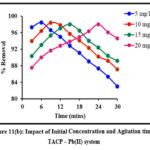 |
Figure 11(b): Impact of Initial Concentration and Agitation time – TACP – Pb(II) system |
Impact of Dosage
Sorbent dosage is a crucial parameter and must be optimized so as to achieve an efficient metal removal. Batch studies carried out at specific sorbent dosages are depicted in fig 12 (a) and 12 (b). A gradual increase in the peaks corresponding to the removal of Pb(II) ions in TMCB and TACP systems are observed, linearly with the sorbent dose of 200 mg and 150 mg, respectively. Further, downgrading in the removal suggest the saturation of active sites.
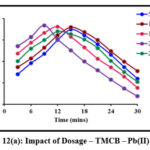 |
Figure 12(a): Impact of Dosage – TMCB – Pb(II) system |
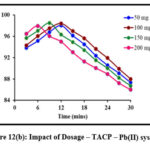 |
Figure 12(b): Impact of Dosage – TACP – Pb(II) system |
Impact of pH
pH of a solution influences the surface charge of and the adsorbate species which, in turn, impose a substantial effect on the sorption behaviour of any studied system. Experimental observations for pH effect are indicated by the inverted parabolic curves in Fig 13. A maximum Pb(II) ions removal had occurred at pH 5 in both the cases. Competent nature of H+ ions, ahead of Pb(II) ions under acidic conditions and complex forming nature of Pb(II) ions in alkaline medium shall be the driving force for decreased sorptive nature of the divalent ion.
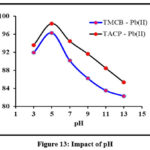 |
Figure 13: Impact of pH |
Impact of Temperature
Temperature has a significant control over the adsorption capacity, adsorption kinetics and desorption in the sorption processes, depending on the specific system and application. The sorption of the selected divalent ion was found to increase gradually with rise in temperature from 273 K – 313 K for TMCB / 273 K – 303 K for TACP as indicated by the smooth inclination patterns in figure 14. Extending the temperature beyond the respective temperature environs for TMCB – Pb(II) / TACP – Pb(II) systems inhibited sorption, may be due to the denaturing of reaction characteristics as implied by the downfall in the curves.
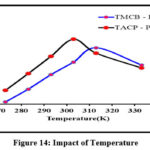 |
Figure 14: Impact of Temperature |
Impact of Cations/Anions/Co-ions
Unavoidable existence of cations, anions, co-ions ions in natural and industrial water streams, had led to their impeccable study in the process of optimization. Presence of these ions deteriorates the chelating ability of the sorbents as evidenced from the variation of the block heights of the bar chart (fig 15). Amongst the studied cations, sorption efficacy was highly subdued due to the presence of K+ than Na+,the reason can be the small ionic radii of K+ and its higher hydration energy. Anionic inhibition is largely extended by Cl– due to the preferential stable chloro complex formation by Pb(II). Pb(II) with larger ionic radii (0.118 nm) than co-metal ions [Zn(II) – (0.074 nm) and Cr(VI) – (0.04 nm)], exhibit appreciable solvation property resulting in a dip in the percentage removal of the former.
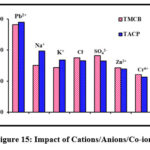 |
Figure 15: Impact of Cations/Anions/Co-ions |
Isothermal Studies
Adsorption process is quantified by the adsorption isothermal studies, as they provide deep insights about the interaction between the sorbate and sorbent surfaces.Langmuir and Freundlich isothermal studies were applied to the obtained experimental data for the studied systems [fig 16(a) and (b)]. Table 1 lists the Langmuir (qm, KL) and Freundlich (1/n, Kf) isothermal coefficients derived from the slope and intercept of the corresponding plots. Tabulated isothermal data show a maximum adsorbing capacity values (qm) [TMCB – 1.89 mg/g; TACP – 17.06 mg/g], along with appreciable sorbate – sorbent interaction (KL) [TMCB – 7.19 L/mg; TACP – 37.68 L/mg] for the studied systems. The qm and KL values is higher for TACP than TMCB, due to its extensive availability of sorption sites and ion exchange capability in the SiO4 structure of the former. However, better fit has been registered for Freundlich model. This may be due to preferential sorption of Pb(II) ions onto the heterogeneous sites of the sorbent matrices, further justified by calculated intensity of adsorption (0≤ 1/n ≤1) and the correlation coefficient values (R2 nearer to unity), as suggested by K.S. Obayomi etal 23.
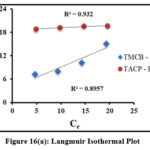 |
Figure 16(a): Langmuir Isothermal Plot |
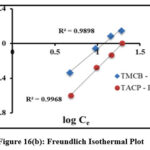 |
Figure 16(b): Freundlich Isothermal Plot |
Table 1: Isothermal Constants
|
System |
Langmuir |
Freundlich |
||||
|
qm (mg/g) |
KL (L/mg) |
R2 |
1/n |
Kf (L/mg) |
R2 |
|
|
TMCB – Pb(II) |
1.89 |
7.19 |
0.8957 |
0.82 |
0.13 |
0.9898 |
|
TACP – Pb(II) |
17.06 |
37.68 |
0.9320 |
0.98 |
0.05 |
0.9968 |
Conclusion
Magnolia champaca BarkandAttapulgite clay powder were collected, modified using 0.1N HCl to maximize their surface area and utilized as an effective sorbents in the chelation of Pb(II) ions from the hydrous environment. The morphological features of the unloaded and loaded samples were analyzed using SEM and EDAX equipment. Peak variations pertaining to functional groups of the modified/ metal laden sorbents were assessed by FTIR spectral studies. Operating factors for the TMCB – Pb(II) / TACP – Pb(II) systems were optimized as 0.18 mm particle size; pH 5 and 20 mg/L initial concentration with a maximum removal of 96% / 98%, with variations in agitation time intervals as 15 mins / 9 mins at temperatures 313 K / 303 K correspondingly. Metal trapping efficiencies of the selected sorbents were hindered in the presence of co-metal ions justified by their corresponding ionic radii property. Linearity and better fit of the experimental data was found to be well in Freundlich isotherm model. Treated Magnolia champaca BarkandAttapulgite clay powder exhibited excellent isolation property towards the noxious Pb(II) ions, thereby their efficacy shall be explored in the confiscation of other toxic metals.
Acknowledgment
Authors acknowledge the DST FIST, New Delhi, India for the infrastructure provided.
Conflict of Interest
The authors declare no conflict of interest.
References
- Ukaogo P. O., Ewuzie U., Onwuka C. V., Microorganisms for Sustainable Environment and Health, Elsevier, 2020, 419 – 429
CrossRef - Tran H. N., Chao, Environ Sci Pollut Res, 2018, 1 – 13
- Speight J. G., Natural Water Remediation Chemistry and Technology, Butterworth – Heinmann, 2020, 165 – 198
CrossRef - Ledezma C. Z., Bolagay D. N., Figueroa F., Ledezma E. Z., Ni M., Alexis F., Guerrero V. H., Environ. Technol. Innov. 2021, 22, 1-26
- Sen A., Pereira H., Olivella M. A., Villaescusa I., Int. J. Environ. Sci. Technol., 2014, 1 – 14
- Khokhar A., Siddique Z., Misbah, J. Environ. Chem. Eng., 2015, 1 – 9
- Raj K., Das A. P., Envtl. Chem. And Ecotox., 2023, 5, 79 – 85
CrossRef - Kumar A., Pinto, M.M.S.C., Chaturvedi A. K., Shabnam A. A., Subrahmanyam G., Mondal R., Gupta D. K., Malyan S. K., Kumar S. S., Khan S. A. and Yadav K. K., Int. J. Environ. Res. Public Health, 2020, 17, 1- 33
CrossRef - Bashir A., Malik L. A., Ahad S., Manzoor T., Bhat M. A., Dar G. N., Pandith A. H. , Environ. Chem. Lett., 2018, 17 (2019), 729–754
CrossRef - Chai W. S., Cheun J. Y., Kumar P. S., Mubashir M., Majeed Z., Banat F., Ho S., Show P. L., J. Clean. Prod., 2021, 296, 1-16
CrossRef - Anastopoulos I., Pashalidis I., Bandegharaei A. H., Giannakoudakis D. A., Robalds A., Usman M., Escudero L. B., Zhou Y., Colmenares J. C., Delgado A. N., Lima E. C., J. Mol. Liq., 2019, 295, 1- 17
CrossRef - Saxena A., Bhardwaj M., Allen T., Kumar S., Sahney R., Water Sci., 2017, 1-9
- Quyen V., Pham T. H., Kim J., Thanh D., Thang P. Q., Van Le Q., Jung S. H., Kim T. Y., Chemosphere, 2021, 284, 1 – 7
CrossRef - Pathirana C., Ziyath A. M., Jinadasa K.B.S.N., Egodawatta P., Sarina S., Goonetilleke A., Chemosphere, 2019, 234, 488 – 495
CrossRef - Wierzba S., Kłos A., J. Clean. Prod., 2019, 225, 112 – 120
CrossRef - Sadeeka S. A., Negmb N. A., Hefni H.H., Abdel Waha M. M., Int. J. Biol. Macromol., 2015, 81, 400–409
CrossRef - Geetha P., Latha M.S., Pillai S. S, Koshy M., Ecotoxicol. Environ. Saf., 2015, 122, 17–23
CrossRef - Ashfaq A., Nadeem R., Bibi S., Rashid U., Hanif M. A., Jahan N., Ashfaq Z., Ahmed Z., Adil M. and Naz M., Water, 2021, 13, 1 – 15
CrossRef - Rathi B. S., Kumar P. S., Environ. Polln., 2021, 280, 1 – 19
CrossRef - Ewis D., Ba-Abbad , M. M., Benamor A., El-Naas M. H., Appl. Clay. Sci., 2022, 229, 1 – 31
CrossRef - Akpomie K. G., Dawodu F. A., J. Adv. Res., 2015, 6, 1003–1013
CrossRef - Jingyan S., Jing Y., Adv. Mat. Res., 2012, 446-449, 2960-2963
CrossRef - Obayomia K.S., Autab M., Kovo A.S., Desalin. Water. Treat., 2020, 181, 376–384
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.










