A Thermoacoustical and DFT Exploration of Physio-Chemical Properties for N, N-dimethylacetamide (DMA) with Water
Laxmi Kumari1 , Ajaz Hussain2
, Ajaz Hussain2 , Harshit Agarwal3
, Harshit Agarwal3 , Abhishek Mishra3
, Abhishek Mishra3 , Ratindra Gautam4
, Ratindra Gautam4 , Rahul Singh5
, Rahul Singh5 , Yashveer Gautam6
, Yashveer Gautam6 , Monal Singh6
, Monal Singh6 , Leena Sinha3
, Leena Sinha3 , Onkar Prasad3
, Onkar Prasad3 and Manisha Gupta3*
and Manisha Gupta3*
1Department of Humanities and Applied Sciences, School of Management Sciences, Lucknow, India.
2Ewing Christian College, University of Allahabad, Allahabad, India.
3Department of Physics, University of Lucknow, Lucknow, India.
4Department of Humanities and Applied Sciences, Dr RML Avadh University, Ayodhya, India.
5Department of Humanities and Applied Sciences, Ambalika Institute of Management and Technology, Lucknow, India.
6Department of Chemistry, Pandit Prithi Nath PG College, Kanpur, India.
Corresponding Author E-mail: guptagm@rediffmail.com
DOI : http://dx.doi.org//10.13005/ojc/400209
Article Received on : 11 Dec 2023
Article Accepted on :
Article Published : 05 Mar 2024
Reviewed by: Dr. Dinesh Rishipathak
Second Review by: Dr. Neeraj Kumar
Final Approval by: Dr. Ioana Stanciu
For the binary mixture of water and N, N-dimethylacetamide (DMA), observations have been done of the ultrasonic velocity (u), density (ρ), viscosity (η) and refractive index (n) in excess of the whole composition range at various temperature. Deviation in ultrasonic velocity (Δu), excess intermolecular free length (LfE), excess acoustic impedance (ZE), excess molar volume (VmE), deviation in molar refraction (ΔRm), deviation in viscosity (Δη) and excess Gibbs' free energy of activation for viscous flow (ΔG*E) were used to investigate the intermolecular interactions present in the mixture. Derived parameters such as isoentropic compressibility (Ks), the specific heat ratio (γ), and the effective Debye temperature (ϴD) at varying concentrations of DMA have also been computed using experimental data. Various semi-empirical mixing rules have also been applied to the binary mixture. Density functional theory is utilised to optimise the geometry of DMA, DMA with water, and calculation of H-bonded intermolecular interactions.
KEYWORDS:Density functional theory; Derived parameters; Intermolecular interaction; Semi-empirical mixing rules
Download this article as:| Copy the following to cite this article: Kumari L, Hussain A, Agarwal H, Mishra A, Gautam R, Singh R, Gautam Y, Singh M, Sinha L, Prasad O, Gupta M. A Thermoacoustical and DFT Exploration of Physio-Chemical Properties for N, N-dimethylacetamide (DMA) with Water. Orient J Chem 2024;40(2). |
| Copy the following to cite this URL: Kumari L, Hussain A, Agarwal H, Mishra A, Gautam R, Singh R, Gautam Y, Singh M, Sinha L, Prasad O, Gupta M. A Thermoacoustical and DFT Exploration of Physio-Chemical Properties for N, N-dimethylacetamide (DMA) with Water. Orient J Chem 2024;40(2). Available from: https://bit.ly/3uY7nk0 |
Introduction
Examining the relationships between molecules in organic binary mixtures. Alcohols are highly self-linked liquids that have a hydrogen bond network that is three dimensions in size1 and can be related with any other group possessing some degree of polarity 2, therefore having alcohol as one of the components is of certain relevance. Because ultrasonic velocity evaluation are extremely reactive to molecular interactions, they may offer subjective information with the kind and intensity of interaction with molecules in liquid solution3-8.
DMA is miscible with water in all ratios and with the majority of organic solvents, such as ethers, ketones, alcohols, and so forth. The boiling point of DMA, a dipolar aprotic solvent, is 438.2 K, a dipole moment of 3.72 D, which is higher than that of water, and a medium dielectric constant of 37.78 at 298.15 K9. Because of its exceptional solvent power and outstanding water solubility especially for high molecular weight polymers and resins. DMA is a widely used solvent in the manufacturing of synthetic fibers and polyurethanes. It functions well as a catalyst and a result for a variety of processes. In addition, it works well as an oil and gas extraction agent and plasticizer for cosmetics and pharmaceutical intermediates.
The experimental basis for selecting the suitable solvents for specific applications is provided by the estimation of ultrasonic velocity, density, refrective index, viscosity and their fluctuation with various proportion composition of liquid and temperature and composition 10-20. Thus, analysis of molecular association in system of water and N, N-dimethylacetamide (DMA) has been attempted in this work. The mixture’s intermolecular interactions were examined. VmE , Δu, ΔRm, LfE, ZE, Δη and ΔG*E. The linear regression polynomial equation for Curve Expert 1.3 has been fitted with these excess parameters. Using experimental data for the system, derived parameters have also been determined, including isoentropic compressibility (ks), specific heat ratio (γ) and the effective Debye temperature (ϴD) at varying concentrations of DMA.
The binary mixture has also been subjected to a several rule and theories, including the Flory’s statistical theory (FST), collision factor theory (CFT), Nomoto, Vandeal and Junjie’s theories for the theoretical estimation of ultrasonic velocities. The APD (average percentage deviation) values determined from the observed values has been used to compare the comparative merits of the different mixing methods and models. Furthermore, the strength of the hydrogen bonding between molecules has been examined using density functional theory (DFT).
DFT Calculations and models
Numerous researchers21-24 have used density functional theory to calculate molecular system properties, with remarkable understanding between experimental and theoretical results. DFT 25 has been functional to compute H-bonded intermolecular interactions using Gaussian 09W26-29 and to optimize the geometry of DMA and DMA in relation to water. The outcomes were visualized using GaussView 5.0 software30.
The Natural Bond Orbital (NBO) has been utilized to quantify hyperconjugation or intermolecular delocalization31. The intermolecular delocalization of the DMA + water binary mixture has been studied by means of second-order perturbation theory using the Fock matrix in the NBO basis. The structure of DMA + water is given in Fig.1
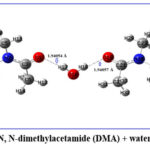 |
Figure 1: N, N-dimethylacetamide (DMA) + water structure. |
Experimental Details
Apparatus and Procedure
Ultrasonic velocity has been determined using the interferometric technique. The ultrasonic velocities were reproducible within an error margin of ±0.08%.
A pycnometer with a single capillary calibration has been used to measure densities. The graduation on the pcynometer stem was 0.01 ml. The reproducibility of the densities was within ±0.01%.
The LVDVII+Pro Brookfield viscometer has been used to measure viscosity. Brookfield Rheocalc 32 Software allows for total PC control over the instrument. The equipment has a repeatability of ±0.2% and an accuracy of ±1.0% of the full-scale range.
An accurate refractive index of ±0.001 unit was obtained with a thermostated Abbe’s refractometer, which had a range of 1.300 to 1.700 for the liquid mixtures.
Results and Discussion
Thermo dynamic parameters
Table 1 presents the experimentally measured values for DMA+ water solution with mole fraction (x1) at varying temperatures.
Table 1: Experimentally measured parameters for the system DMA + water with x1 of DMA at varying temperatures.
|
x1 |
u (ms-1) |
ρx 10-3(kg m-3) |
η(cp) |
n |
|
293.15K |
||||
|
0.0000 0.0999 0.1998 0.2999 0.3998 0.5000 0.6975 0.7949 0.9000 1.0000 |
1483.0 1768.5 1741.1 1722.8 1664.9 1639.0 1550.1 1523.8 1498.5 1472.0 |
0.9982 1.0000 0.9994 0.9961 0.9865 0.9739 0.9597 0.9533 0.9464 0.9398 |
1.002 2.930 4.634 4.918 4.072 3.340 1.840 1.475 1.219 1.012 |
1.333 1.379 1.405 1.419 1.427 1.431 1.435 1.436 1.436 1.437 |
|
303.15K |
||||
|
0.0000 0.0999 0.1998 0.2999 0.3998 0.5000 0.6975 0.7949 0.9000 1.0000 |
1518.0 1718.6 1702.8 1690.5 1639.7 1601.3 1521.9 1481.1 1459.0 1432.0 |
0.9956 0.9932 0.9924 0.9866 0.9771 0.9686 0.9511 0.9438 0.9374 0.9315 |
0.797 2.089 3.138 3.356 2.871 2.295 1.470 1.220 1.034 0.871 |
1.332 1.377 1.401 1.415 1.422 1.427 1.430 1.431 1.431 1.432 |
|
313.15K |
||||
|
0.0000 0.0999 0.1998 0.2999 0.3998 0.5000 0.6975 0.7949 0.9000 1.0000 |
1529.0 1689.7 1676.0 1658.9 1600.9 1555.2 1481.2 1448.9 1417.3 1402.0 |
0.9922 0.9866 0.9851 0.9776 0.9680 0.9598 0.9420 0.9352 0.9284 0.9234 |
0.653 1.615 2.342 2.474 2.229 1.839 1.259 1.067 0.924 0.769 |
1.331 1.374 1.398 1.411 1.418 1.422 1.425 1.426 1.426 1.427 |
For calculating isoentropic compressibility (ks), specific heat ratio (γ) and effective Debye temperature (ϴD),following relations are used,
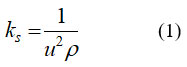
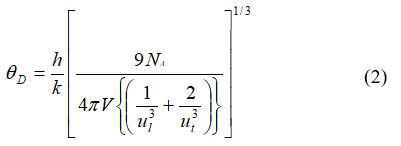

where γ, βT and GT are specific heat ratio, isothermal compressibility and modulus of rigidity respectively.
It can be seen from Table 1 that at all three temperatures, ultrasonic velocity (u ) attains a maxima at .0999 mole fraction. As the concentration increases we see the ultrasonic velocity decreases. In liquid mixtures, the strength of the molecular interaction become maximum at the concentration where the velocity maxima take place. These observations agree with the earlier reported data 11.
The isoentropic compressibility (ks) reaches a least amount and then again increases with an amplify in concentration and have an opposite relationship with the velocity. The mixture under study exhibits compressibility minima in the same concentration range as the velocity maxima, as would be predicted (fig 2a). At or very close to the concentrations where complex formation takes place, a decrease in ks and an increase in u are the results of the hydrogen bond formation strengthening the inter-molecular forces. Similar variations in ultrasonic velocity and isoentropic compressibility have also been reported earlier7.
The derived parameters, viz.,effective Debye temperature (ϴD), and specific heat ratio (γ) provide inclusive information about the interactional and structural characteristics of the mixture at the microscopic level. It can also be seen from figures 2b and 2c that the variation of effective Debye temperature (ϴD) and specific heat ratio (γ) with concentration is also non-linear, which further help the phenomenon of complex formation between dissimilar molecules 32,33.
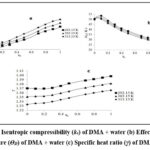 |
Figure 2: (a) Isentropic compressibility (ks) of DMA + water (b) Effective Debye’s temperature (ϴD) of DMA + water (c) Specific heat ratio (γ) of DMA + water |
Excess Parameters
Various forms of interactions between component molecules can account for the excess parameter values for binary mixtures. Non-specific van der Waal forces, dipole-dipole interactions, hydrogen bonding, donor-acceptor interactions between unlike molecules, and smaller molecules occupying the gaps left by larger molecules are a few examples of these interactions. The sign and magnitude of excess parameters are very important for figuring out how molecules in a liquid mixture interact with each other and cause molecules to move around.
Figs. 3a and 3b illustrate the deviation in ultrasonic velocity (Δu) and excess acoustic impedance (ZE) for the system of DMA with respect to the mole fraction at varying temperatures. Analyzing the curves, it can be observed that both Δu and ZE have positive values. The strong specific interactions (hydrogen bonding between DMA and water) is present and the filling of smaller water molecules into the voids created by larger DMA molecues are likely responsible for the positive values of Δu and ZE (figs. 3a and 3b). Fort and Moore 34, as well as many other researchers 16,17,35, contend that positive values of Δu and ZE signify the presence of strong intermolecular interaction between different molecules. The high positive values like Δu and Z E indicates the DMA is interactive with water.
 |
Figure 3: (a) Deviation in ultrasonic velocity (Δu) of DMA+water (b) Excess acoustic impedance (ZE) of DMA+water (c) Excess intermolecular free length (LfE) of DMA+water. |
Figs 3c, 3d, and 3e demonstrate that the values of excess parameters like Lf, VmE and ΔRm are all negative at all three temperatures. The hydrogen bonds with water is thought to be the reason behind DMA’s ability to take protons. The molecules in the mixture varying sizes could possibly be the cause of the reported negative values. The molar volumes of DMA and water at 298.15 K are 92.9 cc /mol and 18.06 cc/mol respectively. The larger difference in molar volume between DMA and water would result in contraction in volume due to the component molecules need to fit with other structures. The difference in component molecules sizes was also taken into account by Ali and Nain36 and Pikkarainen 10 to explain the negative values of VmE in binary mixtures of DMA + ethanol and DMA + methanol/ethanol/propanol, respectively.
The development of strong hydrogen bonds between DMA and water molecules is further supported by the positive values of deviation in viscosity (∆η) and excess free energy of activation for viscous flow (∆G*E) for the DMA + water system (figs 3f and 3g). The values of ∆η and ∆G*E extrema decreasewith elevation in temperature, indicating that the interaction between molecules is temperature sensitive and weakens as temperature rises. Similar patterns in ∆η and ∆G*E have also been observed in the cases of DMA + methanol 5,10 and water + sulfonane37. Table 2 provides the standard deviation (σ(YE)) and coefficient ai as determined by the curve expert 1.3 software.
Table 2: ai for excess parameters and their σ(YE) for binary system at three temperatures
|
Functions |
a1 |
a2 |
a3 |
a4 |
a5 |
σ(YE) |
|||
|
DMA + Water |
|||||||||
|
T=293.15 K |
|||||||||
|
VmEx106 (m3mol-1) |
0.0439 |
-8.5174 |
13.8936 |
-7.0029 |
1.5601 |
0.0961 |
|||
|
Δη(cp) |
-0.1577 |
33.6014 |
-93.8688 |
87.5647 |
-27.0823 |
0.2677 |
|||
|
ΔRm |
-0.1243 |
-66.2275 |
179.5525 |
-183.0507 |
69.9332 |
0.2055 |
|||
|
ΔG*E(kJmol-1) |
0.0263 |
38.9811 |
-107.9595 |
106.4839 |
-37.5455 |
0.0719 |
|||
|
Δu(ms-1) |
21.8986 |
3044.8484 |
-11094.579 |
13804.9984 |
-5786.9105 |
36.0507 |
|||
|
ZE(kgm-2s-1) |
0.1963 |
33.4995 |
-122.1126 |
151.5864 |
-63.2677 |
0.3212 |
|||
|
LfE(A0) |
-0.0049 |
-0.7438 |
2.6412 |
-3.2391 |
1.3488 |
0.0081 |
|||
|
T=303.15 K |
|||||||||
|
VmEx106 (m3mol-1) |
0.0257 |
-6.9623 |
6.1435 |
5.2908 |
-4.5181 |
0.0450 |
|||
|
Δη(cp) |
-0.0967 |
21.9662 |
-62.3313 |
60.0138 |
-19.5212 |
0.1635 |
|||
|
ΔRm |
-1.2300 |
-65.3446 |
175.8656 |
-177.8814 |
67.5653 |
0.2053 |
|||
|
ΔG*E(kJmol-1) |
0.0158 |
35.8636 |
-101.4766 |
103.3124 |
-37.7320 |
0.0336 |
|||
|
Δu(ms-1) |
13.8508 |
2290.4042 |
-8014.7883 |
9629.9490 |
-3925.4604 |
23.0465 |
|||
|
ZE(kgm-2s-1) |
0.1267 |
24.1115 |
-83.9868 |
99.9696 |
-40.2773 |
0.2125 |
|||
|
LfE(A0) |
-0.0033 |
-0.5731 |
1.9308 |
-2.2627 |
0.9096 |
0.0054 |
|||
|
T=313.15 K |
|||||||||
|
VmEx106 (m3mol-1) |
0.0229 |
-6.5455 |
4.9527 |
7.0597 |
-5.4994 |
0.0432 |
|||
|
Δη(cp) |
-0.0554 |
15.3968 |
-42.9074 |
40.9669 |
-13.3799 |
0.0911 |
|||
|
ΔRm |
-0.1325 |
-66.2452 |
179.3324 |
-182.2330 |
69.3677 |
0.2213 |
|||
|
ΔG*E(kJmol-1) |
0.0244 |
33.3978 |
-94.6918 |
97.7223 |
-36.4664 |
0.0421 |
|||
|
Δu(m.s-1) |
10.1288 |
1994.9188 |
-7123.6009 |
8604.9702 |
-3493.1798 |
17.4459 |
|||
|
ZE (kgm-2s-1) |
0.0887 |
20.5102 |
-73.1977 |
87.7487 |
-35.2135 |
0.1553 |
|||
|
LfE(A0) |
-0.0024 |
-0.5116 |
1.7424 |
-2.0321 |
0.8054 |
0.0042 |
|||
The values of the APD (Average percentage deviation) of ultrasonic velocity (u) anticipated by different semi-empirical rules are shown in Table 3. It shows that the CFT relation gives the minimum APD and Vandeal’s relation gives the maximum APD for this system. However, the APD values predicted by Nomoto, Jungie, and FST are nearly equal.
Table 3: APD of several theories utilized for determination of u.
|
Temperatures |
CFT |
FST |
Nomoto |
Vandeal |
Junjie |
|
DMA+Water |
|||||
|
293.15K |
5.8192 |
9.5936 |
7.7528 |
21.1827 |
7.7760 |
|
303.15K |
4.3209 |
7.3986 |
7.2443 |
19.1958 |
7.3438 |
|
313.15K |
3.1629 |
5.1687 |
6.6258 |
17.8053 |
6.7998 |
Theoretical study
Natural bond orbital (NBO) study
NBO examination relies on technique that provide a collection of algorithms that make it possible to extract the basic bonding concept through DFT calculations31. NBO study is an crucial tool for evaluating the molecular interaction in molecules, in addition to given that a useful establishment for analyzing conjugative interaction or charge transfer in molecular systems. Strong electrostatic hyperconjugative interactions are revealed by the NBO study38. The NBO study of DMA has been already mentioned in published work24 and for DMA + water the interaction energy of LP1(N2) with anti-bonding π*(O1-C3) increases to 67.47 kcal/mol showing higher stabilization of the system. The interaction of LP(2) of O1 and O19 with s*(O16-H17) and s*(O16-H18) respectively results in similar stabilization energy of 5.45 kcal/mol.
DFT investigation of H-bonded intermolecular interaction
To compute the intermolecular hydrogen bonding strength using Bader’s Atoms in Molecules (AIM) hypothesis39 has been mentioned in published work24. In this work to determined the hydrogen bond strength for the DMA and water binary mixture. It was computed to be 5.79825 kcal/moland 5.78869 kcal/mol at B3LYP corresponding to two hydrogen bonds formed between water and DMA molecules. Table 4 lists the topological parameters.
Table 4: Topological parameters for DMA+water
|
Parameters |
O19—H18 |
O1—H17 |
|
Electron density (ρBCP) a.u. |
0.02481 |
0.02481 |
|
Laplacian of electron density a.u. |
0.09056 |
0.09507 |
|
Lagrangian kinetic energy G(r) a.u. |
0.02055 |
0.02055 |
|
Hamiltonian kinetic energy K(r) a.u. |
-0.00209 |
-0.00209 |
|
Potential energy density V(r) a.u. |
-0.01846 |
-0.01845 |
|
Eigen value λ1 |
-0.01846 |
-0.01845 |
|
Eigen value λ2 |
-0.03252 |
-0.03252 |
|
Eigen value λ3 |
0.15679 |
0.15680 |
|
H-Bond energy (kcal/mol) |
5.79825 |
5.78869 |
To depict the distribution of electron densities, electron localised field (ELF) graph has been constructed, as shown in fig. 4. The overlapping of the blue zone in Fig. 4 depicts interaction with the oxygen atom of DMA molecules and the H atoms of water molecules in the binary mixture. The figure provides sufficient theoretical support for such intermolecular bonds between DMA and water.
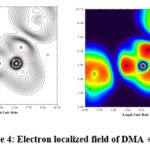 |
Figure 4: Electron localized field of DMA + water. |
HOMO and LUMO (fig 5) plots have been drawn using the optimized structure. HOMO has been distributed over water and DMA molecules, while LUMO is confined to DMA molecules.
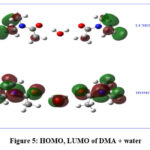 |
Figure 5: HOMO, LUMO of DMA + water. |
The total density of states (TDOS) and partial density of states (PDOS) plots seen in Fig. 6 were plotted using the Gauss-Sum 2.2 Program40. Although the PDOS plot shows a group’s relative contributions towards each molecular orbital, the TDOS plot showing population assessment per orbital provides the allocation of molecular orbitals under a certain spectral region.
 |
Figure 6: TDOS and PDOS plotsof DMA + water. |
Conclusions
The excess parameters were calculated from experimentally measured u, ρ, η and n at varying temperatures. The presence of strong interactions in DMA + water systems is indicated by the observed negative values of LfE, VmE, ΔRm and positive values of ∆u, ZE, ∆η and ΔG*E . The applicability of semi-empirical relations and theories is demonstrated by comparing the average percentage deviation of estimated and experimental values of ultrasonic velocity. Excellent agreement between experimental and theoretical values can be seen in the root mean square deviations for density prediction derived from the density models. The DFT/B3LYP method’s computed data was used to confirm all of the experimental results.
Acknowledgment
One of the authors (Laxmi Kumari) gratefully acknowledges to the UGC, New Delhi and Government of India for providing financial support under BSR fellowship (F-25-1/2014-15 (BSR)/7-177/2007/(BSR) dated 7th October, 2015 during the research.
Conflicts of Interests
The authors declare no competing interests.
References
- Kumari, L.; Gupta, S.; Singh, I.; Prasad, O.; Sinha, L.; Gupta, M.; Thermodynamic, spectroscopic and DFT studies of binary mixtures of poly(vinylpyrrolidone) (PVP) with ethanol, 1-propanol and 1-butanol. J. Mol. Liq. 2020, 299, 1-13.
CrossRef - Yasmin, M.; Gupta, M.; Shukla, J. P.; Acoustical study of molecular interactions in polymer solutions through various thermodynamical parameters and flory’s theory at 298.15 K. Phy. Chem. Liq. 2010, 48, 682-697.
CrossRef - Nain, A. K.; Densities, ultrasonic speeds, viscosities and excess properties of binary mixtures of methyl methacrylate with N, N–dimethylformamide and N, N–dimethylacetamide at different temperatures. J. Chem. Thermodyn. 2013, 60, 105-116.
CrossRef - Kumari, L.; Sinha, L.; Prasad, O.; Gupta, M.; Study of molecular association in binary mixtures of poly(vinyl pyrrolidone) (PVP) with ethanol, 1-propanol and 1-butanol through thermo-acoustical, FT-IR, UV–Vis spectroscopy and DFT studies. Eur. Phys. J. D. 2021, 75, 1-11.
CrossRef - Mrad, S.; Lafuente, C.; Hichri, M.; Khattech, I.; Density, Speed of sound, refractive index, and viscosity of the binary mixtures of N, N‑dimethylacetamide with methanol and ethanol. J. Chem. Eng. Data 2016, 61, 2946−2953.
CrossRef - Vibhu, I.; Singh, A. K.; Gupta, M.; Shukla, J. P.; Ultrasonic and IR investigation of N–H-N complexes in ternary mixtures. J. Mol. Liq. 2004, 115, 1-3.
CrossRef - Gupta, M.; Shukla J. P.; Ultrasonic investigation of molecular association in binary mixtures-Dioxan+formic acid, salicylic acid and benzoic acid. Indian J. Pure Appl. Phys. 1996, 34, 769-772.
- Singh, S.; Vibhu, I.; Gupta, M.; Shukla, J. P.; Excess acoustical and volumetric properties and the theoretical estimation of the excess thermodynamic functions of binary liquid mixtures. Chin. J. Phys. 2007, 45, 412-424.
- Marcus, Y.; Introduction to liquid state chemistry. Wiley Interscience 1977.
- Pikkarainen, L.; Densities and viscosities of binary mixtures of N, N–Dimethylacetamide with aliphatic alcohols. J. Chem. Eng. Data 1903, 28, 344-347.
CrossRef - Kazimierczuk, M. J.; Szydlowski, J.; Physicochemical properties of solutions of amides in H2O and in D2O. J. Solut. Chem. 2001, 30, 623-640.
CrossRef - Shukla, D.; Singh, S.; Parveen, S.; Gupta, M.; Shukla, J. P.; A corresponding state model for the prediction of the viscosity of pure liquids and liquid mixtures. J. Mol. Liq. 2007, 136, 111-116.
CrossRef - Rastogi, M.; Awasthi, A.; Gupta, M.; Shukla, J. P.; Molecular association of aliphatic ketones and phenol in a nonpolar solvent-ultrasonic and IR study. J. Mol. Liq. 2003, 107, 185-204.
CrossRef - Das, D.; Messaâdi, A.; Barhoumi, Z.; Ouerfelli, N.; The Relative reduced redlich-kister equations for correlating excess properties of N, N-dimethylacetamide+water binary mixtures at temperatures from 298.15 K to 318.15 K. J. Sol. Chem. 2012, 41, 1555-1574.
CrossRef - Villca, G.; Justel, F. J.; Jimenez, Y. P.; Water activity, density, sound velocity, refractive index and viscosity of the {(NH4)6Mo7O24 + poly(ethylene glycol) + H2O} system in the temperature range from 313.15 to 333.15 K: Experiment and modeling. J. Chem. Thermodyn. 2020, 142, 1-11.
CrossRef - Singh, K. P.; Agarwal, H.; Shukla, V. K.; Vibhu, I.; Gupta, M.; Shukla, J. P.; Ultrasonic velocities, densities, and refractive indices of binary mixtures of polyethylene glycol 250 dimethyl ether with 1-propanol and with 1-butanol. J. Solut. Chem. 2010, 39, 1749-1762.
CrossRef - Yasmin, M.; Gupta, M.; Thermodynamical study of alcoholic solutions of Poly (ethylene glycol) Diacrylate and Poly (ethylene glycol) Dimethacrylate. Int. J. Thermodyn. 2012, 15, 111-117.
CrossRef - Shukla, D.; Singh, S.; Parveen, S.; Gupta, M.; Shukla, J. P.; Acoustical properties of binary mixtures of heptane with ethyl acetate or butyl. Chin. J. Phys. 2010, 28, 371-377.
CrossRef - Mundhwa, K. M.; Alam, R.; Henni, A.; Volumetric properties, viscosities, and refractive indices for aqueous 2-((2-Aminoethyl)amino) ethanol solutions from (298.15 to 343.15). J. Chem. Eng. Data 2006, 51, 1268-1273.
CrossRef - Sivakami, K. U.; Vaideeswaran, S.; Rosevenis, A.; Thermodynamic properties and interaction abilities of ternary liquid mixtures at 308.15 K. J. Environ. Nanotech. 2018, 7, 22-33.
- Singh, I.; El-Emam, A. A.; Pathak, S. K.; Srivastava, R.; Shukla, V. K.; Prasad, O.; Sinha, L.; Experimental and theoretical DFT (B3LYP, X3LYP, CAM-B3LYP and M06-2X) study on electronic structure, spectral features, hydrogen bonding and solvent effects of 4-methylthiadiazole-5-carboxylic acid. Mol. Simul. 2019, 45, 1029-1043.
CrossRef - Sinha, L.; Prasad, O.; Chand, S.; Sachan, A. K.; Pathak, S. K.; Shukla, V. K.; Karabacak, M.; Asiri, A. M.; FT-IR, FT-Raman and UV spectroscopic investigation, electronic properties, electric moments, and NBO analysis of anethole using quantum chemical calculations. Spectrochim. Acta A, 2014, 133, 165-177.
CrossRef - Kumari, L.; Kumar, U.; Sinha L.; Prasad, O.; Yadav, B. C.; Gupta, M.; Surface modifcation and characterization of h‑BN‑doped PVP thin flm and its application as humidity sensor with theoretical DFT calculations. Chem. Pap. 2021, 75, 4055-4068.
CrossRef - Agarwal, H.; Kumari, L.; Sinha, L.; Prasad, O.; Gupta, M.; Thermoacoustic and DFT Analysis of N, N‑Dimethylacetamide (DMA) with 1‑propanol and methanol at 293.15, 303.15, and 313.15 K. Braz. J. Phys. 2021, 51, 515-526.
CrossRef - Kohn, W.; Sham, L. J.; Self-consistent equations including exchange and correlation effects Phys. Rev. 1965, 140, A1133-A1138.
CrossRef - Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G. A.; et. al. Gaussian Inc. Wallingford CT. 2009.
- Becke, A. D.; Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648-5652.
CrossRef - Lee, C.; Yang, W.; Parr, R. G.; Development of the colle-salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B, 1988, 37, 785-789.
CrossRef - Miehlich, B.; Savin, A.; Stoll, H.; Preuss, H.; Results obtained with the correlation energy density functionals of becke and Lee, Yang and Parr. Chem. Phys. Lett. 1988, 157, 200–206.
CrossRef - Frisch, E.; Hratchian, H. P.; Dennington, R. D.; II, et al. Gaussian Inc., GaussView Version 5.0.8. Sci. 2009, 7, 1505-1517.
- Glendening, E. D.; Landis, C. R.; Weinhold, F.; Natural bond orbital methods, WIREs Comput. Mol. Sci. 2012, 2, 1-42.
CrossRef - Parveen, S.; Yasmin, M.; Gupta, M.; Shukla, J. P.; Thermoacoustical and excess properties of binary mixtures of ethyl butyrate with methanol and vinyl acetate. Int. J. Thermodyn. 2010, 13, 59-66.
- Parveen, S.; Singh, S.; Shukla, D.; Yasmin, M.; Gupta, M.; Shukla, J. P.; Study of molecular interactions in binary mixtures of aniline with carboxylic acids at 293.15 K, 303.15 K and 313.15 K. J. Solution Chem. 2012, 41, 156-172.
CrossRef - Fort, R. J.; Moore, W. R.; Adiabatic compressibilities of binary liquid mixtures. J. Chem. Soc. Faraday Trans. 1965, 61, 2102-2111.
CrossRef - Kavitha, R.; Jayakumar, S.; Uma, R.; Ultrasonic velocity, density and viscosity of binary liquid mixtures of acetone with toluene, chlorobenzene and nitrobenzene. Int. J. Chem. Applicat. 2011, 3, 19-33.
- Ali, A.; Nain, A. K.; Interactions in binary N, N-dimethylacetamide + ethanol and ternary lithium nitrate + N, N dimethylacetamide + ethanol mixtures. Phys. Chem. Liq. 1999, 37, 161-174.
CrossRef - Aminabhavi, T. M.; Gopalakrishna, B.; Density, viscosity, refractive index, and speed of sound in aqueous mixtures of NJV-dimethylformamide, dimethyl sulfoxide, NJV-dimethylacetamide, acetonitrile, ethylene glycol, diethylene glycol, 1,4-dioxane, tetrahydrofuran, 2-methoxyethanol, and 2-ethoxyethanol at 298.15 K. J. Chem. Eng. Data 1995, 40, 856-861.
CrossRef - Chand, S.; Fatmah, A. M.; Omary, A.; Emam, A. A. K.; Shukla, V. K.; Prasad, O.; Sinha, L.; Study on molecular structure, spectroscopic behavior, NBO, and NLO analysis of 3-methylbezothiazole-2-thione. Spectrochim. Acta A Mol. Biomol. Spectrosc., 2015, 146, 129–141.
CrossRef - Bader, R. F. W.; A quantum theory of molecular structure and its applications. Chem. Rev. 1991, 91, 893–928.
CrossRef - Boyle, N. M. O.; Tenderholt, A. L.; Langer, K. M.; Cclib: A library for package-independent computational chemistry algorithms. J. Comput. Chem. 2008, 29, 839– 845.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









