Computational Elucidation of Novel Synthetic Scheme for Erlotinib
Arun B. Chavan1* , Sanjeev M. Reddy2
, Sanjeev M. Reddy2 and G. Krishna Chaitanya1
and G. Krishna Chaitanya1
1School of Chemical Sciences, Swami Ramanand Teerth Marathwada University, Nanded India.
2Gramin ACS Mahavidyalaya, Vasant Nagar, Mukhed, India.
Corresponding Author E-mail: arunchavan1121@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/400233
Article Received on : 23 Jan 2024
Article Accepted on :
Article Published : 15 Apr 2024
Reviewed by: Dr. Hamid Reza Ghorbani
Second Review by: Dr. Amol khandebharad
Final Approval by: Dr. Abdelwahab Omri
The current study focusses on the use of quantum chemistry to elucidate the novel synthetic route for erlotinib from methyl 4,5-dihydroxy-2-isocyanobenzoate, which includes oxidative coupling, nucleophilic addition, cyclization and Williamson’s ether synthesis. The overall reaction requires three intermediate and produces 13 transition states [TS]. Which are less than the earlier reported synthetic schemes. The energies of each reactant, intermediate and products were calculated using DFT (density functional theory) and B3LYP/6-311+G* as a basis set. The energies diagram obtained indicates the novel proposed scheme could follow the easy path to obtain the product, moreover, the energy barrier required to overcome the transition state is low indicating, very less activation energy is required for every reactant to take part in chemical reaction.
KEYWORDS:Activation Energy; Basis Set; Density Functional Theory; Erlotinib; Non Small Cell Lung Cancer; Minimization; Quantum Chemistry; Synthesis
Download this article as:| Copy the following to cite this article: Chavan A. B, Reddy S. M, Chaitanya G. K. Computational Elucidation of Novel Synthetic Scheme for Erlotinib. Orient J Chem 2024;40(2). |
| Copy the following to cite this URL: Chavan A. B, Reddy S. M, Chaitanya G. K. Computational Elucidation of Novel Synthetic Scheme for Erlotinib. Orient J Chem 2024;40(2). Available from: https://bit.ly/3UixOLh |
Introduction
Erlotinib is a targeted therapy used in the treatment of non-small cell lung cancer (NSCLC) and pancreatic cancer. It belongs to the class of drugs known as tyrosine kinase inhibitors (TKIs), specifically targeting the epidermal growth factor receptor (EGFR) 1–3.
EGFR is a protein involved in regulating cell growth and division. In cancers like NSCLC and pancreatic cancer, EGFR can be overactive, leading to uncontrolled cell growth. Erlotinib works by inhibiting the tyrosine kinase activity of EGFR, thereby blocking the signalling pathways that promote cancer cell growth and proliferation. Clinical studies, such as the pivotal BR.21 trial for NSCLC, have demonstrated the efficacy of erlotinib in improving progression-free survival and overall survival in certain patient populations, especially those with EGFR mutations 4,5. Additionally, it has shown benefits in advanced pancreatic cancer, either as a single agent or in combination with other chemotherapy drugs 6.
Quantum chemistry plays a pivotal role in elucidating reaction mechanisms by providing detailed insights into the molecular-level processes involved in chemical reactions. It involves applying principles of quantum mechanics to study the behaviour of atoms and molecules, their interactions, and the distribution of electrons within them 7. Quantum chemistry contributes to understanding reaction mechanisms. it is widely used in calculating energies, geometries of reactants, intermediates, transition states, and products involved in a reaction. Understanding these structures and their energy changes helps identify stable and transient species and elucidate the energy profiles of reactions. It also helps in determine transition states—the fleeting states between reactants and products 8–10. By characterizing transition states, researchers can assess activation energies and reaction rates, crucial for understanding reaction mechanisms. Quantum calculations provide information about bond energies, bond lengths, and electron densities involved in bond-breaking and bond-forming processes during reactions. Quantum chemistry helps to explore various possible pathways a reaction might take. Calculations reveal the most favourable routes and intermediates, aiding in understanding the sequence of steps involved in a reaction. It also provides mechanistic insights by analysing electronic structures, charge distributions, and orbital interactions, quantum chemistry offers mechanistic insights, explaining how electrons rearrange and how molecular properties change during a reaction 11. In summary, quantum chemistry provides a theoretical framework to understand reaction mechanisms by exploring molecular structures, energies, transition states, and electronic properties. These computational methods complement experimental observations, aiding in the elucidation and prediction of complex chemical reactions 12.
The common method for erlotinib consist of six to seven steps starting from 3,4, dihydroxy benzoic acid or its derivatives as mentioned in scheme 1. The key intermediates and their preparations involve a series of reaction and use of highly flammable gas such as hydrogen at high pressure, and platinum oxide a costly reagent 13,14.
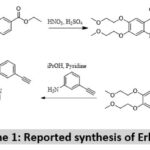 |
Scheme 1: Reported synthesis of Erlotinib. |
Keeping the drawback of known synthetic schemes we propose a new scheme can shorten the reaction steps and can lead to the higher yield of the product, in addition the new scheme eliminates the danger associated with the use of flammable catalyst such as hydrogen gas 15.
Material and methods
The chemical structures and the reaction mechanism were drawn using ChemDraw and saved in mol2 format. All quantum calculations were performed using Gaussian 09 program suit. Structures (reactants, intermediates and products) were optimized to locate the minima. Transition states were identified by finding only one negative eigenvalue. B3LYP/6-311+G* level was used to optimize the reaction pathway followed by single-point calculations at MP2 level using the same basis set. Solvent influence on relative energies of the reactants, intermediates and transition states is also calculated at MP2/6-311+G*:PCM//B3LYP/6- 311+G level using continuum solvent model. The relative energies of the species were calculated with respect to the starting material 4,5-dihydroxy-2-isocyanobenzoate 16.
Result and discussion
The reaction mechanism involves four major steps and proceeds with three intermediate formations. Overall reaction proceeds with stages involving cyclization, halogenation followed by Williamson synthesis and finally amination or C-N coupling. Schematic reaction path is depicted in figure 1.
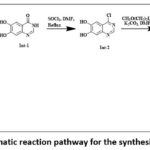 |
Figure 1: Schematic reaction pathway for the synthesis of erlotinib. |
Cyclization
This step consists of various sub steps starting from oxidative coupling in which partially negativecarbonatom of iso thiocyanate is attacked by diacetoxycopper to form a bond between copper and partially charged carbon, which is then attacked by ammonia base to undergo nucleophilic addition reaction followed by reductive elimination and generation of various transition states which are in resonance with each other. These resonating structure/transition states undergo intramolecular cyclization mediated by ammonia to form intermediate-1. The schematic representation for the synthesis of intermediate-1 is shown in figure 2. The starting material was minimized using aforementioned method, energy obtained for the compound-I was -82.1 Kcal/mol, whereas as observed from energy diagram compound-I is unstable (97.3Kcal/mol) and immediately releases energy to converted in compound-III which is quite stable (-152.2Kcal/mol). Compound-IV absorbs the energy and is converted to TS-1 (180.5Kcal/mol), the energy difference between compound-3 and TS-1 is -332.7Kcal/mol indicating the reaction is endothermic and requires energy. The two transition states formed, TS-1 and TS-II shows somewhat similar energy indicating both the structures are unstable and are interconvertible, their energy values are 180.5Kcal/mol and 179.8Kcal/mol respectively. The formation of Int-1 from TS-II is exothermic in natures, releases about 269.4Kcal.mol heat which is highly stable. The overall energy diagram for the formation of Int-1 from compound-1 is shown in figure 3.
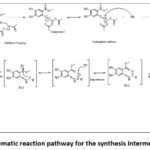 |
Figure 2: Schematic reaction pathway for the synthesis intermediate (Int) 1 |
 |
Figure 3: Energy diagram for the formation of Int-1 from Compound-1 |
Halogenation
6,7-dihydroxyquinazolinone and its resonating structures undergo nucleophilic attack via sulphonyl chloride to form an unstable compound TS-3, loss of one chlorine atom results in compound TS-4, which further undergo nucleophilic attack to form TS-5 followed by intermolecular rearrangement to form Int-2. Schematic representation of overall cyclization process in given in figure 4. As per the energy diagram enormous amount of heat is absorbed to form three unstable transition state compounds. The energy diagram for the three transition states are 87.8Kcal/mol, 102.2Kcal/mol and 130.8Kcal/mol for TS-II, TS-III and TS-IV respectively. As discussed, these intermediates undergo further changes to form stable compound Int-2 (-80.4Kcal/mol). The energy diagram for the formation of Int-II from Int-I is shown is figure 5.
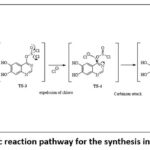 |
Figure 4: Schematic reaction pathway for the synthesis intermediate (Int) 2 |
 |
Figure 5: Energy diagram for the formation of Int-2 from Int-1 |
Williamson ether synthesis
Usually it involves SN2reaction where an alkoxide ion interacts with primary alkyl halide. Reaction of Intermediate-2 with ammonia and potassium carbonate result in the formation of unstable compound which eliminates 2 mol of hydrogen carbonate (HCO3). The product formed thus react with 2 mol of KI and undergo SN2 reaction to form Int-3. Schematic representation of overall reaction process in given in figure 6. Similar toInt-2 formation, formation of Int-III generates four unstable transition states, TS-6, TS-7, TS-8 and TS-9 with the energy of 95.2Kcal.mol, 98.8Kcal/mol, 93.2Kcal/mol and 108.4Kcal/mol respectively. These TS states undergo reaction with potassium iodide to form stable Int-3 (-91.8Kcal/mol). The energy diagram for their formation shown is figure 7.
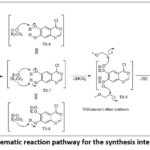 |
Figure 6: Schematic reaction pathway for the synthesis intermediate Int-3 |
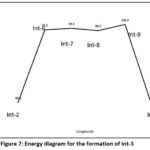 |
Figure 7: Energy diagram for the formation of Int-3 |
Amination (nucleophilic aromatic substitution)
Intermediate-3 reacts with 3-ethynylaniline to form TS compound which undergo rearrangement to form Erlotinib. Schematic representation of overall reaction process in given in figure 8. The formation of erlotinib from Int-3 proceeds with the formation of three Transition states compounds. TS-10, TS-11, TS-12 with the energy of 87.8Kcal/mol, 102.2Kcal/mol, 130.8Kcal/mol. The final product (erlotinib) shows the energy of -80.4Kcal/mol, which is quite stable shown in figure 9.
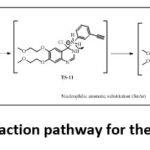 |
Figure 8: Schematic reaction pathway for the synthesis of erlotinib. |
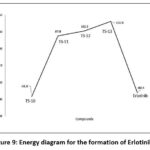 |
Figure 9: Energy diagram for the formation of Erlotinib. |
Conclusion
Erlotinib, (Tarceva), is a reversible and extremely selective EGFR tyrosine kinase inhibitor. It is used to treat pancreatic cancer and non-small cell lung cancer (NSCLC).[4] It is specifically used to treat NSCLC caused by mutations in the EGFR (epidermal growth factor receptor). In 2004, erlotinib received FDA approval for use in medicine. It is included in the List of Essential Medicines by the World Health Organization. The reported synthetic schemes of Erlotinib consist of six-seven steps, which are highly expensive. Moreover, use of flammable catalysts and hydrogen gas in the current synthesis process again raise the associated safety concerns. In the current manuscript, we propose a new synthetic scheme for the preparation of erlotinib. This novel scheme undergo the product formation in fewer steps and does not require or produce any harmful reagents or byproducts. We validated the scheme by calculating the energies using Quantum calculations. The current proposed scheme could be adopted in the synthesis of erlotinib.
Acknowledgements
Authors are thankful to the Management and Principal of Rahemaniya Junior College, Nilanga for giving permission to carry this research work. He is also thankful to Professor Dr. K. Chaitanya, School of Chemical Sciences, S R T M U, Nanded and his guide Dr. Sanjeev M. Reddy, Gramin ACS Mahavidyalaya, Vasant Nagar, Mukhed Dist. Nanded without whose kind guidance and patients towards him in carrying out the said research work despite their hectic, busy academic and Research schedule.
References
- Bareschino, M. A.; Schettino, C.; Troiani, T.; Martinelli, E.; Morgillo, F.; Ciardiello, F. nt. Annals of Onco. 2007, 18, vi35–vi41.
CrossRef - Tsao, M.-S.; Sakurada, A.; Cutz, J.-C.; Zhu, C.-Q.; Kamel-Reid, S.; Squire, J.; Lorimer, I.; Zhang, T.; Liu, N.; Daneshmand, M.; Marrano, P.; Da Cunha Santos, G.; Lagarde, A.; Richardson, F.; Seymour, L.; Whitehead, M.; Ding, K.; Pater, J.; Shepherd, F. A. N Engl J Med 2005, 353 (2), 133–144.
CrossRef - Abdelgalil, A. A.; Al-Kahtani, H. M.; Al-Jenoobi, F. I. Academic Press, 2020, 45, 93–117.
CrossRef - Robertson, J.; Barr, R.; Shulman, L. N.; Forte, G. B.; Magrini, N. Bulletin of the World Health Organization, 2016, 94 (10), 735.
CrossRef - Thatcher, N.; Chang, A.; Parikh, P.; Pereira, J. R.; Ciuleanu, T.; Von Pawel, J.; Thongprasert, S.; Tan, E. H.; Pemberton, K.; Archer, V. The Lancet, 2005, 366 (9496), 1527–1537.
CrossRef - Moore, M. J.; Goldstein, D.; Hamm, J.; Figer, A.; Hecht, J. R.; Gallinger, S.; Au, H. J.; Murawa, P.; Walde, D.; Wolff, R. A. Journal of clinical oncology, 2007, 25 (15), 1960–1966.
CrossRef - Ivancevic, V. G.; Ivancevic, T. T. Quantum Neural Computation, 2010, 151–217.
CrossRef - Cheng, G.J.; Zhang, X.; Chung, L.W.; Wu, Y.D., J. Am. Chem. Soc. 2015,137,5, 1706-1725.
CrossRef - Wiebe, N.; Reiher, M.; Svore, K.; Wecker, D.; Troyer, M. APS March Meeting Abstracts; 2017, 2017, 52-009.
- Chirkina, E.; Korchevin, N.A.; Structural Chemistry, 2023, 34(6), 1-10.
CrossRef - Tanaka, A.; Maekawa, K.; Suzuki, K. Org. Syn. Res. Labo. 2013, 2013.
- Lehtola, S.; Karttunen, A. J. WIREs Comput Mol Sci 2022, 12 (5), e1610.
CrossRef - Barghi, L.; Aghanejad, A.; Valizadeh, H.; Barar, J.; Asgari, D. Advanced pharmaceutical bulletin, 2012, 2 (1), 119.
- Smith, J. Clinical therapeutics 2005, 27 (10), 1513–1534.
CrossRef - Schnur, R. C.; Arnold, L. D. 1998. https://patents.google.com/patent/ US5747498A/en (accessed 2024-01-05).
- Tirado-Rives, J.; Jorgensen, W. L. J. Chem. Theory Comput. 2008, 4 (2), 297–306.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









