Synthesis, Characterization, and In Silico Assessment of Novel Pyrazole Carbaldehyde for Antidepressant Activity
1Department of Chemistry H.N.G.U., Patan Gujarat India.
2Department of Chemistry The HNSB. Ltd. Science College, Himatnagar, India.
Corresponding Author E-mail: arunmalviya145@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/400132
Article Received on : 28 Oct 2023
Article Accepted on : 02 Jan 2024
Article Published : 16 Jan 2024
Reviewed by: Dr. Kiritkumar Damor
Second Review by: Dr. G. D Acharya
Final Approval by: Dr. Naresh Batham
This research delves into the uncharted territory of pyrazole derivatives as potential antidepressants, despite their versatile biological activities. The study primarily focuses on a novel antidepressant designed as a selective serotonin reuptake inhibitor (SSRI) and involves the synthesis of six new pyrazole derivatives through a conventional heating method. These compounds were then subjected to pharmacokinetic prediction and molecular docking studies at the active site of the human serotonin transporter protein enzyme (PDB ID: 5I73) using AutoDock Vina 1.2.3. SwissADME software was utilized to forecast pharmacokinetics, while PreADMET software assessed toxicity. The findings suggest that these derivatives exhibit promising antidepressant properties in comparison to established drugs. The convergence of docking, SwissADME, and toxicity results implies potential avenues for the development of effective antidepressants based on pyrazole derivatives, thereby shedding light on a novel class of compounds with potential applications in mental health treatment.
KEYWORDS:Carbaldehydes; In silico activity; Pre ADMET; Pyrazole; Swiss ADME
Download this article as:| Copy the following to cite this article: Malaviya A. R, Gadhawala Z. H, Panchal V. Synthesis, Characterization, and In Silico Assessment of Novel Pyrazole Carbaldehyde for Antidepressant Activity. Orient J Chem 2024;40(1). |
| Copy the following to cite this URL: Malaviya A. R, Gadhawala Z. H, Panchal V. Synthesis, Characterization, and In Silico Assessment of Novel Pyrazole Carbaldehyde for Antidepressant Activity. Orient J Chem 2024;40(1). Available from: https://bit.ly/4b13bA7 |
Introduction
Depression is a widespread, chronic condition that often resists drug treatments, impacting people’s quality of life and productivity, The World Health Organization reported a significant 18.4% increase in the global prevalence of depression from 2005 to 2015, reaching 322 million individuals by 2017, Studies in the Middle East and North Africa have shown depression rates between 13% to 18%, with a higher incidence in women1. The COVID-19 pandemic has exacerbated psychological issues, including depression, stress, and emotional distress, The causes of depressive disorders are multifaceted, involving changes in brain neurotransmitters, especially norepinephrine, serotonin, and dopamine2,3. A decrease in serotonin levels4,5 is considered a crucial factor in depression’s onset. Antidepressants, like SSRIs, aim to increase serotonin availability, with noradrenergic and dopaminergic systems also playing a role.6–8 Despite adequate treatment options, social stigma remains a significant barrier to seeking and continuing treatment for depression and anxiety disorders, with individuals often perceiving the stigma as worse than their mental health condition. Research has shown this to be the case.9 SSRIs are a commonly prescribed medication class utilized for managing mood disorders such as depression and cardiovascular conditions,the rising prevalence of depression has prompted the pharmaceutical industry to develop various drugs for its treatment, currently, the primary approach to address depression involves inhibiting the binding of serotonin to the enzyme, This mechanism is commonly employed to target and manage symptoms of depression10. The serotonin transporter serves as a crucial mechanism for the reuptake of extracellular serotonin, effectively terminating neurotransmission by removing serotonin from the synaptic cleft10 neuropsychiatric disorders, including anxiety and depression, are often associated with a deficiency of extracellular serotonin. The insufficient levels of serotonin in the brain are believed to contribute to the development and manifestation of these disorders.Several SSRI drugs, including citalopram, escitalopram, fluoxetine, fluvoxamine, and paroxetine, have demonstrated success in the treatment of both anxiety and depression.11,12 These medications effectively modulate serotonin levels by inhibiting its reuptake, leading to improved symptoms and overall relief for individuals experiencing anxiety and depression. These drugs, including fluoxetine and paroxetine, exert their therapeutic effects by binding to the serotonin transporter and preventing the binding of serotonin.12,13 However, it is important to note that while these drugs have shown effectiveness, they also possess certain pharmacokinetic and pharmacodynamic properties that can have adverse effects on the body.12,14 Furthermore, common side effects associated with the mentioned drugs include headaches, nausea, and constipation.15 Due to the observed negative effects and limitations of current drugs, there is a need for the development of new and improved designs with the potential to minimize side effects. Research and advancements in pharmacology aim to address these challenges by formulating medications that can provide therapeutic benefits while reducing adverse reactions. The goal is to optimize drug efficacy and safety profiles, offering better treatment options for individuals suffering from anxiety and depression.16–18 With the recognition of the importance of aromatic carbaldehyes, the objective of the present study is to create new substituted pyrazole-based carbaldehyes as inhibitors of Serotonin Computational investigations were carried out to assess the binding affinity of the synthesized compounds towards the target protein with Crystal structure of receptor (PDBID -4IAR)19.
Experimental
Every chemical used in the investigation was purchased from for-profit vendors, such as Sigma-Aldrich and Merck. These chemicals were employed as received without undergoing additional purification steps. Solvents such as methanol, ethanol, dimethylformamide (DMF), n-hexane, and ethyl acetate were utilized in the study. The Preparation involving 4-Chlorophenylhydrazine, 4-Methylphenylhydrazine, 2,5 Di methylphenylhydrazine, 4-Hydroxyacetophenone, 4-Aminoacetophenone and phosphorousoxichloride. FT-IR spectra were collected using a Perkin Elmer Spectrum BXw FT-IR Spectrometer, which covered the range of 400-4000 cm-1. An Agilent 400 MR apparatus operating at 400 MHz for 1H NMR and 100 MHz for 13C NMR was used to acquire NMR spectra. The internal standard was TMS, and the solvent was DMSO-D6-. Mass spectra were recorded using a Waters LC-MS 8040 Model Spectrometer. TLC plates, specifically Merck Silica Gel 60 F254 plates, were employed to monitor the progress of reactions and assess the purity of the products.
General Method for the preparation of Hydrazone (A-1 to A-6)
To initiate the reaction, a mixture comprising 10 mmol of different substituted acetophenones, substituted phenylhydrazine, and 1 ml of glacial acetic acid as a catalyst was dissolved in 20 mL of methanol. The solution was then stirred for 4-5 hours at a temperature of 60°C, allowing the reaction to proceed. To determine the reaction’s completion, TLC was conducted, providing visual confirmation. Once the reaction was confirmed to be complete after adding the reaction mixture to the ice, causing the formation of a precipitate. The resulting hydrazone intermediates, denoted as A-1to A-6, were separated from the mixture through filtration. The filtrate was then washed with water to remove any impurities present. Immediates are transfer to next step without further recrystallization.20–22
General method for the preparation of carbaldehydes (SSRI) containing pyrazole (B-1 to B-6)
To perform the next step, a hydrazone compound (A-1 to A-6, 4 mmol) was put into the reagent Vilsmeier-Haack. The Vilsmeier-Haack reagent was prepared by slowly adding 3 mL of POCl3 to 15 mL of DMF (dimethylformamide) that was chilled on ice. The resulting mixture was stirred for 4-6 hours at a temperature of 70°C, allowing the reaction to take place. To confirm the completion of the reaction, TLC was conducted. Once the reaction was verified to be complete, the mixture was poured into crushed ice to facilitate cooling and precipitation of the reaction products. To neutralize the mixture, a solution of bicarbonate was added. This helped achieve a neutral pH and stabilize the reaction mixture. The crude product was then isolated from the mixture, possibly through filtration or other appropriate separation techniques. To further purify the product, it was subjected to crystallization from ethanol.
1-(4-chlorophenyl)-3-(4-hydroxyphenyl)-1H-pyrazole-4-carbaldehyde (B1)
Yield: 72%, Orange, mp 189-193ºC; Rf :0.37 (Ethyl Acetate:n-Hexane 1:4 ); IR(KBr,cm-1): 3308 (OH), 1678 (-C=O), 1598 (C=N); 1H NMR(DMSO-D6,ppm) δ: 9.95(1H, s), 9.83 (1H, s), 9.30 (1H, s); 8.00-8.09 (2H, d), 7.67-7.77 (2H, d) 7.53-7.62 (2H, d) 6.88-6.90 (2H, d) 13C(DMSO-D6,ppm) δ: 184.4, 158.5, 192.9, 137.3, 134.64, 131.61, 130.01, 129.53, 129.43, 129.33, 121.95, 121.75, 120.58, 115.28; EI-MS: m/z [M+H]+ 298.0 for C16H11ClN2O2
3-(4-aminophenyl)-1-(4-chlorophenyl)-1H-pyrazole-4-carbaldehyde(B2)
Yield: 63 %; Orange, mp 203-207 ºC; Rf : 0.52 (Ethyl Acetate:n-Hexane 1:4 ); IR(KBr, cm-1): 3381 (-NH2), 1683 (C=O), 1596 (C=N); 1H NMR(DMSO-D6, ppm): δ 9.96 (1H, s), 9.81 (1H, s) 9.29(1H, s) 7.79-8.02(2H ,d) 7.52-7.67(2H, d) 7.42(2H, d) 7.29(1H, s) 6.88-6.90(2H, d); 13C NMR(DMSO-D6 , ppm) δ : 184.48, 158.48, 152.97, 137.32, 134.60, 131.60, 130.02, 129.42, 121.65, 121.76, 120.57, 115.27; MS: m/z [M+H]+ 298.05 for C16H12ClN3O
3-(4-hydroxyphenyl)-1-(4-methylphenyl)-1H-pyrazole-4-carbaldehyde (B3)
Yield: 59%; Buff White, mp 133-137ºC; Rf :0.45 (Ethyl Acetate:n-Hexane 1:4 ); IR(KBr, cm-1): 3334 (-OH), 1661 (C=O), 1588 (C=N); 1H NMR(DMSO-D6, ppm): δ 9.95 (1H, s), 9.78 ( 1H, s), 9.24 (1H, s), 7.75-7.82(4H, q) 7.41-7.45(1H, t)7.22-7.24(1H, d) 6.87-6.89(2H, d) 2.41(3H,s) ; 13C NMR(DMSO-D6,ppm) δ : 184.47, 158.37, 152.70, 139.22, 138.49, 134.40, 130.01, 129.31, 128.01, 122.00, 121.69, 119.47, 116.06, 115.24; EI-MS: m/z [M+H]+ 279.0 for C17H14N2O2
3-(4-aminophenyl)-1-(4-methylphenyl)-1H-pyrazole-4-carbaldehyde (B4)
Yield: 78 %; Buff White , mp 144-149ºC; Rf :0.30 (Ethyl Acetate:n-Hexane 1:4 ); IR(KBr, cm-1): 3413 (NH2), 1674 (C=O), 1596 (C=N); 2918 (-CH3); 1H NMR(DMSO-D6, ppm): δ 11.51(1H, s) 9.98 (1H, s),9.28-9.32(1H, s) 8.5(1H, s),7.90-8.00(2H, d)7.79(1H, s) 7.74(1H, d)7.45-7.48(3H, t)7.24-7.26(1H, d),2.42(3H, s); 13C(DMSO-D6,ppm) δ : 184.51, 162.39, 152.40, 151.36, 139.29, 138.43, 138.39, 135.49, 134.83, 129.67, 129.57, 128.26, 126.33, 121.97, 119.56, 117.01, 116.19 EI-MS: m/z [M-H]+ 277 for C17H15N3O
1-(2,5-dimethylphenyl)-3-(4-hydroxyphenyl)-1H-pyrazole-4-carbaldehyde(B5)
Yield: 62 %; Buff White , mp 201-206ºC; Rf :0.42 (Ethyl Acetate:n-Hexane 1:4 ); IR(KBr, cm-1): 3325 (-OH), 1684(C=O), 1593(C=N); 2923(-CH3); 1H NMR(DMSO-D6, ppm): δ 10.00 (1H, s), 8.19 (1H, s), 7.67(2H, d), 7.19-7.21(3H. q), 6.88-6.90(2H, d), 2.26-2.35(7H, d); 13C(DMSO-D6,ppm) δ : 185.95, 157.38, 154.57, 138.63, 137.13, 135.68, 131.59, 130.70, 130.45, 130.40, 126.73, 123.53, 121.35, 116.02, 77.68, 77.26, 76.83; EI-MS: m/z [M+H]+ 293.1 for C18H16N2O2
1-(4-chlorophenyl)-3-(3,4-dimethoxyphenyl)-1H-pyrazole-4-carbaldehyde (B6)
Yield: 69 %; Orange, mp 210-215ºC; Rf :0.28 (Ethyl Acetate:n-Hexane 1:4 ); IR(KBr, cm-1): 1103 (-OCH3), 1677(C=O), 1597 (C=N); 1H NMR(DMSO-D6, ppm): δ 9.98 (1H, s), 9.33(1H, s), 8.05(2H, d), 7.63-7.66(2H, d), 7.53-7.57(2H, t), 7.01(1H, d),3.69-3.84(6H, d); 13C(DMSO-D6,ppm) δ: 184.49, 152.60, 149.71, 148.49, 137.28, 153.05, 131.67, 129.44, 123.45, 122.14, 121.38, 120.67, 111.94, 111.46, 55.44, 55.41 EI-MS: m/z [M+H]+ 343.4 for C18H15ClN2O3
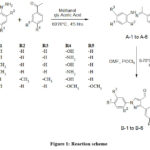 |
Figure 1: Reaction scheme |
Molecular docking methodology
Understanding how ligands and receptors interact can be accomplished with the help of molecular docking23,24. Auto dock vina 1.2.3 was used to carry out the molecular docking investigation25.The Protein Data Bank provided the crystal structure of the receptor (PDBID: 5I73).26 An in-depth comprehension of the numerous interactions among the ligand and the active site of the enzyme was obtained by a molecular docking investigation of the produced molecule. Using Chemdraw 11.0, the synthesized chemical-based was generated. The 2D structure was then converted into 3D structures with the help of Avogadro software27 and also energy of all 3D structures are minimized and was saved as PDB. In order to prepare the protein file, water molecules got rid of, polar hydrogens were included, and other linked ligands were eliminated. The site of binding used in the present investigation was chosen based on the amino acid residues found in the protein data bank that participate in binding with the receptor complexed with s-citalopram. This region is believed to be the the majority likely to be accurate since it has been solved by experimental crystallographic data. The Auto Dock VINA software was utilized to execute the docking technique for the synthesized chemical specified in Table III, adhering to standard operating procedures.28 The Auto Dock VINA software was utilized to execute the docking technique for the synthesized chemical specified in Table III, adhering to standard operating procedures.
Dynamical (ADME) prognosis and drug-likeness.
The online program SwissADME (http://www.swissadme.ch) was employed to ascertain the pharmacokinetic characteristics and drug-likeness of the suggested derivatives, The compounds were anticipated to be druglike using Lipinski’s rule of 5, The purpose of the guideline was to set the ground circumstances for the drug-likeness of unique molecular entities, Molecules meeting the following criteria: molecular weight more than 500; log P (iLog P) higher than Five; a hydrogen bond donors greater than 5; and H-bond acceptors bigger than Ten, Additional features include the number of rotatable bonds (nRotb), which was reported to have low absorption, and the topologically polar surface area (TPSA) for B5, which is 55.12 Å2 < 140 Å2. Molar refractivity (MR), log of skin permeability (log Kp), penetration of the blood-brain barrier (BBB), permeability the glycoprotein (Pgp) substrate, GI (gastrointestinal) absorption, and inhibitors of the human cytochrome the P450 enzyme (CYP450) enzymes CYP1A2, CYP2C9, and CYP2C19 are a few of the pharmacokinetic characteristics that need to be ascertained.29
Toxicity summary
Using the PreADMET scheme, the toxicity features of the two substances paroxetine and the created analogs were estimated in silico, In comparison to the trademarked medication, we chose compounds with toxicity profiles which were either superior or similar.30
Result and discussion
Chemistry
The title compounds were created using the process outlined in Scheme 1.. The compound A1 – A6 were obtained by condensation of Substituted Phenyl hydrazine and substituted Aromatic Ketone. Compound A1-A6 were further treated with Vilsmerheack reagent to afford compound B1-B6. All of the synthesized compounds’ formations were determined using spectrum that was information obtained from IR, 1H and 13C-NMR, and ESI-MS.
Docking Study
To comprehend the interactions between ligands and receptors, we conducted a detailed analysis involving the most potent compound (B2). This compound was strategically Docked within the active site of the receptor31 (PDBID -5I73).Notably, the benzene ring with primary amine group displayed a robust Pi-alkyl interaction with the amino acid PRO561. Additionally, a benzene ring bearing an alkyl substituent exhibited a pronounced pi-alkyl interaction with ALA331, a Pi-cation interaction with ARG104, and an interaction with ARG104 facilitated by a substituted alkyl group. Furthermore, another benzene ring engaged in dual potent pi-alkyl interactions, involving ALA331 and PRO561. The stability of this complex was further reinforced through notable interactions: a strong hydrogen bond formed between the primary amine group (-NH2) and SER555. The synthesized compounds showed promising docking results, with compound B2 exhibiting the most potent interactions within the active site. Notable interactions included Pi-alkyl interactions forming hydrogen bonds with important residues of amino acids. The docking results indicated that the synthesized compounds have potential antidepressant activity. The interactions observed between the ligands and the receptor provided insights into their binding mechanisms.
Table 1: Binding affinity of all synthesized molecules and control drug
|
No |
Sample code |
Binding Affinity (kcal/ mol) |
|
1 |
Citalopram |
-7.522 |
|
2 |
Fluoxetine |
-8.727 |
|
3 |
Fluvoxamine |
-7.613 |
|
4 |
Sertraline |
-9.320 |
|
5 |
B1 |
-9.187 |
|
6 |
B2 |
-9.232 |
|
7 |
B3 |
-9.355 |
|
8 |
B4 |
-9.436 |
|
9 |
B5 |
-9.448 |
|
10 |
B6 |
-8.750 |
 |
Figure 2: 2D interaction of compound B2 |
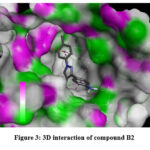 |
Figure 3: 3D interaction of compound B2. |
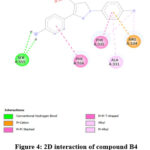 |
Figure 4: 2D interaction of compound B4. |
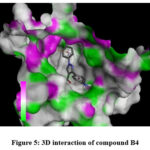 |
Figure 5: 3D interaction of compound B4. |
Swiss ADME
Each and every chemical is blood-brain barrier (BBB) permeable, that which needs to happen for antidepressant effectiveness to be proven. According to the preceding study, our substance B2 is not as hazardous based on all logP values, and the TPSA score of 55.12 Å2 suggests very little toxicity throughout the drug-design process. Every substance that are supportive of our molecule also has a Lipinski violation of zero. The chemical may be easily found in the body, as indicated by the bioavailability score of 0.55. Ultimately, the anticipated medical investigation makes it abundantly evident that the molecular structure itself exhibits lead similarity, a certain sign of drug-likeness.
Table 2: Predicated ADME parameters by swissADME web tool
|
Compound |
Parameters |
||||||||
|
nHBA (1) |
nHBD (2) |
TPSA (3) |
logP (4) |
p-gp S (5) |
LRO5 (6) |
GIA (7) |
BBBP (8) |
BAS (9) |
|
|
B1 |
3 |
1 |
55.52 |
2.38 |
No |
Yes |
High |
Yes |
0.55 |
|
B2 |
2 |
1 |
60.91 |
2.38 |
No |
Yes |
High |
Yes |
0.55 |
|
B3 |
3 |
1 |
55.12 |
2.12 |
No |
Yes |
High |
Yes |
0.55 |
|
B4 |
2 |
1 |
60.91 |
2.12 |
No |
Yes |
High |
Yes |
0.55 |
|
B5 |
3 |
1 |
55.12 |
2.35 |
No |
Yes |
High |
Yes |
0.55 |
|
B6 |
4 |
0 |
53.35 |
2.29 |
No |
Yes |
High |
Yes |
0.55 |
|
(1)No. of H Bond acceptor (2) No. of H bond donor (3) Topological polar surface area (4)calculated lipophilicity (5) p-gp substrate (6) Lipinski rule of 5 (7) Gastrointestinal absorption (8) Blood Brain barrier permeant (9) Bioavailability Score |
|||||||||
 |
Figure 6: Boil egg model. |
Toxicity profile
Parent compound was evaluated in PreADMET32 by examining two rodent carcinogenicity studies and an S. typhimurium genetic mutation test (Ames Test). Three of the candidates scored positive for at least one of the two cancer-causing capacity assays, based on the results (B3,B4 and B6) and four, including Fluoxetine, were negative on both (B1,B2 and B6). On the other hand, only three of the possibilities (B1, B2, and B6) showed a negative result from the cancer-causing the ability in mice, whereas fluoxetine showed a positive result. Based on these results, candidates B1, B2, as well as B6 shown a less hazardous toxicological profile than fluoxetine(Table 3).
Table 3: Paroxetine and several of its competitors’ in silico toxicity evaluation.
|
Compound |
TOXICITY |
||
|
Mice’s carcinogenicity |
Rats’ propensity for cancer |
Metric Ames |
|
|
Fluoxetine |
Positive |
Negative |
Mutagenic |
|
B1 |
Negative |
Negative |
Mutagenic |
|
B2 |
Negative |
Negative |
Mutagenic |
|
B6 |
Negative |
Negative |
Mutagenic |
Conclusion
Within this investigation, we synthesized and confirmed the structures of a series of six compounds labeled as B1-B6, featuring substituted carbaldehydes. This structural confirmation was achieved through a battery of analytical techniques, including IR, 1H and 13C-NMR and ESI-MS. With that, we used In Silico approaches to evaluate each of the generated molecules for probable antidepressant effectiveness. Furthermore, molecular docking was employed to analyze receptor interactions. Our findings revealed that carbaldehydes featuring phenyl and substituted phenyl rings exhibited varying degrees of antidepressant activity. Interestingly, the highest activity was observed with the compound containing a primary amine substituent (B2). Future investigations will delve into additional substitution on the benzyl ring and explore the impact of varying spacer/linker lengths. It is noteworthy that certain compounds within this study displayed superior antidepressant activity when compared to the standard drug fluoxetine.
Conflict of interest
There is no conflict of interest.
Acknowledgment
The PG Department of the HNSB Ltd. science college in Himmatnagar is acknowledged by the authors of this paper for giving all the tools needed to finish the research.
References
- Razzak, H. A.; Harbi, A.; Ahli, S. Depression: Prevalence and associated risk factors in the United Arab Emirates. Oman Med J 2019, 34, 274–283.
- Kim, G. W.; Kim, B. C.; Park, K. S.; Jeong, G. W. ;Sci Rep 2020, 10.
- Phillips, T. O.; Castro, M.; Vas, R. K.; Ferguson, L. A.; Harikumar, A.; Leal, S. L. ;Front Hum Neurosci 2023, 17.
- Anguelova, M.; Benkelfat, C.; Turecki, G. ;Mol Psychiatry 2003, 8, 646.
- Lewis, V.; Bonniwell, E. M.; Lanham, J. K.; Ghaffari, A.; Sheshbaradaran, H.; Cao, A. B.; Calkins, M. M.; Bautista-Carro, M. A.; Arsenault, E.; Telfer, A.; Taghavi-Abkuh, F. F.; Malcolm, N. J.; El Sayegh, F.; Abizaid, A.; Schmid, Y.; Morton, K.; Halberstadt, A. L.; Aguilar-Valles, A.; McCorvy, J. D. ;Cell Rep 2023, 42.
- Acquas, E.; Tanda, G.; Di Chiara, G. ;Neuropsychopharmacology 2002, 27, 182.
- Roodenrys, S.; Booth, D.; Bulzomi, S.; Phipps, A.; Micallef, C.; G.Dip.App.Psyc; Smoker, J. ;Neuropsychopharmacology 2002, 27, 279.
- de Almeida, R. M. M.; Miczek, K. A. ;Neuropsychopharmacology 2002, 27, 171.
- Alemayehu, Y.; Demilew, D.; Asfaw, G.; Asfaw, H.; Alemnew, N.; Tadesse, A. ;Psychiatry J 2020, 2020, 1.
- Coleman, J. A.; Green, E. M.; Gouaux, E. ;Nature 2016, 532, 334.
- Edinoff, A. N.; Akuly, H. A.; Hanna, T. A.; Ochoa, C. O.; Patti, S. J.; Ghaffar, Y. A.; Kaye, A. D.; Viswanath, O.; Urits, I.; Boyer, A. G.; Cornett, E. M.; Kaye, A. M. Selective serotonin reuptake inhibitors and adverse effects: A narrative review. Neurol Int 2021, 13, 387–401.
- Achyutuni, K. G.; Adhikari, S.; Huang, P.; Siegel, J. B. ;bioRxiv 2020, 2020.08.21.262006.
- Ng, C. W. M.; How, C. H.; Ng, Y. P. ;Singapore Med J 2017, 58, 459.
- Bergstrom, R. F.; Lemberger, L.; Farid, N. A.; Wolen, R. L. ;The British Journal of Psychiatry 1988, 153, 47.
- Kaye, C. M.; Haddock, R. E.; Langley, P. F.; Mellows, G.; Tasker, T. C. G.; Zussman, B. D.; Greb, W. H. ;Acta Psychiatr Scand 1989, 80, 60.
- Dechant, K. L.; Clissold, S. P. ;Drugs 1991, 41, 225.
- Chen, W.; Zhou, Y.; Chen, G.; Wu, Y.; Tu, B.; Liu, F.-Z.; Huang, L.; Ng, A. M. C.; Djurišić, A. B.; He, Z. ;Adv Energy Mater 2019, 9, 1803872.
- Syed, I.; Ahmad, I.; Niveditha, P. ;Int J Pharm Investig 2014, 4, 38.
- Yin, W.; Zhou, X. E.; Yang, D.; De Waal, P. W.; Wang, M.; Dai, A.; Cai, X.; Huang, C. Y.; Liu, P.; Wang, X.; Yin, Y.; Liu, B.; Zhou, Y.; Wang, J.; Liu, H.; Caffrey, M.; Melcher, K.; Xu, Y.; Wang, M. W.; Xu, H. E.; Jiang, Y. ;Cell Discov 2018, 4.
- Sivaramakarthikeyan, R.; Iniyaval, S.; Saravanan, V.; Lim, W. M.; Mai, C. W.; Ramalingan, C. ;ACS Omega 2020, 5, 10089.
- Arshad, M.; Khan, M. S.; Nami, S. A. A.; Ahmad, D. ;SN Appl Sci 2019, 1.
- Nandurkar, Y.; Bhoye, M. R.; Maliwal, D.; Pissurlenkar, R. R. S.; Chavan, A.; Katade, S.; Mhaske, P. C. ;Eur J Med Chem 2023, 258, 115548.
- Kanhed, A.; Zambre, V.; Pawar, V.; Sharma, M.; Giridhar, R.; Yadav, M. R. ;Medicinal Chemistry Research 2014, 23.
- Bhosale, S. M.; Suryawanshi, M.; Gaikwad, M.; Bhosale, P.; Kim, J. H.; Moholkar, A. ;Mater Lett 2014, 129, 153.
- Eberhardt, J.; Santos-Martins, D.; Tillack, A. F.; Forli, S. ;J Chem Inf Model 2021, 61, 3891.
- Berman, H. M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T. N.; Weissig, H.; Shindyalov, I. N.; Bourne, P. E. ;Nucleic Acids Res 2000, 28, 235.
- Hanwell, M. D.; Curtis, D. E.; Lonie, D. C.; Vandermeersch, T.; Zurek, E.; Hutchison, G. R. ;J Cheminform 2012, 4, 17.
- Berman, H. M.; Bhat, T. N.; Bourne, P. E.; Feng, Z.; Gilliland, G.; Weissig, H.; Westbrook, J. ;Nat Struct Biol 2000, 7, 957.
- Dubal, G. G.; Vachchharajani, P. R.; Solanki, M. J.; Shah, V. H. ;Russ J Gen Chem 2022, 92, 2161.
- Jaramillo, D. N.; Millán, D.; Guevara-Pulido, J. ;European Journal of Pharmaceutical Sciences 2023, 183, 106403.
- Senior, T.; Botha, M. J.; Kennedy, A. R.; Calvo-Castro, J. ;ACS Omega 2020, 5, 17223.
- Loiodice, S.; Nogueira da Costa, A.; Atienzar, F. ;Drug Chem Toxicol 2019, 42, 113.

This work is licensed under a Creative Commons Attribution 4.0 International License.










