Development and Utilization of Newly Synthesized Tamarind-6-Amino Hexanoic Acid (TAMHA) Resin for Removal of Toxic Heavy Metals Ions from Industrial Effluents
Chandra Prakash* , Ganesh Kumar Choudhary
, Ganesh Kumar Choudhary and Vimla Chowdhary
and Vimla Chowdhary
Department of Chemistry, Jai NarainVyas University, Jodhpur, Rajasthan, India.
Corresponding Author E-mail: cp26490@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/400235
Article Received on : 15 Jan 2024
Article Accepted on :
Article Published : 17 Apr 2024
Reviewed by: Dr. Ammar Dawood
Second Review by: Dr. Ponnusamy Thillaiarasu
Final Approval by: Dr. Tanay Pramanik
Tamarind Kernel Powder (TKP) and its biosorbents that went through chemical modification are crucial for removing heavy metals from industrial effluents including Fe2+, Cd2+, Pb2+, Cu2+, Zn2+. TKP is a natural polymer that is fully non-toxic, biocompatible, and biodegradable and belongs to the group of natural gums. It has a matrix made of hydrophilic polysaccharides, which has been utilized for the generating chelating resins. The chelating resin, based on tamarind kernel powder and containing a 6-Amino hexanoic acid, has been created in the current work. FTIR, TGA, SEM, ion exchange capacity and other physicochemical characteristics were used to characterize the TAMHA resin. At different pH the preferences, the "Kd" values of these dangerous metal ions were also computed.
KEYWORDS:6-Amino hexanoic acid; Chelating resin; Industrial effluent; Tamarind kernel powder
Download this article as:| Copy the following to cite this article: Prakash C, Choudhary G. K, Chowdhary V. Development and Utilization of Newly Synthesized Tamarind-6-Amino Hexanoic Acid (TAMHA) Resin for Removal of Toxic Heavy Metals Ions from Industrial Effluents. Orient J Chem 2024;40(2). |
| Copy the following to cite this URL: Prakash C, Choudhary G. K, Chowdhary V. Development and Utilization of Newly Synthesized Tamarind-6-Amino Hexanoic Acid (TAMHA) Resin for Removal of Toxic Heavy Metals Ions from Industrial Effluents. Orient J Chem 2024;40(2). Available from: https://bit.ly/49GJg7Z |
Introduction
The presence of harmful heavy metal ions in wastewater pollution brought on by careless dumping is a worry for the environment on a global scale. The environment is negatively impacted by improper management of wastewater and waste products from the metal and metal restoring industries and represents an imminent threat to every form of life, including animals and people. Heavy metals toxicological effects, even at extremely minimal concentrations, pose a serious health threat to all creatures living, making heavy metal pollution an international issue. Heavy metals aggregate in soft tissues of the human body and may lead to many kinds of disorders, such as blindness, irritation, paralysis, neurological damage, liver and kidney damage, chronic respiratory illness, anemia, hepatitis, and nephritic syndrome 1-3. Scientific community is compelled to search out the source of energy to feed with non-polluting nature [4]. If these heavy metals accumulate in substantial quantities in a food chain, they may be dangerous to an individual’s health. There are a number of numerous forms of metal ions in the environment. Whereas some of these are hazardous, others are not. Industries release poisonous metal ions into the water. They enter the food chain of humans through the environment. Once within our body, they interfere with basic functions and can sometimes have catastrophic consequences 5-7. The separations of mixture of metal ions on GHBA resin on the basis of their distribution coefficient at various pH were also achieved using column chromatography [6]. The metal ions are relevant to development and Utilization of newly synthesized progress in polymer science 7. Some of the contaminants that are exceedingly dangerous, poisonous, and toxic within the PPB (parts per billion) limit are lead, cadmium, chromium, especially hexavalent chromium; nickel, barium, cadmium; mercury; cobalt; vanadium; selenium; arsenic; grease and oils; etc. 8. As a result of their natural surroundings resistance and endurance, the treatment of toxic metals is of particular significance 9-10. Heavy metals can be removed from wastewater using a variety of methods, such as the use of ion exchange, adsorption, electro dialysis, lime treatment, chemical precipitation, the coagulation process and the process of precipitation photo catalytic processes, reduction, filtration through membranes (including the ultra-filtration process, Nano filtration, and reverse osmosis), and electro flotation procedures 11-14. Many strategies have been researched recently in an effort to create more affordable and efficient adsorbents that comprise natural polymers. Among these, polysaccharides like starch 15-17 chitin 18-20 and tamarind kernel powder 21-23 are particularly noteworthy. Several innovative low-cost adsorbents can be made from natural materials, synthetic biopolymers, industrial residues, or waste from farming. They’re referred to as bio sorbents. These substances can be utilized to make cheap sorbents for metal ion cleaning up. since they are highly effective, affordable, and renewable sources of biomass. The current work outlines the chemical modification of TKP resin’s production and their use in detoxifying water bodies with toxic metal ions. There has been considerable usage of processes.
Object
TKP 6-aminohexazoic acid resin will be created and characterized using polysaccharide from natural sources and 6-aminohexazoic acid acting as a functional group. The resin will then be added to industrial waste and effluent to remove harmful metal ions. The goal of adopting TKP as the polysaccharide matrix in this case is to make use of its straightforward availability from farming products as well as practical and affordable methods for treating and decontaminating industrial effluent.
Materials and methods
Materials
Table 1
|
S.No. |
Chemicals |
Specifications |
|
1 |
Dioxane (AR) |
Ases Chemical work, Jodhpur, Rajasthan |
|
2 |
Hydrochloric acid |
Ases Chemical work, Jodhpur, Rajasthan |
|
3 |
Sodium Hydroxide (NaOH) |
Sarabhai Chemicals, Baroda, India |
|
4 |
6-aminohexanoic acid |
Loba Chemie Pvt. Ltd, Mumbai |
|
5 |
TKP |
Ases Chemical work, Jodhpur, Rajasthan |
|
6 |
Epoxy chloropropane |
Sarabhai Chemicals, Baroda, India |
|
7 |
Methanol |
Ases Chemical work, Jodhpur, Rajasthan |
Methods
Preparation of epoxypropyl ether of 6-Aminohexanoicacid
13.117 g (0.1 mol) of 6-aminohexanoic acid were added to a 200 mL round-bottom flask. After that, 9.25 g (0.1 mol) of epoxy chloropropane (epichlorohydrin) was then added, and a magnetic stirrer was used to constantly agitate the entire mixture for five hours at 60°C. To turn it alkaline, a 50% water-based solution of sodium hydroxide in water was added drop by drop until the pH dropped to 8.5.
Synthesis of TKP derived TAMHA resin
Dioxane was introduced to a round-bottom flask containing 0.1 mol of TKP and swirled for five hours at 60°C. Afterwards, while stirring, a 50% sodium hydroxide (aqueous solution) was added during synthesis. Following this, epoxy propane ether of 6-aminohexanoic acid was added, and the reaction mixture was continually agitated for 4.5 hours at 65 degrees Celsius before being allowed to settle overnight. To get rid of inorganic contaminants, the product was vacuum-filtered before being cleaned with 90% methyl alcohol and a few drops of inorganic acid (HCl). In the end, pure methanol was used to wash it. The yield of the 6-Aminohexanoic acid (TAMHA) resin was 162.2 g, and the end result was a free-flowing, light yellow powder. It was resin made by method of Singh et al 26.
Adsorption method
Batch adsorption method was implemented in order to remove heavy metal ions from wastewater from industries. TAMHA resin (0.1 mol.) was added to a 250 mL conical flask with a capacity of 100 mL of the established metal ion concentrations, which corresponded to 2 to 15 mg/L. Sodium bicarbonate and hydrochloric acid were used in the conventional way to modify the pH, and the mixture was stirred continually for the conceived equilibrium time of ninety minutes utilizing a magnetic stirrer. After the mixture reached equilibrium, it was filtered by Whatman-42 paper filters, and an atomic absorption spectroscopy (Perkin-Elmer device 2380) was used to assess the filtrate. For the ions Pb2+, Cd2+, Fe2+, Cu2+and Zn2+, plots of the corresponding calibration curves have been generated. The percentage of Pb2+, Cd2+, Fe2+, Cu2+and Zn2+ adsorption on TAMHA resin as well as the Kd (distribution coefficient) values were derived.

Result and Discussions
Thermogravimetric analysis (TGA)
For determining the thermal degradation of resin, the model number Q 500 V 6.7 Build 203 thermogravimetric instrument will frequently use. The resin sample was properly dried in a vacuum desiccator after being powdered to the same typical mesh size. For analysis, the boat was uniformly stocked. The model number Q 500 V 6.7 Build 203 thermo gravimetric analyzer is frequently used to measure the thermal decomposition of resin. After being ground into a powder with the same specified particle size, the resin sample was appropriately dried in a desiccator with a vacuum while the boat was continuously being prepared for analysis. Under a stationary atmosphere, the system was constantly warmed at a rate of 20°C/min1 until it reached complete decomposition for the dynamics measurement. Up to 250°C, the TAMHA resin was found to be stable, but deterioration was shown to be quick after that. Figure 1 showcases the TAMHA resin TGA graph.
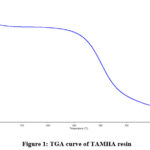 |
Figure 1: TGA curve of TAMHA resin. |
Scanning Electron Microscopy (SEM)
Instruments made by Zeiss were used for SEM. The results of the SEM clearly show the uneven and porous surface of the resin. Comparatively speaking to TKP, synthetic resin has a more abrasive texture. SEM findings and adsorption observations of metal ions in effluent are in good agreement. Figure 2 shows the micrographs of TAMHA resin that were so obtained. SEM images showing how voids have appeared in resins to the same proportion as the area of their surface on the surface of manufactured natural resin. Surfaces display structural variety and variability. The nature of the adsorbent is porous.
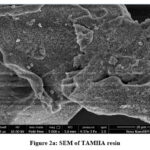 |
Figure 2a: SEM of TAMHA resin |
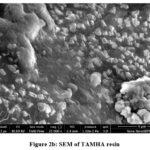 |
Figure 2b: SEM of TAMHA resin |
FTIR spectra of TAMHA resin
FTIR spectra of TAMHA resin were recorded on Agilent technologies FTIR instrument. Due to the stretching of the -OH molecule, the TAMHA resin’s FTIR spectrum displays a broad band with a peak between 3600 and 3200 cm-1. The peak at 2922.2 cm-1 is caused by C-H stretching vibrations, the peak at 1625.1 cm-1 is caused by N-H bending vibrations, and the peak at 1401.51 cm-1 is caused by C-H bending vibrations. A noticeable peak at 1006.10 cm-1 indicates the C-O stretching vibration.
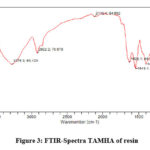 |
Figure 3: FTIR-Spectra TAMHA of resin. |
Effect of pH on distribution coefficient (Kd)
The distribution’s coefficient is the ratio of the substance’s concentrations in all of its states—ionized and unionized—in a mixture of two immiscible states at equilibrium. These coefficients represent the variance in the compound’s solubility between these two phases. Measurements for the ‘Kd’ are significantly impacted with the pH. Table 2 lists the variations in distribution coefficient (Kd) values of several metal ion with H+ ion. Based on the information gathered, the Kd values increase as the acidity of the aqueous solution declines, and the most suitable pH ranges are 5-8. TAMHA resin begins functioning when the pH reaches to a level where the strongest acidic ion exchange sites begin to interchange hydronium ions (H+) for metal ions. When any of the heavy metal ion adsorption on its functional group can form complexes synthesized guar gum diamino benzoic acid (GDABA) resin for elimination of hazardous waste metal ions from industrial effluents 24. The relevant work was also conducted by various researchers exchange method 25. The reduction in ‘Kd’ distribution coefficient values after the neutral and alkaline region maximum can be attributed to the complex growth of TAMHA resin with toxic metal ions. High distribution coefficient values suggest that the metal has been adsorbent Ly held by the resin.
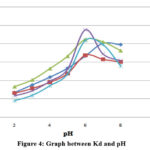 |
Figure 4: Graph between Kd and pH |
Table 2: The significant features of industrial waste water.
|
Description |
Visibility: Turbid, solution Color: Green, Odor: Unpleasant, pH: 4.8, Total hardness: 970 |
||||||
|
Metal ions |
Ca2+ |
Mg2+ |
Fe2+ |
Cd2+ |
Pb2+ |
Cu2+ |
Zn2+ |
|
Concentration mg/L |
175.4
|
101.5
|
3.12
|
0.98
|
1.14
|
3.99
|
5.82
|
Table 3: Metal ions distribution coefficient
|
Metal ion distribution coefficient (Kd) Kd ×102 |
|||||
|
pH |
Fe2+ |
Zn2+ |
Cu2+ |
Cd2+ |
Pb2+ |
|
2 |
13.45 |
13.18 |
16.77 |
11.77 |
9.00 |
|
3 |
17.61 |
15.75 |
20.45 |
14.50 |
11.92 |
|
4 |
21.83 |
19.10 |
26.60 |
19.69 |
17.14 |
|
5 |
26.70 |
23.83 |
33.36 |
26.29 |
23.52 |
|
6 |
33.33 |
33.75 |
42.50 |
47.64 |
41.81 |
|
7 |
40.32 |
31.57 |
41.15 |
34.54 |
39.56 |
|
8 |
39.52 |
30.13 |
36.39 |
30.83 |
28.00 |
Elimination of metal ions from effluent of Steel Industry
Metal ion elimination through TAMHA resin necessitates prior complex synthesis. Since this impacts the metal ions’ solubility, the opposite ion’s concentration, and subsequent extraction of metal ions, the creation of metal-chelates is specifically influenced by the pH of the aqueous phase. Ions on the functional groups of the adsorbent and the degree to which it transforms during a reaction. The consequences of pH in the 2.0–8.0 range of pH on the development of complexes and metal ion separation from effluent were studied using HCl and NaOH. The largest percentage of the metal ions that were tested was eliminated at pH ranges of 5.0-8.0. The reported table makes it abundantly evident that as pH rises, the fraction of metal ions removed first rises and subsequently declines. This depicts how the pH of the solution influences the metal ions’ selectivity. This results in the maximal elimination of harmful metal ions. The H+ ions of the TAMHA resin are rapidly exchanged with metal ion in an acidic solution.
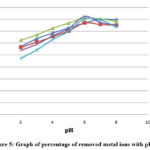 |
Figure 5: Graph of percentage of removed metal ions with pH |
Table 4: Percentage of metal ions extracted
|
pH |
Fe2+ (%) |
Zn2+ (%) |
Cu2+ (%) |
Cd2+ (%) |
Pb2+ (%) |
|
2 |
57.37 |
56.70 |
62.65 |
54.08 |
47.36 |
|
3 |
63.78 |
61.16 |
67.16 |
59.18 |
54.38 |
|
4 |
68.58 |
65.63 |
72.68 |
66.32 |
63.15 |
|
5 |
72.75 |
70.44 |
76.94 |
72.44 |
70.17 |
|
6 |
76.92 |
77.14 |
80.95 |
82.65 |
80.70 |
|
7 |
80.12 |
75.94 |
80.45 |
77.55 |
79.82 |
|
8 |
79.80 |
75.08 |
78.44 |
75.51 |
73.68 |
Conclusion
At pH 6, the maximum adsorption of Pb2+, Cd2+, Cu2+, Zn2+ were noted, whereas the maximum Fe2+ was also noted at pH 7. The highest lead absorption has been determined at pH 6. TAMHA resin is currently thought to be one of the most promising methods for removing heavy metal ions because of its speed, price, and sustainability. By deploying different concentrations of HCl solution to effectively remove the metal ions from the adsorbed TAMHA resin, the resin could be washed with pure distilled water and acidic 10 times to regenerate it into its (H+) cationic exchanger state. The recently developed TAMHA resin is recyclable and hydrophilic, allowing for the safe disposal of old resins following effluent treatment without posing any environmental hazards. Therefore, our research shows that newly synthesized Industrial wastewater may be efficiently treated to remove problematic heavy metal ions utilizing TAMHA resin.
Acknowledgements
The authors are thankful to the Head, Department of Chemistry, Jai Narain Vyas University, Jodhpur, Rajasthan for providing necessary facilities during the experimental work.
Conflict of Interest
All authors have no competing interests.
Reference
- Ajmal, M.; Khan, AS.; AhmadS.; Ahmad, A., Water Research, 1998, 32(10),3085.
CrossRef - Dakiky, M.; Khamis, M.; Manassra, A.; Mereb, M., Adv in Environ Res., 2002, 6(4),533.
CrossRef - Kozlowski, CA.; Walkowiak, W., Water Research, 2002, 36,4870.
CrossRef - Lal, M.; Gangotri, KM., Environ Sci Pollut Res., 2023, 30(44),98805–98813.
CrossRef - Shah, PU.; Raval, NP.; Shah NK., J Mater. Environ. Sci. 2015, 6(9),2573-2582.
- Singh, AV.; Kumawat, IK., Polym Eng Sci, 2013,53: 546-554.
CrossRef - Gregorio, C., Progress in Polymer Science. 2005, 30(1),38-70.
CrossRef - Zakariyah JA.; Tawfik SA., Shaikh, AA., J Hazardous Materials, 2017, 327,44-54.
CrossRef - Hua, S.; Yang, H.; Wang, W; Wang, A., Appl. Clay Sci., 2010, 50,112.
CrossRef - Zhao, M.; Duncan, JR.; Van Hille, RP., Water Res., 1999, 33, 1516.
CrossRef - Srivastava, V.; Weng, CH; Singh, VK.; Sharma, YC., J Chem. Eng. Data., 2011,56,1414.
CrossRef - Srivastava, VC.; Mall, ID.; Mishra, IM., Chem. Eng. J., 2006, 117,79.
CrossRef - Mohan, S.; Gandhimathi, R., J Hazard. Mater., 2006, 169-351.
CrossRef - Ahmad, MA.; Ahmad, N.; Bello, OS., Water. Air. Soil Pollut., 2014, 225:1.
CrossRef - Yuryev, VP.; Cesaro, A; Bergthaller, WJ., New York: Nova Science Pub., Inc., 2002.
- Wurzburg, OB., CRC Press: Boca Raton Florida,1986.
- Sandford, PA.; Baird, J., Vol. 2. New York: Academic Press,1983, 411-90.
CrossRef - Synowiecki, J., Al-Khateeb NA., J Crit Rev Food Sci Nutr., 2003, 43:145-71.
CrossRef - Kumar, R., J React Funct Polym., 2000, 46(1),1-27.
CrossRef - Bailey, SE.; Olin, TJ.; Bricka, RM., Adrian, DD., J Water Res., 1999, 33,2469-2479.
CrossRef - Singh, AV.; Singh, R., J Environ Prog and Sust Energy, 2011, 32(1):103-08.
CrossRef - Sharma, M.; Rani, B., J Adv Sci Res 2011, 2(3),48-51.
CrossRef - Singh, AV.; Karel, G.; Musyuni, P., Int J Appl Biol Pharm Tech., 2010, 1(3),897-02.
- Rathore, M.; Singh, AV., Orient J. Chem., 2023, 39(1),216-221.
CrossRef - Choudhary, GK.; Anju; Prakash, C.; Kumari, S.; Choudhary, M.; Chowdhary, V., Orient J Chem., 2023;39(4),990-995.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









