Spectroscopic Identification of Isolated Bioactive Compounds from Methanolic Extract of Psychotria dalzellii leaves
Preeti N Tallur1* , Abhishek M2
, Abhishek M2 , Vinayak M Nayik3, Shivanand S Bhat4
, Vinayak M Nayik3, Shivanand S Bhat4 and Pragasam A1*
and Pragasam A1*
1Department of chemistry, Maharani's Science College for women, Mysore, Karnataka, India.
2Research and Development Centre, Bharathiar University Coimbatore, Tamilnadu, India.
3Department of chemistry, Government First Grade College, Kumata, Karnatka, India.
4Department of Botany Smt. Indira Gandhi Government First Grade Women's College, Sagar, Karnataka, India.
Corresponding Author E-mail: brightbright56@gmail.com, preetiksh2003@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/400137
Article Received on : 13 Dec 2023
Article Accepted on : 15 Feb 2024
Article Published : 15 Feb 2024
Reviewed by: Dr. Kanmani Vincentraj
Second Review by: Dr. K. P. Srivastava
Final Approval by: Dr. Nenad Ignjatovic
Plant sources plays vital role in the medicinal field as new bioactive compounds are found to be isolated. Present work was focused on isolation and structure elucidation of compounds from Psychotria dalzellii(family-Rubiaceae) leaves in methanol extract. The extract was fractionated through thin layer chromatography followed by column chromatography. Spectroscopic technique like FT-IR, NMR and LC-MS has been used to identify the fractionated compounds. Spectral data supports the structural elucidation of compounds like Harmaline, Emetine, Dimethyltryptamine and Psychollatine. These lead molecules are responsible for high anti-diabetic property and antioxidant property. The lead molecules are conspicuous in showing bioactivity and have future scopes.
KEYWORDS:Dimethyltryptamine; Emetine; Harmaline; Psychotria dalzellii; Psychollatine
Download this article as:| Copy the following to cite this article: Tallur P. N, Abhishek M, Nayik V. M, Bhat S. S, Pragasam A. Spectroscopic Identification of Isolated Bioactive Compounds from Methanolic Extract of Psychotria dalzellii leaves. Orient J Chem 2024;40(1). |
| Copy the following to cite this URL: Tallur P. N, Abhishek M, Nayik V. M, Bhat S. S, Pragasam A. Spectroscopic Identification of Isolated Bioactive Compounds from Methanolic Extract of Psychotria dalzellii leaves. Orient J Chem 2024;40(1). Available from: https://bit.ly/3OMcQ45 |
Introduction
Plants have been integral to human well-being, serving as both food and medicine since ancient times1. Approximately 6.4 billion people in developing countries heavily rely on traditional plant-based medicines for their primary healthcare2(WHO, 2002). The transmission of herbal medicine knowledge from generation to generation is a cultural practice among tribal communities3. A significant portion, around 10%, of the world’s higher plant species known for treating animal diseases have reported from Indian origin4, 5.
The foundation for numerous drugs addressing critical human diseases is derived from plants and their by-products6. Plants produce two main categories of metabolites: primary and secondary. Primary metabolites, such as amino acids and fatty acids, are essential for plant growth, while secondary metabolites, including flavonoids and alkaloids, offer additional benefits7, 8. Over 90% of traditional medicine remedies worldwide are based on natural products, particularly those of plant origin, showing promise in upcoming drug development and disease control strategies 9, 10.
Emphasizing their importance, plant-based diets, rich in fatty acids and sugars, play a significant role in promoting beneficial health effects11. Natural products are known for their lower toxicity and their effectiveness in treating various ailments, including hypoglycaemic, antidiabetic, antioxidant, anti-inflammatory, anti-carcinogenic, anti-malarial, anti-cholinergic, and anti-leprosy properties12, 13, 14. Pharmacologically active compounds are sourced directly or indirectly from plants, contributing to the production of clinically beneficial drugs. Phytomedicine-based drugs are widely used worldwide to address conditions such as colds, burns, headaches, depression, and diarrhoea15, 16.
Several examples illustrate the significance of plant-derived compounds in drug development, such as Podophyllotaxin from Podophyllum emodi Wall, Paciltaxel and taxotere from Taxus brevifolius, Quinoline and comptothecin alkaloids from Comptotheca acuminate, and Teniposide and etoposie from Juniperus communis17, 18, 19, 20. This underscores the immense potential of natural products in providing effective and less toxic solutions for a wide array of health issues. Therefore, the present study is aimed isolate bioactive compounds from Psychotria dalzellii leavesis plant
Methodology
Chemicals
Analytical grade solvents such as ethylacetate , methanol, Petroleum ether, chloroform, acetone, were procured from Sisco Laboratories (SRL), Bangalore, India.
Plant collection and processing
The plant, Psychotria dailzelli was collected from the foot hills of Billiranga Karnataka state, India. The plant was identified and authenticated by botanist from University of Mysore, Mysore, Karnataka state, India. The herbarium of Psychotria dailzelli plant was submitted and accession number was obtained from National Ayurveda, Bangalore, India. Leaves of the plant were separated and infected region were removed. The material was washed with running tap to remove the dust and adhered soil particles. Leaves were shadow dried and the dried material was homogenized in an electric blender to fine powder. The sieved powder was stored in airtight container for the further use.
Preparation of plant extracts
Exactly 100 g of sample was separately weighed and de-fatted using petroleum ether in soxhlet apparatus. The de-fatted sample was extracted for 8-10 hours using various solvents such as chloroform, acetone, ethyl acetate, and methanol. Solvent of each extract was evaporated using rotor vaccum evaporator. The extracts were collected in screw cap tubes and kept at 4 degrees Celsius for further use.
Thin Layer Chromatography (TLC)
The methanol leaf extracts of (0.1ml) of Psychotria dalzellii species was subjected thin layer chromatography plate (TLC Silica gel 60 F254 25 Aluminium sheets 20×20 cm) solvent system using the ratio 2:1 n-hexane and acetone has a mobile phase. Rf values of the compounds were calculated.
Column Chromatography
The Column chromatography technique was used to separate the compounds based on its polarity. The length of the silica gel packed glass column 30 cm and width 4 cm was used. The weight of 3gm methanol crude extract first eluted with n-hexane and then eluted with different ratio of n-hexane: acetone. Fractions collected in 100ml beaker were labelled as A, B, C and D then evaporation was carried out using rotary vacuum pump.
Fourier-transform infrared spectroscopy (FT-IR)
Fourier-transform infrared (FT-IR) instrument used was Shimadzu, IR Affinity Japan and spectra were recorded using KBr disk method (2 mg sample in 200 mg KBr). Scanning range was 400-4000 cm-1 with resolution 2 cm-1.
NMR: 1H NMR instrument used was Bruker AM-500 spectrometer.
LC-MS
The instrument used is Agilent 1100 HPLC (Agilent, Palo Alto, CA) coupled with DAD and mass analyzer (MSD, model SL). Experimental set up was as described below.
Column: XTerra MS 50 mm×4.6 mm i.d., 3.5 μm, symmetry C18 column
Flow rate: of 0.75mL min-1 at 25°C
Mobile phase: A (0.01% Acetic acid) and B (Acetonitrile). The gradient was T/%B at 0/10, 1.5/90, 5.5/95, 6/10, 7/10.
Recorder: The DAD was set to record UV/Vis spectra from 200-400nm.
Mass spectra were recorded using electron ionization in positive and negative ionization modes at low and high fragmentation volts in the range of m/z 100-1000 and LC system was directly coupled without stream splitting.
Results and discussion
Biologically important bioactive compounds were isolated and characterized in methanol leaf extract of Psychotria dalzellii. Structural elucidation was confirmed by spectroscopic techniques such as TLC, H1NMR, FTIR and LC-MS. The vital bioactive compounds isolated were listed as below.
Harmaline
The basic structure of Harmaline is a harmala alkaloid which is very similar to two ring indole tryptamine. However, third ring has been formed via a carbon reconnected to indole through ethylamine side chain. Harman skeleton is methoxy-substituted at C-7 and it has been reduced across the 3, 4 bond. It act as oneirogen and the structure is derived from a hydride of Harman and elucidated by spectral data as shown below figures.
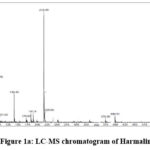 |
Figure 1a: LC-MS chromatogram of Harmaline |
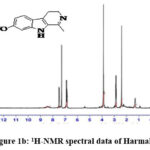 |
Figure 1b: 1H-NMR spectral data of Harmaline. |
1H NMR (CDCl3): 6.80 – 7.50 (m, 3H, Aromatic (5, 6 & 8)); 3.88 (s, 3H, -OCH3 (10)); 2.84 – 2.89 (t, 2H, -CH2– (4)); 2.41 (s, 3H, -CH3 (11)); 1.28 – 1.45 (b, 2H, -CH2– (3)).
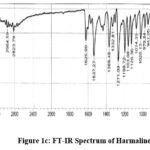 |
Figure 1c: FT-IR Spectrum of Harmaline. |
FTIR spectra: 2964.59 (NH stretching), 2823.79 (CH2 and CH stretches), 1625.99 (NH bending), 1537.27 (C=C str), 1271.09 (C-N str), 1026.13 (C-O ether str). Mass Spectra: m/z 214.89 (M+H)+
Emetine
Emetine is a pyridoisoquinoline which compose emetam with methoxy group at the 6′-, 7′-, 10- and 11 carbons. It is an isoquinoline and pyridoisoquinoline alkaloid and is derived from cephaeli whose structure is spectroscopically elucidated as recorded below.
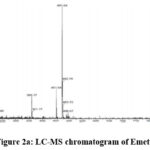 |
Figure 2a: LC-MS chromatogram of Emetine. |
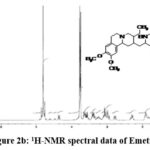 |
Figure 2b: 1H-NMR spectral data of Emetine. |
1H NMR (D2O): 6.75 – 6.90 (m, 4H, Aromatic (8, 11, 5′ & 8′)); 4.80 (m, 1H, -CH- (11b & Water in D20)); 4.40 (b, 1H, -CH- (1′)); 3.80 (m(s), 12H, -(OCH3)4 (15, 16, 9′ & 10′)); 2.90-3.70 (m, 10H, -CH2– (3′, 4′, 4, 6 & 7)); 2.80 & 1.45 (b, 2H, -CH2– (14)); 2.50 (t, 2H, -CH2– (1)); 1.90 (m, 2H, -CH- (2 and 3)); 1.65 and 1.25 (m, 2H, -CH2– (12)); 0.90 (m, 3H, -CH3(13)).
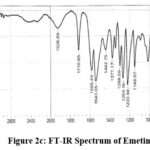 |
Figure 2c: FT-IR Spectrum of Emetine. |
FTIR spectra: 3340.71 (NH stretching), 1716.65 (C=C Stretching, alkene), 1585.49 and 1543.05 (C=C bond stretching), 1220 (C-N stretch), 1149.57(C-O ether stretch), 975.98 (=C-H, out-of-plane bending). Mass Spectra: m/z 481.64 (M+H)+
Dimethyl tryptamine (DMT)
Dimethyl tryptamine is an indole alkaloid consists of the tryptamine core structure in which two hydrogen atoms of ethylamine of tryptamine side chain has been replaced by two methyl groups. The structure elucidation has done by spectroscopic methods as follows.
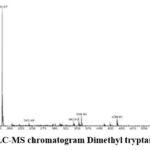 |
Figure 3a: LC-MS chromatogram Dimethyl tryptamine (DMT). |
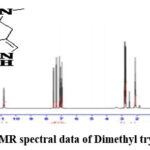 |
Figure 3b: 1H-NMR spectral data of Dimethyl tryptamine (DMT). |
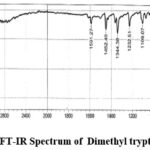 |
Figure 3c: FT-IR Spectrum of Dimethyl tryptamine DMT. |
IR Spectra: 3342.64(Amine), 1591.27, 1452.40 and 1344.38 (C-C Stretching), 1232.51 (N-H Bending), 740.67, 802.39 (C-H bending). Mass Spectra: EIMS: m/z 189.87 (M+H)+
Psychollatine
Psychollatine is an alkaloid and the structure is elucidated using the spectroscopic techniques as recorded below.
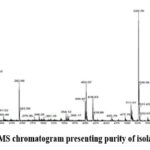 |
Figure 4a: LC-MS chromatogram presenting purity of isolated Psychollatine |
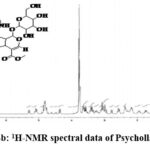 |
Figure 4b: 1H-NMR spectral data of Psychollatine. |
1H NMR(D2O):6.78 -7.18 (m,5H, Aromatic +alkene (9,10, 11, 12 & 17)); 5.21-5.36 (d, 1H, alkene (18); 5.0 – 5.18 (m, 2H, -CH- (21 & 25)); 4.36 – 4.40 (d, 1H, -CH- (3)); 3.78 – 3.82(s, 3H, -OCH3 (23)); 3.68 – 3.70 (m, 2H, -CH2– (30)); 3.38-3.42 (m, 2H, -CH- (26 & 29)); 2.96-3.18(m, 2H, -CH- (27 & 28)); 2.5 – 2.60 & 2.78-3.0 (d, m, 2H, -CH2-5)); 2.36 – 2.40 (m, 1H, -CH- (20)); 2.18 – 2.10 (m, 1H, -CH- (15)); 1.72 – 1.80 & 1.82-2.0 (m, 2H, -CH2– 6)); 1.28- 1.38 (d, d 2H, -CH2– (14)).
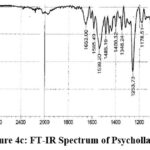 |
Figure 4c: FT-IR Spectrum of Psychollatine |
FTIR spectra: 3275.13 (NH and OH stretching), 2924.09 (CH2 and CH stretches), 1653.00 (C=O stretching), 1539.20 (C=C aryl double bond stretching), 1253.73 (C-N bond stretching), 1072.42 (C-O ether stretching). Mass Spectra: m/z 529.70 (M+H).+
Although potential compounds have been identified in the methanolic extracts of leaf, which compound particularly contribute to the observed antioxidant and antidiabetic properties needs to be elucidated.
Discussion
The isolated and elucidated compounds show a vital biological activities and the literature survey imparts research interest in this field. Researchers reported that harmaline alkaloid is a biologically active compound21, 22, 23 which belongs to sub class of beta-carboline alkaloids24. Harmaline is almost twice as toxic as harmine and in moderate doses causes tremors and clonic convulsions but with no increase in spinal reflex excitability 25. These alkaloids act as a reversible monoamine oxidase inhibitor26 and in common with other beta carboline binds to 5 hydroxy tryptamine (HT) receptors 27. Harmaline are reported to induce spasmolytic effects on guinea – pig isolated trachea with interaction to muscarinic, histaminic and beta adreno receptors 28. It also possesses anti – proliferative effect29 and analgesic activity30.
The Emetine is a natural product alkaloid native to Brazil, occur in three angiosperm plant families namely Rubiaceae, Alangiaceae and Icacinaceae. One of the major sources of emetine is Psychotria ipecacuanha 31. The inhibition of protein biosynthesis and interaction with DNA are considered the sources of emetine cytotoxicity32, 33. Emetine has been reported to prevent heat shock response (HSR) in cancer cells34. It has an antiprotozoal and emetic property and prevents replication of Zika, SARS-CoV-2 and Ebola virus. It also shows antiamoebic, antimalarial and antineoplastic properties. Many research reports that it can act as an antiprotozoal, antiviral, emetic, antimalarial, a protein synthesis inhibitor, antineoplastic agent, autophagy inhibitor, anti-infective agent and expectorant and anti-corona viral agent.
Dimethyl tryptamine is an indole alkaloid, psychodysleptic compound that has been found in several organisms35. It is present as endogenous compounds in animals36 and also many plants around the world. Major plant genera containing DMT include Phalaris, Delosperma, Acacia, Desmodium, Mimosa, Virola and Psuchotria37. DMT are mediators of several neurotransmitters.
Psychollatine is a monoterpene indole alkaloid and it has shown several biological activities 38. Psychollatine show significant biological activities such as mild analgesic effects, anxiolytic, antidepressive and amnesic effects in mice models39, 40.
Conclusion
Plant sources plays vital role in the medicinal field as new bioactive compounds are found to be isolated. Experiment was focused on isolation and structure elucidation of compounds from psychotria dalzellii leaves in methanol extract, it is belonging to the family Rubiaceae. The lead molecule which is responsible for high anti-diabetic property and antioxidant property was identified as Harmaline, Emetine, Dimethyltryptamine (DMT) and Psychollatine. As per the discussion these compounds are biologically very important and play a crucial role in controlling number of diseases. The lead compounds were isolated and characterised from psychotria dalzellii leaves. The availability and cost of materials is easily affordable and identification of compounds lot of scope in the medicinal field.
Acknowledgement
Authors are sincerely thankful to the Principal of Maharani’s Science College for Women, Mysore, for providing facilities available to carry out this work.
Conflict of interest
The authors declare have no conflict of interest.
Funding Sources
There is no funding sources.
REFERENCES
- Pugazharasi, G.; Meenakshi, S.A.; Ramesh, K.N.; Bastin, C.M.; Natarajan, E. J Basic Appl Biol., 2009, 3, 43-9.
- WHO, World Health Organization. WHO traditional medicine strategy, 2002‐2005, Geneva.
- Sandhya, B.; Thomas, S.; Isabel, W.; Shenbagarathai, R. African Journal of Traditional, Complementary and Alternative Medicines., 2006, 3(1), 101-14.
CrossRef - Vanwyk, B.E.; Gericke, N. People’s plants: A guide to useful plants of Southern Africa. Briza publications., 2000
- Prakasha, H.M.; Krishnappa, M.; Krishnamurthy, Y.L.; Poornima, S.V. Indian Journal of Traditional knowledge., 2010, 9(1), 55-60.
- Geldenhuys, W.J.; Gaasch, K.E.; Watson, M., Allen, D.D.; Vander Schyf,C.J. Drug Discov Today. 2006, 11(3-4), 127-32.
CrossRef - Croteau, R.; Kutchan, T.M.; Lewis, N.G. Biochemistry and molecular biology of plants. 2000, 24, 1250-319.
- Keeling, C.I.; and Bohlmann, J. New Phytologist, 2006, 170(4), 657-75.
CrossRef - Sharifi-Rad, M.; Salehi, B.; Sharifi-Rad, J.; Setzer, W.N.; and Iriti, M. Cell. Mol. Biol. 2018, 64, 18-21.
CrossRef - Abayomi Sofowora, Eyitope Ogunbodede and Adedeji Onayade. Afr J Tradit Complement Altern Med., 2013, 10(5), 210-229.
CrossRef - McMacken, M.; Shah, S.A. J. Geriatr. Cardiol., 2017, 14, 342.
- Kossuga, M.H.; MacMillan, J.B.; Rogers, E.W.; Molinski, T.F.; Nascimento, G.G. F.; Rocha, R.M.; Berlinck, R.G.S. J. Nat. Prod., 2004, 67, 1879
CrossRef - Newman, D.J.; Cragg, G.M. Journal of natural products., 2007, 70(3), 461-77.
CrossRef - Negi, J.S.; Singh, P.; Rawat. B. Curr Res Chem., 2011, 3( 1), 1-5.
- Veeresham, C.; Chitti, P. Natural Products Chemistry and Research., 2013, 1, 4.
CrossRef - Calixto, J.B. Brazilian Journal of medical and Biological research., 2000, 33(2), 179-89.
CrossRef - Singh, P.K.; Kumar, R.; Sharma, A.; Arora, R.; Chawla, R.; Jain, S.K.; Sharma, R.K. Integrative cancer therapies., 2009, 8(3), 261-72.
CrossRef - Samy, R.P.; Gopalakrishnakone, P. Nature precedings., 2007, 28, 1-1.
- Lorence, A.; Nessler, C.L. Phytochemistry., 2004, 65(20), 2735-49.
CrossRef - Gordien, A.Y.; Gray, A.I.; Franzblau, S.G.; Seidel, V. Journal of ethnopharmacology., 2009, 126(3), 500-5005.
CrossRef - Ross, S.A.; Megalla, S.E.; Bishay, D.W.; Awad, A.H. Fitoterapia., 1980, 51, 309-312.
- Rommelspacher, H. Pharmacopsychiatry., 1981, 14(04), 117-25.
CrossRef - Harsh, M.L.; and Nag, T.N. Journal of natural products. 1984, 47(2), 365-7.
CrossRef - Allen, J.R.; Holmstedt, B.R. The simple b-carboline alkaloids [a review]. Phytochemistry., 1980
CrossRef - Budavari, S.; O’Neil, M. J. The Merck Manual., 1996, 4644-4645.
- Abdel-Fattah, A.F.; Matsumoto, K.; Murakami, Y.; Gammaz, H.A.; Mohamed, M.F.; Watanabe, H. The Vascular System., 1997, 28(3), 405-9.
CrossRef - Abdel-Fattah, A.F.; Matsumoto, K.; Gammaz, H.A.; Watanabe, H. Pharmacology Biochemistry and Behavior., 1995, 52(2), 421-6.
CrossRef - Shi, C.C.; Liao, J.F.; Chen, C.F. Pharmacology and toxicology., 2001, 89(5), 259-64.
CrossRef - Moongkarndi, P.; Kosem, N.; Luanratana, O.; Jongsomboonkusol, S.; Pongpan, N. Fitoterapia., 2004, 75(3-4), 375-7.
CrossRef - Abourashed, E.A.; Plank, J.V.; Khan, I.A.. Pharmaceutical Biology., 2003, 41(2), 100-106.
CrossRef - Wiegrebe, W.; Kramer, W.J.; Shamma, M. (1984). Journal of Natural Products., 1984, 47(3), 397-408.
CrossRef - Grollman, A.P. Proceedings of the National Academy of Sciences of the United States of America., 1966, 56(6), 1867.
CrossRef - Grollman, A.P. Inhibitors of protein biosynthesis V. Effects of emetine on protein and nucleic acid biosynthesis in HeLa cells. Journal of Biological Chemistry., 1968, 243(15), 4089-94.
CrossRef - Neznanov, N.; Gorbachev, A.V.; Neznanova, L.; Komarov, A P.; Gurova, K V.; Gasparian, A V.; Banerjee, A K.; Almasan, A.; Fairchild, R.L.; Gudkov, A.V.; Cell cycle., 2009, 8(23), 3960-70.
CrossRef - Jacob, M.S.; Presti, D.E. Medical hypotheses., 2005, 64(5), 930-7.
CrossRef - Saavedra, J.M.; Axelrod, J. Science., 1972, 175(4028), 1365-1366.
CrossRef - Servillo, L.; Giovane, A.; Balestrieri, M.L.; Cautela, D.; Castaldo, D. J Agric Food Chem., 2012, 60, 9512-9518.
CrossRef - Pasquali, G.; Porto, D.D.; Fett-Neto, A.G. J Biosci Bioeng., 2006, 101(4), 287-96.
CrossRef - Nepokroeff, M.; Bremer, B.; Sytsma, K. Syst Bot., 1999, 24, 5-27.
CrossRef - Both, F.L.; Meneghini, L.; Kerber, V.A.; Henriques., AT.; Elisabetsky, E. J Nat Prod., 2006,69, 342-5.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









