An Experimental Study on the Evaluation of Boerhavia Diffusa in the Treatment of Psoriasis in Albino Mice
Vinay Kumar1* , Pawan Prajapati1
, Pawan Prajapati1 , Gauri Goyal1
, Gauri Goyal1 , Virendra Pratap Singh Rathore2
, Virendra Pratap Singh Rathore2 and Ashutosh Mishra3
and Ashutosh Mishra3
1Department of Pharmacology, KIET Group of Institutions (KIET School of Pharmacy), Delhi-NCR, Meerut Road, Ghaziabad, (UP), India.
2Department of Pharmacology, Maharana Pratap College of Pharmacy, Kanpur, (UP), India.
3Mankind Research Center, 191 E sector 4 IMT Manesar, Gurgaon, Haryana, 122052 India.
Corresponding Author E-mail: vinaykumarpatel@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/400224
Article Received on : 29 Jan 2024
Article Accepted on :
Article Published : 27 Mar 2024
Reviewed by: Dr. Bhavini Gharia
Second Review by: Dr. Gisele Barbosa
Final Approval by: Dr. Mohan Maruga Raja
Psoriasis, impacting 2-3% of the population, poses a chronic challenge. Conventional treatments having limited effectiveness and adverse effects; recent immunological insights foster targeted therapies, while herbal medications gain attention for safety and accessibility.
The current research aimed to assess the efficacy of Boerhavia diffusa in mitigating psoriasis induced by imiquimod in mice. Study induced psoriasis in albino mice using 5% imiquimod cream for 5 days, then treated with Boerhavia diffusa (1% and 3%) and Tazarotene (0.1%). Parameters were assessed in the study viz. PASI score, spleen weight/body weight ratio, serum inflammatory markers (IL-17, IL-23, and TNF-α), and skin histopathology. Mice treated with imiquimod exhibited noteworthy elevations in PASI score, spleen weight/body weight ratio, and serum inflammatory markers, which were effectively suppressed by Boerhavia diffusa and Tazarotene applications. Topically application of Boerhavia diffusa significantly ameliorated psoriasis, suggesting it as a promising, well-tolerated alternative to enhance the quality of life for affected individuals.
Boerhavia diffusa; Inflammatory markers; Molecular targets; PASI; Psoriasis
Download this article as:| Copy the following to cite this article: Kumar V, Prajapati P, Goyal G, Rathore V. P. S, Mishra A. An Experimental Study on the Evaluation of Boerhavia Diffusa in the Treatment of Psoriasis in Albino Mice. Orient J Chem 2024;40(2). |
| Copy the following to cite this URL: Kumar V, Prajapati P, Goyal G, Rathore V. P. S, Mishra A. An Experimental Study on the Evaluation of Boerhavia Diffusa in the Treatment of Psoriasis in Albino Mice. Orient J Chem 2024;40(2). Available from: https://bit.ly/3TI03Sa |
Introduction
Psoriasis, an autoimmune disorder driven by T-cells, remains a medical enigma, with its precise origins still unknown. When plasmacytoid and myeloid dendritic cells (DCs) are stimulated, certain cytokines like type I interferons come into play. In the realm of biological responses, an unfortunate phenomenon unfolds when the delicate equilibrium is disturbed: the prominent role is played by the overproduction of tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6). These two notorious agents, known for their pivotal roles in inflammatory processes, emerge as the protagonists of a hyperactive production saga 1.
Elevated cytokine levels trigger IL-12 and IL-23 secretion, sparking robust IL-17 generation by pathogenic T helper cells. This IL-17 prompts keratinocyte activity, driving a cycle of epidermal hyperplasia and immune response activation. A cascade ensues, ramping up proinflammatory cytokines like IL-1β, IL-6, and IL-8, amplifying the inflammation in affected epidermal tissue. This molecular interplay forms a feedback loop, intensifying the response with each iteration. It’s a dramatic cellular drama onstage, showcasing the intricate symphony of inflammation in psoriasis 2,3.
Psoriasis affects about 125 million people 4, manifesting in striking red, scaly plaques amidst otherwise normal skin—a vivid, unmistakable signature of the condition. These patches resemble artistic expressions, visually narrating the body’s internal struggle. This condition’s origins lie in a complex blend of genetic predisposition and environmental influences. Genetics set the stage for susceptibility, while environmental factors intricately shape the unfolding narrative 4,5.
T-helper (Th17) cells and interleukin-23 (IL-23), two possible causes of the psoriatic phenotype, have been proposed. The production of proinflammatory cytokines on keratinocytes by dermal dendritic cells, which can trigger Th17 lymphocyte development, leads to parakeratosis and epidermal hyperplasia 6.
Presently, managing psoriasis primarily involves using topical treatments, systemic medications, and phototherapy. Unfortunately, these approaches often lead to various side effects like relapses and adverse drug reactions. Due to an incomplete understanding of psoriasis’ underlying mechanisms, there remains a scarcity of universally accepted and safe therapeutics with high effectiveness. Therefore, finding a treatment option with evident benefits and minimal side effects for psoriasis is an urgent necessity 7.
While psoriasis lacks a cure, established protocols exist for its management. Mild to moderate cases commonly begin with topical therapies incorporating a mix of glucocorticoids, vitamin D analogous, and phototherapy. Moderate to severe instances typically necessitate systemic therapies. Unfortunately, prolonged use of these systemic treatments often brings about harmful side effects, including liver and kidney issues, high blood pressure, tremors, electrolyte imbalances, cancers, poor long-term patient adherence, and immune responses against the drugs. As a result, there is an ongoing need for alternative therapeutic approaches catering to high-risk or unresponsive patients 8.
Boerhavia diffusa is utilized for conditions such as arthritis, cramps, pain in the joints, rheumatic discomfort, and renal pain, acting as an anti-inflammatory agent by infusing stems with leaves. Moreover, the decoction made from its spongy roots is employed in addressing the condition of cirrhosis, yellowing of the skin (jaundice), and inflammation of the liver (hepatitis).9. The decoction of Boerhavia diffusa roots is employed as a substance with diuretic properties, addressing urinary problems including gall bladder stones, albumin in the urine, glomerulonephritis and copious urine 10. Additionally, it is utilized for conditions like ischemia, inflammation of the liver, icterus, inflammation of the bladder, abdominal dropsy, thiamine deficiency disease, gonorrhea 10, anaemia, heart troubles, palpitations and jaundice 11. Powdered Boerhavia diffusa root, when mixed with butter or oil, is used in the treatment of abdominal tumors 11. The decoction made from Boerhavia diffusa roots is used for respiratory condition like asthma, oliguria, inflammatory conditions, hemorrhoids, vaginal discharge, musculoskeletal pain, abdominal pain, lymphatic filariasis, addressing sexual debility and regulating hypertension 12. The leaf infusion serves as an anticonvulsant, antiasthmatic, expectorant, and emetic 13. It is also utilized for conditions like asthma, cholera, and paludism 14. The whole plant decoction of Boerhavia diffusa is employed for treating gonorrhea, nephritis, and edema, while an infusion of its leaves is utilized as an appetizer and for alleviating joint pain 15. Boerhavia diffusa roots, whether chewed or boiled, address gastroenteritic problems; a tea brewed from the roots treats prolapsed uterus, while applying the sap extracted from the root onto the neck and throat, alleviates symptoms of mumps, laryngitis, and burns 11. The freshly extracted juice or infusion of Boerhavia diffusa foliage is also employed for its pain reliever and anti-swelling attributes 16. Therefore, the current study was conducted to evaluate Boerhavia diffusa against the imiquimod-induced psoriasis in mice.
Materials and Methods
Experimental Animals
Experimental animals were employed following the approval of the experimental protocol (IAEC/KSOP/2022-23/05) by the Institutional Animal Ethics Committee (IAEC) of KIET School of Pharmacy, Ghaziabad (UP), India (Reg. No.: 1099/PO/RE/S/07/CPCSEA). The study involved thirty albino mice (30-35 g) of both sexes, housed in standard conditions of room temperature, relative humidity (25 ± 2˚C and 50 ± 15%), and a 12-hour light-dark cycle.Top of Form
Drugs and chemicals
Boerhavia diffusa was obtained as a gift sample. Imiquimod 5% and Tazarotene cream were obtained from Glenmark pharmaceuticals Ltd. Formalin, Diethyl ether and Tween 80 were procured from Qualigens. ELISA kits for interleukins (IL-17 and IL-23) were purchased from G. Biosciences. The experiment employed chemicals and reagents of analytical grade exclusively.
Treatment schedule
The animals were categorized into five groups (n = 6) as follows: group I (Normal Control) received no treatment, while group II (Psoriasis group) received 5% imiquimod topical application for 5 days to induce psoriasis, group III (Herbal Formulation Low Dose) received Boerhavia diffusa (1%) at low dose along with Psoriasis induction, group IV (Herbal Formulation High Dose) received Boerhavia diffusa (3%) at high dose along with Psoriasis induction and group V (Standard Group) received Tazorac Cream (Tazarotene; 0.1%) along with psoriasis induction [4,17,18,19].
Psoriasis Area and Severity Index (PASI) Assessment
The assessment of skin irritation severity utilized the PASI score. However, the affected skin area was not factored into the overall score in accordance with the clinical PASI score; instead, adjustments were made based on the mouse model. The overall grading of erythema and scaling conditions ranged from 0 to 4: 0 for none, 1 for faint, 2 for moderate, 3 for marked, and 4 for extremely marked 4.
Spleen weight vs body weight ratio
After dissection, spleen was taken out and washed with normal saline. Then the spleen was dried and dry weight of the spleen was measured, followed by the calculation of the spleen-to-body weight ratio [20].
Estimation of inflammatory markers (IL-17, IL-23 and TNF-α)
The concentrations of IL-17, IL-23, and TNF-α in the serum were measured using commercially accessible ELISA kits (Abbkine) in accordance with the manufacturer’s recommendations 4.
Histopathological Analysis
Using skin tissue, a histopathological study was conducted. After being removed, the skin was submerged in a 10% formalin solution. Haematoxylin-eosin stains were used to prepare and stain the tissue in 5-µm slices21.
Statistical Analysis
The presentation of results is in the form of mean ± SEM. Statistical analysis was conducted using GraphPad Prism Version 5.0 software (San Diego, CA, USA). To determine the significance among groups, one-way ANOVA tests were applied, and subsequent specific multiple comparison Tukey’s post-hoc tests were performed for each observed parameter as indicated. P<0.05 was set as a significant value.
Results
Effect of the Boerhavia diffusaon PASI score
After induction of psoriasis in animals, the PASI score was significantly (P<0.001) increased in imiquimod treated animals while Boerhavia diffusa (1%, 3%),and tazarotene (0.1%) treatment significantly decreased the PASI score as compared to imiquimod treated animals as shown in Fig. 1. Findings showed that treatment has prominent outcome in psoriasis treatment.
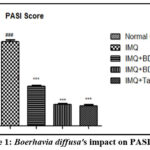 |
Figure 1: Boerhavia diffusa’s impact on PASI score. |
The results are presented as Mean ± SEM, n=6, ###P<0.001 vs Normal Control group, and ***P<0.001 vs IMQ group. Tukey’s post-hock analysis was conducted after the one-way ANOVA. IMQ- Imiquimod; BD- Boerhavia diffusa; PASI- Psoriasis Area and Severity Index.
Evaluation of Spleen weight vs body weight ratio
The average spleen weight/body weight ratio significantly increased by approximately 1.5 times following 5 days of IMQ treatment compared to the normal group, indicating a substantial accumulation of immune cells. However, the application of IMQ+BD (1%), IMQ+BD (3%), and IMQ+Tazarotene (0.1%) led to a specific decrease in spleen weight/body weight ratio compared to the IMQ group. Furthermore, when comparing the treatment groups with the normal controls, there was a notable reduction in spleen weight, approaching normal levels (P< 0.001). Fig. 2 depicts these trends in spleen weight changes across the experimental conditions.
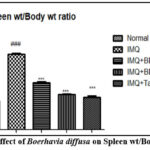 |
Figure 2: Effect of Boerhavia diffusa on Spleen wt/Body wt ratio. |
The results are presented as Mean± SEM, n=6, ###P<0.001 against Normal Control group, ***P<0.001 vs IMQ group, and **P<0.01. Tukey’s post-hock analysis was conducted after the one-way ANOVA. IMQ- Imiquimod; BD- Boerhavia diffusa.
The impact of Boerhavia diffusa on inflammation indicators (IL-17, IL-23 and TNF-α)
The levels of inflammatory markers in the serum, specifically IL-17, IL-23, and TNF-α, showed a noteworthy (P<0.001) elevation in animals treated with imiquimod when compared to the Normal control group. The treatment of these animals with Boerhavia diffusa at the concentration (1%, and 3%), and Tazarotene at the concentration 0.1%, significantly shows decrease in the concentrations of these inflammation indicators as depicted in Fig. 3,4 and 5 respectively.
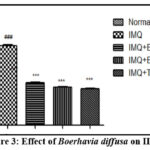 |
Figure 3: Effect of Boerhavia diffusa on IL-17. |
The results are presented as Mean± SEM, n=6, ###P<0.001 vs Normal Control group, and ***P<0.001 vs IMQ group are used to represent the results. The one-way ANOVA was followed by Tukey’s post-hock analysis. IL-17- Interleukin-17; IMQ- Imiquimod; BD- Boerhavia diffusa.
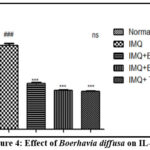 |
Figure 4: Effect of Boerhavia diffusa on IL-23. |
The results are presented as Mean SEM, n=6, ###P<0.001 vs. Normal Control group, and ***P<0.001 vs. IMQ group. Tukey’s post-hock analysis was conducted after the one-way ANOVA. IL-23- Interleukin-23; IMQ- Imiquimod; BD- Boerhavia diffusa.
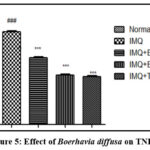 |
Figure 5: Effect of Boerhavia diffusa on TNF-α. |
The results are presented as Mean ±SEM, n=6, ###P<0.001 against Normal Control group, ***P<0.001 vs IMQ group, and **P<0.01. Tukey’s post-hock analysis was conducted after the one-way ANOVA. TNF-α- Tumor necrosis factor-alpha; IMQ- Imiquimod; BD- Boerhavia diffusa.
Histopathological Examination of Skin
The results of the histology analysis are shown in below figure (Fig. 6). The normal control group showed no keratosis symptoms, indicating that their skin was healthy and intact. The skin was thick (coated with white desquamation), keratosis and hyperkeratosis with scales and erythema in the IMQ group. The changes were reversed after topical treatments with Boerhavia diffusa (1% and 3%) and Tazarotene (0.1%). These changes are depicted in Fig. 6 (A.B.C.D and E) respectively.
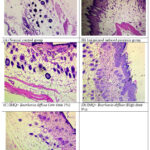 |
Figure 6: Histopathological examination of skin of different groups. |
Discussion
Psoriasis is a persistent inflamed condition of the skin that is impacted by both hereditary and environmental causes. Its prevalence tends to rise with advancing age and exerts a substantial influence on the quality of life for affected individuals 4.
Increased T helper 1 cell (Th-1), Th-17 infiltration, and angiogenesis, aberrant keratinocyte differentiation, and epidermal hyperproliferation are histological hallmarks of the chronic inflammatory skin condition psoriasis. Th17 cells and interleukin-23 (IL-23) have been proposed as potential elements contributing to the manifestation of psoriasis. Dermal dendritic cells can secrete IL-23, which can cause Th17 lymphocyte differentiation and the release of proinflammatory cytokines on keratinocytes, resulting in parakeratosis and epidermal hyperplasia 22,23.
In the present investigation, we utilized an IMQ-induced model to explore the impact of Boerhavia diffusa on psoriasis-like dermatitis. The IMQ-induced model is widely employed in psoriasis research due to its cost-effectiveness, practicality, ease of use, and swift induction of skin inflammation24,25. This model, however, has some limitations, including non-standardized procedures, the generally non-specific nature of the induced skin irritation, and unsuitability for long-term use. Furthermore, the genetic make-up of the mice would have an impact on the psoriasis-like inflammation generated by IMQ 19.
Boerhavia diffusa, a widely recognized plant in traditional and ethnobotanical medicinal practices worldwide, harbors a diverse array of chemical compounds that exhibit notable therapeutic properties. These include anti-inflammatory, hepatoprotective, anticancer, and immunomodulatory effects. Despite its impressive medicinal potential, Boerhavia diffusa has yet to secure a prominent position in the herbal market. The phytochemical chemical constituents from Boerhavia diffusa viz. Boeravinones are phenolic compounds with antioxidant and anti-inflammatory action 9,26.
In this research study, Imiquimod had increased the spleen weight of the animals. In the progression of psoriasis-like dermatitis triggered by IMQ, the spleen underwent noticeable changes, including a significant increase in length from day 2 onwards, reaching a marked difference compared to controls (p < 0.0001). Spleen mass showed a substantial rise starting from day 3, reaching a four-fold increase by day 7, signifying an expansion of immune cells. The lymph nodes also exhibited a three-fold increase in total area, supporting their involvement in the immune response. Presence of germinal center formation in treated mice on day 7 emphasized the connection between immune response severity and psoriasis progression. Visual examination confirmed spleen enlargement, reinforcing its active role in psoriasis-associated immune processes. These observations underscore the significance of studying secondary lymphoid organs in understanding the complex immunological mechanisms driving psoriasis progression27.
After induction of psoriasis in animals, IL-17 initiates immune responses and resulting in the recruitment of neutrophils to the tissue. Imiquimod treated animals shows the decrement in the immune response and increment in the number of neutrophils which kill the dead cell which results in the cause of the psoriasis28. Boerhavia diffusa act as interleukin 17 inhibitor and help in the increase of the immune response and downward the formation of the neutrophils and help in decreasing the psoriasis in the groups which is compared with the disease induced groups, and if the comparison is done with the normal group the treatment is much more effective and shows the positive results.
IL-23 increases the inflammation which causes the psoriasis symptoms. Boerhavia diffusa act as the IL-23 inhibitor which helps in the decreases of the formation of the inflammation28,29. In Fig. 4 we can easily see the result of the medication (treatment groups) that shows the impactful results against the disease group. If the comparison is done then the normal groups the results are well and helpful in blockade of the formation of the interleukin 23.
In the present study, TNF-α was also an important molecular target in a psoriasis-like model. Results consistently showed positive effects in treated groups, reducing visible signs of skin inflammation linked to TNF-α. This underscores the potential of TNF-α-targeted therapies in managing psoriasis-associated inflammation. Further research is crucial to understand mechanisms, long-term effects, and ensure safety for broader application in skin-related inflammatory conditions30.
Mice subjected to different treatments for IMQ-induced psoriasis-like symptoms showed varying responses. The normal control group (Group A) had no symptoms, while the psoriasis group (Group B) displayed skin alterations. Microemulsion and Boerhavia diffusa extract treatments (Groups C & D) had limited effects, with slight improvements. However, Tazarotene at 0.1% concentration (Group E) showed significant protection, notably reducing erythema, swelling, and keratosis. These results indicate Tazarotene’s superior efficacy compared to microemulsion and Boerhavia diffusa extract in alleviating IMQ-induced psoriasis-like symptoms in mice.
Conclusion
In summary, psoriasis-like symptoms were effectively induced in albino mice through the administration of 5% IMQ. The findings of this study highlight the potential efficacy of Boerhavia diffusa in managing psoriasis in mice. It is essential to acknowledge the limitations of this research, given its small sample size and relatively short duration. Consequently, further extensive investigations are necessary to thoroughly understand the underlying mechanisms and confirm the therapeutic viability of Boerhavia diffusa for psoriasis in human subjects.
Acknowledgment
The authors express gratitude to the KIET School of Pharmacy for granting access to research facilities.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Author contribution
Vinay Kumar: Review and Editing; Pawan Prajapati: Experimental work, Analysis, Data compilation; Gauri Goyal: Writing and Editing; Virendra Pratap Rathore: Drafting and Analysis
Data sharing statement
The authors possess the original data, and it will be provided upon request.
References
- Mylonas, A.; Conrad, C. Front. immunol. 2018, 9, 2746.
CrossRef - Lowes, M.A.; Suarez-Farinas, M.; Krueger, J.G. Annu. Rev. Immunol. 2014, 32, 227-255.
CrossRef - Zeini, M.S.; Haddadi, N.S.; Shayan, M.; Zeini, M.S.; Kazemi, K.; Solaimanian, S.; Abdollahifar, M.A.; Hedayatyanfard, K.; Dehpour, A.R. Int. Immunopharmacol. 2021, 100, 108160.
CrossRef - Li, Y.; Zhang, G.; Chen, M.; Tong, M.; Zhao, M.; Tang, F.; Xiao, R.; Wen, H. Biomed. Pharmacother. 2019, 109, 1876-1883.
CrossRef - Nograles, K.E.; Krueger, J.G. Exp. Cell Res. 2011, 317, 1293-1300.
CrossRef - Gudjonsson, J.E.; Johnston, A.; Dyson, M.; Valdimarsson, H.; Elder, J.T. J. Invest. Dermatol. 2007, 127, 1292-1308.
CrossRef - Jiang, M.; Fang, H.; Shao, S.; Dang, E.; Zhang, J.; Qiao, P.; Yang, A.; Wang, G. FASEB J. 2019, 33, 13241-13253.
CrossRef - Zhang, C.; Yan, K.; Diao, Q.; Guo, Q.; Jin, H.; Yang, S.; Chen, X.; Lei, T.; Wu, J.; Yu, H.; Zheng, M. J. Am. Acad. Dermatol. 2022, 87, 95-102.
CrossRef - Mishra, S.; Aeri, V.; Gaur, P.K.; Jachak, S.M. Biomed. Res. Int. 2014, 2014, 808302.
CrossRef - Gour, R. Int. J. Pharm. Sci. Med. 2021, 6, 25-41.
- Patil, K.S.; Bhalsing, S.R. J. Ethnopharmacol. 2016, 182, 200-220.
CrossRef - Mitra, R.; Gupta, R.C. Applied botany abstracts, economic botany information service. NBRI, Lucknow, Uttar Pradesh, India. 1997, 17, 209-227.
- Adesina, S.K. Fitoterapia. 1982, 53, 147-162.
- Adefokun, D.I.; Iwalewa, E.O.; Omisore, N.O.; Obuotor, E.; Idowu, I.J. Br. J. Pharm. Res. 2015, 8, 1-14.
CrossRef - Silva, O.; Caldeira, G.; Serrano, R. Eur. J. Integr. Med. 2020, 39, 101211.
CrossRef - Hiruma-Lima, C.A.; Gracioso, J.S.; Bighetti, E.J.B.; Robineou, L.G.; Brito, A.S. J. Ethnopharmacol. 2000, 71, 267-274.
CrossRef - Cheng, H.M.; Chen, F.Y.; Li, C.C.; Lo, H.Y.; Liao, Y.F.; Ho, T.Y.; Hsiang, C.Y. J. Agric. Food Chem. 2017, 65, 10233-10242.
CrossRef - Van Der Fits, L.; Mourits, S.; Voerman, J.S.; Kant, M.; Boon, L.; Laman, J.D.; Cornelissen, F.; Mus, A.M.; Florencia, E.; Prens, E.P.; Lubberts, E. J. Immun. 2009, 182, 5836-5845.
CrossRef - Lo, H.Y.; Li, C.C.; Cheng, H.M.; Liu, I.C.; Ho, T.Y.; Hsiang, C.Y. Food Chem. Toxicol. 2019, 129, 365-375.
CrossRef - Shinno-Hashimoto, H.; Eguchi, A.; Sakamoto, A.; Wan, X.; Hashimoto, Y.; Fujita, Y.; Mori, C.; Hatano, M.; Matsue, H.; Hashimoto, K. Sci. Rep. 2022, 12, 14738.
CrossRef - Ibad, S.; Heibel, H.D.; Cockerell, C.J. Am. J. Dermatopathol. 2021, 43, 678.
CrossRef - Guo, J.W.; Cheng, Y.P.; Liu, C.Y.; Thong, H.Y.; Huang, C.J.; Lo, Y.; Wu, C.Y.; Jee, S.H. Pharmaceutics. 2020, 12, 457.
CrossRef - Balak, D.M.; Hajdarbegovic, E. Psoriasis-targets th. 2017, 87-94.
CrossRef - Mehta, C.S.; Dave, A.R.; Shukla, V.D. Ayu. 2011, 32, 333.
CrossRef - Girhepunje, K.S.; Gupta, V.; Srivastava, V.K.; Pandey, A.K.; Prasad, R.; Singh, O.P. J. Ayurveda Integr. Med. 2022, 13, 100533.
CrossRef - Das, S.; Singh, P.K.; Ameeruddin, S.; Bindhani, B.K.; Obaidullah, W.J.; Obaidullah, A.J.; Mishra, S.; Mohapatra, R.K. Front. Chem. 2023, 11.
CrossRef - Jabeen, M.; Boisgard, A.S.; Danoy, A.; El Kholti, N.; Salvi, J.P.; Boulieu, R.; Fromy, B.; Verrier, B.; Lamrayah, M. Pharmaceutics. 2020, 12, 789.
CrossRef - Ghoreschi, K.; Balato, A.; Enerbäck, C.; Sabat, R. Lancet. 2021, 397, 754-766.
CrossRef - Chen, L.; Deshpande, M.; Grisotto, M.; Smaldini, P.; Garcia, R.; He, Z.; Gulko, P.S.; Lira, S.A.; Furtado, G.C. Sci. Rep. 2020, 10, 8259.
CrossRef - Hu, J.Z.; Billings, S.D.; Yan, D.; Fernandez, A.P. J. Am. Acad. Dermatol. 2020, 83, 71-77.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









