Synthesis and Evaluation of Schiff’s Base Indole Derivatives Against Inflammation Induced by Carrageenan in Rats
1Amity Institute of Pharmacy, Amity University, Noida-201313, Uttar Pradesh, India.
2Sunder Deep Pharmacy College, Dasna, Ghaziabad, Uttar Pradesh, India.
3Glocal School of Pharmacy; Glocal University, Mirza Pur Pole, Saharanpur, Uttar Pradesh.
Corresponding Author E-mail: tsdivass7@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/400113
Article Received on : 11 Nov 2023
Article Accepted on : 10 Jan 2024
Article Published : 19 Jan 2024
Reviewed by: Dr. Vishesh Maurya
Second Review by: Dr. B. S. Chhikara
Final Approval by: Dr. Tanay Pramanik
Inflammation is a complex physiological response that can lead to various health issues. The development of effective anti-inflammatory agents is crucial for managing inflammatory conditions. This study focused on synthesizing and evaluating Schiff's base Indole derivatives for their anti-inflammatory potential. Among several synthesized compounds, C1IN, C2IN, C3IN, C7IN, C8IN, and C11IN demonstrated substantial reductions in paw edema and levels of cytokines of inflammation such as IL-1β and TNF-α. In-silico analysis and molecular docking studies further supported the observed effects, indicating potential interactions with TNF-α. The study highlights the therapeutic potential of Schiff's base Indole derivatives in mitigating inflammatory responses. Hence, Schiff's base Indole derivatives present a novel avenue for future research and the potential development of anti-inflammatory drugs.
KEYWORDS:Computational analysis; Docking Study; Inflammation; Indole Derivatives; Molecular docking analysis; Schiff’s Base; Spectroscopy Analysis
Download this article as:| Copy the following to cite this article: Singh D, Kharb R, Sharma S. K. Synthesis and Evaluation of Schiff’s Base Indole Derivatives Against Inflammation Induced by Carrageenan in Rats. Orient J Chem 2024;40(1). |
| Copy the following to cite this URL: Singh D, Kharb R, Sharma S. K. Synthesis and Evaluation of Schiff’s Base Indole Derivatives Against Inflammation Induced by Carrageenan in Rats. Orient J Chem 2024;40(1). Available from: https://bit.ly/3S1dHPn |
Introduction
Inflammation represents a complex and tightly regulated biological effect to harmful biological activators, such as damaged cells, pathogens, or irritants, aimed at restoring homeostasis and promoting tissue repair. When this process becomes altered, chronic inflammation can lead to numerous ailments including cardiovascular disorders, cancer, neurodegenerative pathophysiological condition, and autoimmune disorders. Therefore, the production of potent and safe anti-inflammatory agents is of significant interest in contemporary pharmacology and medicinal chemistry.1,2 Schiff’s bases, a class of compounds with a general structure of R1-CH=NR2, have demonstrated diverse biological activities, including anti-inflammatory properties. The structural versatility of Schiff’s bases allows for the systematic modification of their aromatic and aliphatic moieties, thereby fine-tuning their biological activities. Among Schiff’s base derivatives, indole-based compounds have garnered attention due to their potential as anti-inflammatory agents. Indole is an aromatic heterocyclic compound present in various natural products and pharmaceuticals, often associated with multiple bioactivities.3,4
This study focuses on the development of novel indole derivatives derived from Schiff’s base as effective anti-inflammatory drugs. The design and development of these compounds aim to harness the structural and chemical diversity of indole to enhance anti-inflammatory activity. The rationale behind this research is to insights the anti-inflammatory potential of indole derivatives of Schiff’s base through molecular modifications, guided by structure-activity relationships. The proposed indole derivatives are synthesized through a well-established and efficient synthetic route, allowing for the introduction of diverse substituents at key positions. These modifications are essential for tailoring the anti-inflammatory efficacy of the compounds, such as inhibiting cytokine mediators or modulating specific inflammatory pathways.5,6
A novel Schiff’s base, in the context of organic chemistry, refers to a specific type of chemical compound formed by the condensation reaction between an amine group (usually derived from primary amines) and an aldehyde or ketone. This reaction results in the creation of a unique class of compounds characterized by a central azomethine (-C=N-) functional group. Schiff’s bases are vital intermediates in organic synthesis and can serve as essential building blocks for a diverse array of compounds with varied biological and pharmacological activities.3,6
The versatility of Schiff’s bases is particularly evident in their widespread use in medicinal chemistry. Researchers have designed novel Schiff’s base compounds with the intent of enhancing their biological activities. Whether it is improving the antimicrobial, anticancer, anti-inflammatory, or antioxidant properties, these structural modifications can fine-tune the compound’s interaction with specific cellular targets or metabolic pathways. Thus, the term “novel Schiff’s base” typically refers to a derivative that has been chemically engineered with the aim of exhibiting distinct, enhanced, or previously unexplored properties compared to its parent compounds.7,8
Moreover, a novel Schiff’s base signifies a chemically tailored compound that leverages the fundamental structure of Schiff’s bases, introducing modifications to create unique properties and functions, particularly within the realm of medicinal chemistry. These compounds hold the potential for new and exciting applications, advancing the fields of drug discovery, organic synthesis, and chemical biology. The evaluation of these indole derivatives’ anti-inflammatory activity is a critical aspect of this study. Standard assays are employed to examine their effectiveness in mitigating the inflammatory response. The anti-inflammatory mechanism of action will also be investigated, shedding light on their potential therapeutic targets and pathways.7,9,10
However, this research holds significant promise in the synthesis of new anti-inflammatory agents with enhanced efficacy and reduced side effects. The development of novel indole derivatives derived from Schiff’s base provides valuable insights into their structure-activity relationships and potential as therapeutic agents in the context of inflammation-related diseases. Furthermore, this work contributes to the broader field of medicinal chemistry, offering a new avenue for the design and development of indole-based pharmacological agents.
The synthesis and evaluation of these indole derivatives represent a novel approach to exploring anti-inflammatory agents’ efficacy. Their potential to modulate inflammatory processes offers exciting prospects for the synthesis of effective therapeutic strategies. The following sections will delve into the experimental methodology, results, and discussions, ultimately presenting a comprehensive assessment of the agents against inflammatory of these newly synthesized indole derivatives derived from Schiff’s base.
Material and methods
Chemicals and reagents
The chemicals were received from Sisco Research Laboratories Pvt. Ltd., India and Sigma-Aldrich Chemicals Pvt. Ltd. The chemicals include, 2,5-Dichloroaniline, Fluoroaniline, 2,5-Dibromoaniline, 3,4,5-trimethoxyaniline, 3,5-Dimethoxyaniline, Anisidine, Ethylene Diamine, Tert-butylamine, 3,5-Bis(Trifluoromethyl)benzylamine, Benzylamine, 4-amino-2-chlorobenzonitrile, Thiophene-2-ethylamine, 3-Amino Pyrazole, 2-Amino Pyrimidine and 2-aminophenol.
Chemistry for synthesis
Indole derivatives synthesis commenced with the preparation of indole-3-carboxaldehyde. Initially, gramine methiodide (348 mg), NaNO2 (239 mg) and DMF (4 ml) solution was prepared. The developed solution was enthused at 25°C for 6 hours with the help of a magnetic stirrer. Subsequently, 15 ml of distilled water was mixed in the solution and 20 ml of water was used to extract the solution. After drying with Na2SO4, the solution was concentrated to yield indole-3-carboxaldehyde. The progression of the reaction was observed via TLC, and its completion was confirmed through IR, NMR, and LCMS analyses. In the subsequent step, equimolar amounts (0.6 mmol) of indole-3-carboxaldehyde and a primary amine were dispersed in methanol (10 ml). The reflux method was used to achieve the reaction at 70⁰C for 1 to 2 hours. Monitoring of the reaction was carried out using Thin Layer Chromatography of the developed products was done using the mobile phase of ethyl acetate: toluene: formic acid (4: 6: 1, v/v/v). The obtained solid content was then cooled, filtered, and subjected to purification through re-crystallization from methanol. The synthetic procedure for indole derivatives derived from Schiff’s base is schematically depicted in Figure 1.
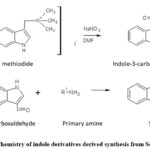 |
Figure 1: Chemistry of indole derivatives derived synthesis from Schiff’s base. |
Characterization and selection of biologically active components
In this study, various spectroscopic techniques, including Fourier-transform infrared spectroscopy (FT-IR), mass spectrometry (MS) and Nuclear Magnetic Resonance (NMR), were applied for the depiction and identification of the synthesized compounds. These methods were conducted following established protocols with some necessary modifications.3,4 Additionally, in-silico analysis was conducted to assess the potential therapeutic components. This interactions profile of compounds was determined with Tumor Necrosis Factor-alpha (TNF-α), with specific protein coordinates (grid dimension center, -3.641, 68.631, 131.508, with the size of 53, 53, 53). The findings of this analysis have been visually depicted in Figure 2. The detailed characterization of these components can be found in Table 1 and 2. Furthermore, the spectroscopic results regarding the therapeutically active compounds are presented as follows, demonstrating their structural and molecular characteristics.
 |
Table 1: chemistry and docking profile of developed indole derivatives. |
Table 2: Spectroscopic characterization of compounds.
|
Sr. No |
Compound name |
MS (ESI) m/z |
FTIR (υmax, cm−1) |
NMR |
|
|
1HNMR (500MHz) |
13CNMR |
||||
|
1. |
C1IN |
239.21 (M+1H) |
3612.99, 3425.14, 3321.22 (=N, -NH), 1624.77 (C=C/-C=N), 2934.95 (CH) 1458.42 (CO), 1005.47 (CH) |
δ7.086 (4H, m), 7.224, 7.723, 8.304, 10.902 (3H, 2d,s), 9.801, (1H, s). |
δ115.323, 116.634 (2C), 119.34, 126.738 (4C), 122.36, 124.784 (3C), 163.764 (1C). |
|
2. |
C2IN |
237.16 (M+1H) |
3618.86, 3498.24, 3345.12 (=N, -NH and OH), 2914.75 (CH), 1623.85, 1587.25, 1451.75 (C-N, C=C/C-H), 1261.99 (C-H vibrations). |
δ6.972 (6H, m), 7.4988, (1H, d), 7.232 and 8.401(2H, s,m), 10.902 and 9.801, (2H, 2s). |
δ115.323, 116.634 (2C), 154.763 (1C), 124.376 (2C), 163.37 (1C). |
|
3. |
C3IN |
281.24 (M+1H); |
3518.42, 3387.55 (=N, -NH), 2989.44 (CH3), 1698.03 (C=O), 1517.43, 1623.73 (C=C), 1408.42 (C-O-C and C-H), 1179.32 (C-H vibrations). |
δ3.682 (6H, s), 6.3001, (3H, s), 6.9422 (3H, m), 7.701, and 8.4001 (2H, s,d), 10.902 and 9.801, (2H, 2s). |
δ59.352 (2C), 101.347, 104.932 (3C), 115.376, 116.688 (2C), 121.376, 124.463 (2C).
|
|
4. |
C7IN |
333.31 (M+Na+) |
3651.42, 3381.37 (=N, -NH), 1247.52 (C-O-C and C-H), 2982.46 (CH3) 1832.47, 1749.52, 1609.48 (C=C/-C=N, C-O). |
δ3.542 and 3.804 (9H, s), 6.211, (2H, s), 6.983 (3H, m), 7.731, and 8.403 (2H, s,d), 10.903 and 9.802, (2H, 2s). |
δ 59.63, 63.252 (2C), 104.63 (2C), 114.78, 115.84 (2C) and 163.36 (1C)
|
|
5. |
C8IN |
251.24 (M+1H) |
3415.43, 3287.96 (=N, -NH), 1253.73 (C-O-C and C-H), 2917.53, 2834.82 (CH3/CH2) 1633.23 (C=C/-C=N). |
δ3.543 (3H, s), 6.481, (2H, s), 6.985 (3H, m), 7.611, 8.009, 8.429 (4H, 2d,s), 11.003 and 9.802, (2H, 2s). |
δ59.345 (1C), 114.634, 115.437 (2C), 118.376, 125.892 (4C), 163.276 (1C).
|
|
6. |
C11IN |
223.18 (M+1H) |
3441.42, 3357.98 (=N, -NH), 1609.73 and 1653.49 (C=C/-C=N), 3085.47, 2967.48 (C-H-C) |
δ6.985 (3H, m), 6.274, 8.426 and 8.872 (5H, 2m,d), 11.023 and 9.322, (2H, 2s). |
δ114.327, 115.234 (2C), 122.76, 124.478 (3C). 161.783, 163.89, 165.302 (3C). |
 |
Figure 2: In-silico computational docking analysis of the compounds with the TNF-α protein, the figure represents 3D (Left) and 2D (in Right ) diagram of interaction of most potent components with the protein that shows their biologically activeness. |
In-vivo studies
Experimental animals
The experimental investigations were conducted using Wistar albino rats procured from the institution. The study was examined in compliance with the ethical procedures and principles of the Institutional Animal Ethics Committee (IAEC) under endorsement number of the letter 163/PO/RE/S/12/CPCSEA/2021-6. The average weight of 190 ± 20 g was found of each animal used in experimental analysis. Polypropylene cages were used to retain the animals in and acclimatized to standard laboratory conditions, including a 12:12 h light-dark cycle, a temperature of 23 ± 2°C, and a relative humidity of 55 ± 5%. The rats were receiving standard pellet food and had normal saline (ad libitum) during treatment period. The CPCSEA and IAEC guidelines were followed during the experimentation period.
Carrageenan-induced rat paw edema model
Forteen groups (n=14) were made and animals were alloted equally in each group (n=6) and carragennan (100 μl of a 1% (w/v), i.p) was used to induced inflammation for acute inflammation using a intraplantar injection of. To assess the therapeutic effects of drugs against carrageenan mediated inflammation, each rat (n=11) received a dosage of 300 mg/kg of the test drug. The measurement of the paw edema volume was taken in both before and after treatment of carrageenan at 0, 30, 60, 120, 180, 240, and 300 min. As a standard drug, indomethacin was employed. After complition of treatment period, the rats were euthanized and measured the dissected carrageenan-induced edema from paws for final assessment and stored at -80°C.11,12 thereafter, blood from each group was withdrawn for biochemical analysis.
Pro-inflammatory markers Measurements
The biomarkers of cytokines involved in inflmattion were assessed in the blood after subsequent to the final paw measurement via anesthetizing the animals through ketamine hydrochloride injection at a dosage of 80-90 mg/kg, and they were then humanely euthanized to obtain blood samples. These samples were utilized for the analysis of IL-1ß and TNF-α and the analysis was performed using an ELISA kit sourced from Abbkine (China). In a summarized procedure, the collected blood samples subjected to centrifugation at 13,000 rpm for 15 minutes. The resultant samples of crude serum were carefully collected and transferred into fresh tubes, after which they were placed at -20°C for subsequent analysis.12,13
Statistical analysis
To assess significant differences and statistically variation in data are summarized as Mean ± SD (n = 6). One-way as well as Two-way ANOVA were used to represent the data in the statistical analysis, and the Tukey test was then performed. The p-value summary and p-value and significance level was determined for statistical difference.
Results
Indole derivatives derived from Schiff’s base were effectively developed and analyzed. By using in-silico docking analysis, the possible active ingredients were identified. Their anti-inflammatory properties were then assessed in relation for examining the anti-inflammatory effect using model of carrageenan.
Anti-inflammatory activity
In this examination, the results demonstrated a substantial effect (*p<0.05) for each drug and group treated with carrageenan were compared to the normal control and positive control group. Notably, out of the six examined indole derivatives, C1IN, C2IN, and C8IN exhibited remarkable protective effects against carrageenan-induced paw inflammation. These effects were comparable to the standard drug, indomethacin. Furthermore, the anti-inflammatory effects of all treated compounds were significant compared to the negative control group. This study showed that compounds C1IN, C2IN, and C8IN hold promise as agents against various inflammatory cytokines responsible for inflammation. The study’s outcomes are depicted in Table 3, illustrating the potential of these compounds in mitigating inflammation.
Table 3: Measurement of paw edema or change in paw edema thickness (mm).
|
Control |
NC |
PC |
T1 |
T2 |
T3 |
T4 |
T5 |
T6 |
T7 |
T8 |
T9 |
T10 |
T11 |
|
|
0 min |
2.143 |
2.142 |
2.146 |
2.139 |
2.146 |
2.143 |
2.144 |
2.142 |
2.144 |
2.145 |
2.133 |
2.161 |
2.156 |
2.183 |
|
30 min |
2.143 |
2.551 |
2.288 |
2.457 |
2.448 |
2.457 |
2.456 |
2.466 |
2.457 |
2.545 |
2.541 |
2.4355 |
2.437 |
2.404 |
|
60 min |
2.146 |
2.846 |
2.468 |
2.647 |
2.628 |
2.667 |
2.726 |
2.541 |
2.532 |
2.745 |
2.743 |
2.442 |
2.742 |
2.819 |
|
120 min |
2.144 |
3.126 |
2.478 |
2.602 |
2.473 |
2.607 |
2.746 |
2.661 |
2.697 |
2.845 |
2.858 |
2.488 |
2.987 |
2.883 |
|
180 min |
2.1455 |
3.391 |
2.208 |
2.573 |
2.353 |
2.677 |
2.395 |
2.546 |
2.362 |
2.975 |
3.174 |
2.659 |
3.116 |
3.090 |
|
240 min |
2.146 |
4.116 |
2.478 |
2.712 |
2.697 |
3.342 |
2.955 |
3.146 |
3.117 |
2.987 |
3.136 |
2.739 |
3.024 |
3.429 |
|
300 min |
2.154 |
4.246 |
2.413 |
2.636 |
2.543 |
3.167 |
3.05 |
3.301 |
3.087 |
3.095 |
3.234 |
2.89 |
3.268 |
3.341 |
Effect of Indole derivatives against inflammation, the information is given as mean ± SD (n = 6). Superscripts (*) indicate a substantial difference between the control and treatment groups, and values with them indicate a substantial alteration between the normal control and toxic control. P < 0.05 is regarded as substantial. The significance level represented as #/* (p < 0.05), ##/** (p < 0.01), or ###/*** (p < 0.001).
*C1IN: T1, C2IN:T2, C3IN: T3, C4IN: T4, C5IN:T5, C6IN:T6, C7IN:T7, C8IN:T8, C9IN:T9, C10IN:T10 C11IN:T11
Pro-inflammatory markers Measurements
In this examination, the compounds found effective counteract against IL-1β and TNF-α. The study’s findings revealed that among the different compounds, C1IN, C2IN, C6IN and C8IN compounds exhibited significant (p<0.05) anti-inflammatory activity, leading to a notable reduction in cytokine levels. These compounds also effectively counteracted the toxic effects induced by carrageenan than standard drug. The results of these findings are succinctly depicted in Figure 3.
 |
Figure 3: Indole derivatives’ anti-inflammatory properties against aggravation generated by carrageenan. Figure (A) illustrates how indole derivatives protect against TNF-α, while Figure (B) shows how they protect against IL-1β. |
Carrageenan is used to induce inflammation, hence used as a suitable model for numerous investigations have been carried out to ascertain how components counteract inflammation due carrageenan.14–16
Bhatt et al. conducted a study in which they synthesized a novel series of indole derivatives. These compounds were subjected to comprehensive evaluation for their potential in various aspects. The synthesized compounds were thoroughly determined based on their chemistry using spectroscopic techniques. The study’s outcomes highlighted that three synthetic compounds (S3, S7, and S14) exhibited substantial anti-inflammatory effects than the effect of indomethacin. However, component S3, in particular, showed promising results by demonstrating stomach-sparing effects while selectively suppressing COX-2 (cyclooxygenase-2). Docking studies suggested that the compounds might cooperate with the COX-2 enzyme. Furthermore, compound S3 was found to establish hydrogen bonds found as like indomethacin between the hydroxyl group of Tyr 355 and the amino group of Arg 120. These findings provide crucial insights into compound S3, indicating its potential as a novel lead molecule with selective COX-2 inhibitory properties and anti-inflammatory efficacy.17
Additionally, Ibrahim et al. synthesized novel non-steroidal anti-inflammatory medications (NSAIDs). Carrageenan and diclofenac used to develop inflammation and Schiff’s condensation reaction was used to create the compounds for determining the anti-inflammatory effect. Next, their ability to reduce inflammation was investigated. Furthermore, virtual screening was conducted using molecular docking research to confirm the anti-inflammatory efficacy. Interestingly, compound M2 exhibited 61.32% inhibition than the effect of diclofenac 51.36% inhibition.5 Additionally, it aids in determining the pathophysiological state by evaluating various cytokine types, including ILs, TNFs, and NF-kB.18 The study also assessed the anti-inflammatory effects of the compounds against pro-inflammatory cytokines found significant and showed that the components such as C2IN, C4IN, C6IN and C9IN exhibited remarkable anti-inflammatory activity. These compounds significantly reduced cytokine levels, effectively mitigating the likelihood of inflammation.
In line with these findings, research by Takada et al. demonstrated the anti-inflammatory properties of indole-3-carbinol. They found that indole-3-carbinol effectively inhibited NF-κB activation in various cell types, including myeloid, leukemia, and epithelial cells. Moreover, indole-3-carbinol was found to inhibit NF-κB activation in leukemia patients, leading to reduced cell proliferation. These observations indicate that indole-3-carbinol effectively suppresses NF-κB activation and its transcription, providing a molecular basis for its potential in reducing carcinogenesis and inflammation.19
The indole derivative also combats degeneration of neurons and oxidative stress-induced inflammation of neurons in Parkinson’s disease. Indole derivatives inhibited NLR family (NLRP3) activation of the inflammasome and decreased IL-1, NO, IL-6, as well as TNF production in vitro. In vivo, MPTP-treated mice given NC009-1 showed reduced oxidative damage, decreased reactivity of microglia and astrocytes, and decreased motor dysfunction and non-motor depression in adding to increased dopamine levels and dopamine transporters in the striatum. Raising SOD2, NRF2, and NQO1 and decreasing CASP1, NLRP3, IL-6, iNOS, TNF and IL-1, allowed for the achievement of these protective effects.20, 21, 22, 23
Conclusion
It has been concluded that Schiff’s base Indole derivatives such as C1IN, C2IN, C6IN, C8IN, and C9IN exhibited most promising therapeutic effect against the noxious effect of carrageenan on hind paw and substantially reduce the inflammation via regulation of IL-1β and TNF-α. The study highlights their potential as alternative components for managing inflammation. The observed anti-inflammatory effects of these compounds, when coupled with their molecular docking and in-silico interactions with target proteins, further support their therapeutic potential. The significant reduction in cytokine levels and paw edema suggests that these derivatives may contribute to the development of novel anti-inflammatory agents, potentially offering new avenues for combating inflammatory conditions. Further research and clinical investigations are warranted to validate their efficacy and safety for future therapeutic applications.
Acknowledgment
The authors thanks to Amity Institute of Pharmacy, Amity University, Noida-201313, Uttar Pradesh, India for providing facilities to compete the study.
Conflict of interest
No conflict of interest is declared by the authors.
Funding Sources
There are no funding sources
References
- Breyer, M.D.; Susztak, K. Semin. Nephrol. 2016, 36, 436–447,
CrossRef - Jiang, S.; Zhang, Z.; Yu, F.; Zhang, Z.; Yang, Z.; Tang, Y.; Ding, G. J. Funct. Foods 2020, 142(6):556-74.
- Yadav, S.; Kumar, N. Orient. J. Chem. 2021, doi:10.13005/ojc/370517.
CrossRef - Ali, Z.; Ain, S.; Kumar, B.; Ain, Q. Orient. J. Chem. 2022, 38, 898–905, doi:10.13005/ojc/380409.
CrossRef - Ibrahim, M.M.; Elsaman, T.; Al-Nour, M.Y. Int. J. Med. Chem. 2018, doi:10.1155/2018/9139786.
CrossRef - Dixit, R.B.; Uplana, R.A.; Patel, V.A.; Dixit, B.C.; Patel, T.S. Sci. Pharm. 2010, 78(4):909-26. doi:10.3797/scipharm.0912-20.
CrossRef - Saipriya, D.; Prakash, A.; Kini, S.G.; Varadaraj Bhatt, G.; Pai, K.S.R.; Biswas, S.; Mohammed Shameer, K. Indian J. Pharm. Educ. Res. 2018, 52. doi:10.5530/IJPER.52.4S.114.
CrossRef - Abba, C.; Betala, S. Res. J. Chem. Environ. 2022, doi:10.25303/ 2612rjce1390145.
CrossRef - Sai Priya, D.; Kini, S.G.; Bhatt, V.G.; Rathi, E.; Muralidharan, A.; Koteshwara, A. Indian Drugs 2018.
- Kadhom, M.; Mohammed, A.; Ghani, H.; Hasan, A.A.; Mousa, O.G.; Abdulla, R.T.; Al-Dahhan, W.H.; Al-Mashhadani, M.H.; Yusop, R.M.; Yousif, E. J. Vinyl Addit. Technol. 2023, doi:10.1002/vnl.22027.
CrossRef - Jargalsaikhan, B.E.; Ganbaatar, N.; Urtnasan, M.; Uranbileg, N.; Begzsuren, D. Biomed. Pharmacol. J. 2019, 12, 1801–1809, doi:10.13005/bpj/1811.
CrossRef - Amdekar, S.; Roy, P.; Singh, V.; Kumar, A.; Singh, R.; Sharma, P. Int. J. Inflam. 2012, doi:10.1155/2012/752015.
CrossRef - Umar, S.; Zargan, J.; Umar, K.; Ahmad, S.; Kant, C.; Khan, H.A. Chem. Biol. Interact. 2012, 197, 40–46, doi:10.1016/j.cbi.2012.03.003.
CrossRef - Maswadeh, H.M.; Semreen, M.H.; Naddaf, A.R. Acta Pol. Pharm. – Drug Res. 2006.
CrossRef - Chaudhari, S.P.; Baviskar, D.T. Adv. Tradit. Med. 2021, doi:10.1007/s13596-020-00502-1.
- Odira, H.O.; Mitema, S.O.; Mapenay, I.M.; Moriasi, G.A. J. Evidence-Based Integr. Med. 2022, doi:10.1177/2515690X221082986.
CrossRef - Bhat, M.A.; Mohamed, A.A.O.; Mohammad, R.; Ansari, M.A.; Abuelizz, H.A.; Bakheit, A.H.; Naglah, A.M. Molecules 2018, 23, doi:10.3390/molecules23061250.
CrossRef - Gaurav; Sharma, I.; Khan, M.U.; Zahiruddin, S.; Basist, P.; Ahmad, S. Biomedicines 2023, 11, doi:10.3390/biomedicines11010168.
CrossRef - Takada, Y.; Andreeff, M.; Aggarwal, B.B. Blood 2005, doi:10.1182/blood-2004-12-4589.
CrossRef - Chiu, Y.J.; Lin, C.H.; Lin, C.Y.; Yang, P.N.; Lo, Y.S.; Chen, Y.C.; Chen, C.M.; Wu, Y.R.; Yao, C.F.; Chang, K.H.; et al. Int. J. Mol. Sci. 2023, doi:10.3390/ijms24032642.
CrossRef - Sharma, S.; Monga, Y.; Gupta, A.; Singh, S. J. Mol. Chem. 2023, 3 (1), 585.
- Monga J, Ghosh NS, Kamboj S, Mukhija M. J. Mol. Chem. 2023, 3 (2), 590.
- Singh, K.; Siwach, P. Chem. Biol. Lett. 2022, 9 (4), 407.
- Irez, T.; Bicer, S.; Sahin, E.; Dutta, S.; Sengupta, P. Chem. Biol. Lett. 2020, 7 (2), 131–139.

This work is licensed under a Creative Commons Attribution 4.0 International License.










