Phytochemical Screening by GCMS Analysis of Leaf Extracts of Ocimum Sanctum & Mentha Arvensis in Different Organic Solvents
1School of Chemistry, Devi Ahilya Vishwavidyalaya, Indore (M.P.) India.
2Department of Chemistry, Govt. Holker Science College, Indore (M.P.) India.
Corresponding Author E-mail: anil.badore12@rediffmail.com
DOI : http://dx.doi.org/10.13005/ojc/400122
Article Received on : 01 Aug 2023
Article Accepted on :
Article Published : 14 Feb 2024
Reviewed by: Dr. Prasong Srihanam
Second Review by: Dr. Chitrasen Gupta
Final Approval by: Dr. Pounraj Thanasekaran
According to estimations from the World Health Organization (WHO), certain Asian and African people already utilize herbal medicine for some component of basic healthcare. In the current work, it was intended to perform phytochemical and FTIR analyses on the leaves of Mentha arvensis and Ocimum sanctum in various organic solvents. The findings of this study make it abundantly evident that ocimum sanctum and mentha arvensis leaves have saponins, flavanoids, steroids, alkaloids, phenols, Tannins, glycosides, and terpenoids in them when they underwent preliminary phytochemical examination. The current work uses FTIR spectroscopy to investigate the leaf extracts of two medicinal plants, Ocimum sanctum and Mentha arvensis, using water, ethanol, hexane, ethyl acetate, and benzene as solvents. The FTIR spectroscopy analyses identified numerous functional chemicals in the extracts with distinct distinctive peak values. The presence of amide, alcohols, phenols, alkanes, carboxylic acids, aldehydes, ketones, alkenes, primary amines, aromatics, esters, ethers, alkyl halides, and aliphatic amines compounds, which showed major peaks, was confirmed by FTIR analysis of ethanol and hexane leaf extracts of ocimum sanctum and mentha arvensis.
KEYWORDS:Mentha arvensis; Ocimum sanctum; Organic Solvents; phytochemical examination
Download this article as:| Copy the following to cite this article: Badore A, Pandit P, Nilosey V. Phytochemical Screening by GCMS Analysis of Leaf Extracts of Ocimum Sanctum and Mentha Arvensis in Different Organic Solvents. Orient J Chem 2024;40(1). |
| Copy the following to cite this URL: Badore A, Pandit P, Nilosey V. Phytochemical Screening by GCMS Analysis of Leaf Extracts of Ocimum Sanctum and Mentha Arvensis in Different Organic Solvents. Orient J Chem 2024;40(1). Available from: https://bit.ly/48fZ3tA |
Introduction
Since most medicinal plants have less adverse effects than synthetic medications, they are frequently utilised as complementary treatment for human and animal illnesses. Knowing the chemical makeup of the phytochemicals found in medicinal plants can help us understand the many functional groups behind those compounds’ therapeutic effects 1.
Due to their capacity to heal a range of illnesses, medicinal plants play a vital part in Siddha, Ayurveda, and the Unani school of medicine. The traditional medical system employs plant sources for medications that have no adverse effects on the human body, according to recent research employing animal models 2. Glucosides are a key metabolite involved in cellular activities. Secondary metabolites, which develop in response to stress and have a more complex structural composition, include alkaloids, flavonoids, terpenoids, steroids, and saponins 3. A critical phase of the drug development process is the screening for phytochemicals. Phytochemicals present in plants, such as saponins, flavanoids, steroids, alkaloids, phenols, Tannins, glycosides, and terpenoids, are a great source of new and exciting medications4-7. The current work, was intended to perform phytochemical and FTIR analyses on the leaves of Mentha arvensis and Ocimum sanctum in various organic solvents.
Methods of Extraction
The plants were gathered from the neighbourhood and washed thoroughly with water. The leaves were dried in shade and powdered for extraction. The dried powder of Mentha arvensis and Ocimum sanctum leaves was kept in contact with the different solvents in a stoppered container for 24 hours with frequent agitation until the soluble matter is dissolved. This method is best suitable for use in case of the thermolabile drugs.
Parameters for characterization
The different solvent extracts of Mentha arvensis and Ocimum sanctum leaves were analysed for the following parameters as shown in Table 1.
Table 1: Experimental methods for phytochemical analysis 8 – 14
|
Sr. no. |
Experiment |
Observation |
Inference |
|
|
1 |
Test for Alkaloids |
|||
|
Mayer’s Test |
Plant extract + 2 drops of Mayer’s reagent along the sides of the test tube |
White creamy ppt. |
Alkaloid present |
|
|
Wagner’s Test |
Plant extract + 2 drops of Wagner’s reagent along the sides of the test tube |
Reddish brown ppt. |
Alkaloid present |
|
|
2 |
Test for Glycosides |
|||
|
Borntrager’s Test |
Plant extract is hydrolysed with concentrated HCl for 2 hours on a water bath and filter. Filtered hydrolysate + 3ml of chloroform and shake well + 10% ammonia solution when chloroform layer is separated |
Pink colouration |
Glycosides present |
|
|
Legal’s Test |
Pyridine dissolved plant extract + sodium nitroprusside solution + 10% NaOH |
Pink colouration |
Glycosides present |
|
|
3 |
Test for Phenolic compounds |
|||
|
Gelatin Test |
Plant extract + 5ml distilled water + 2ml 1% gelatin + 10% NaCl solution |
White ppt. |
Phenolic compound present |
|
|
Lead acetate Test |
Plant extract +distilled water + 3ml 10% lead acetate |
Bulky white ppt. |
Phenolic compound present |
|
|
4 |
Test for Tannins |
|||
|
Ferric Chloride Test |
Dissolve 50mg of pulverized sample in 5ml distilled water and filter + 1% FeCl3 solution |
Brownish green or Blue black colouration |
Tannins present |
|
|
5 |
Test for Flavonoids |
|||
|
Alkaline Reagent Test |
Plant extract + distilled water + 10% NH4OH solution |
Yellow fluorescence |
Flavonoids present |
|
|
Magnesium and HCl Reduction Test |
Alcoholic plant extract + a few fragments of Mg ribbon and concentrated HCl dropwise |
Pink to crimson colouration |
Flavanol glucosides present |
|
|
6 |
Test for Phytosterols |
|||
|
Libermann-Burchard’s Test |
Plant extract + 2ml acetic anhydride + 2 few drops of concentrated H2SO4 along the test tube’s sidewalls |
An array of colour change |
Phytosterols present |
|
|
Ethanolic plant extract + 5ml hot water and allowed to stand for 1hr. and filtered. Using a separating funnel and 2.5ml of chloroform, the filtrate was extracted 0.5 ml of chloroform extract + 1 ml concentrated H2SO4 |
brownish-red interface |
Steroids present |
||
|
7 |
Test for Terpenoids |
|||
|
Ethanolic plant extract + 5ml of boiling water is added, let to stand for 1 hour, and then filtered. A separating funnel was used to extract the filter with 2.5 ml of chloroform. On a water bath, 0.5 ml of chloroform extract is evaporated to dryness + 3ml concentrated H2SO4 + heat |
Grey colouration |
Terpenoids present |
||
|
8 |
Test for Saponins |
|||
|
Plant extract + 20 ml distilled water and boil in the water bath. After 50% reduction shakes vigorously to obtain stable persistent froth. Froth + olive oil |
Emulsion formation |
Saponins present |
||
Results and Discussions
Phytochemical analysis
Tables 2 and 3 in the current study present the results of phytochemical analyses of the following plants: Ocimum sanctum and Mentha arvensis. The positive sign (+) denotes the presence of a specific phytochemical component in plants, whereas the negative sign (-) denotes the absence of that component in plants. Depending on the different extraction solvents (water, ethanol, hexane, ethyl acetate and benzene) utilised, these phytochemical components in various plants exhibit varying results. Saponins, flavanoids, steroids, alkaloids, phenols, Tannins, glycosides, and terpenoids are thought to indicate strong therapeutic value.
Table 2: Phytochemical analysis for different Ocimum sanctum leaf extracts
|
Sr. No. |
|
1. Ocimum sanctum leaf extract |
||||
|
Phytochemicals |
Ethanol |
Hexane |
Water |
Ethyl acetate |
Benzene |
|
|
1 |
Saponins |
+ |
+ |
+ |
+ |
+ |
|
2 |
Flavanoids |
+ |
+ |
+ |
+ |
+ |
|
3 |
Steroids |
+ |
+ |
– |
– |
– |
|
4 |
Alkaloids |
– |
+ |
+ |
– |
– |
|
5 |
Phenols |
+ |
+ |
– |
+ |
– |
|
6 |
Tannins |
+ |
– |
– |
– |
– |
|
7 |
Glycosides |
+ |
+ |
+ |
+ |
+ |
|
8 |
Terpenoids |
+ |
+ |
+ |
+ |
+ |
Table 3: Phytochemical analysis for different Mentha arvensis leaf extracts
|
Sr. No. |
|
2. Mentha arvensis leaf extract |
||||
|
Phytochemicals |
Ethanol |
Hexane |
Water |
Ethyl acetate |
Benzene |
|
|
1 |
Saponins |
+ |
+ |
+ |
+ |
+ |
|
2 |
Flavanoids |
+ |
+ |
+ |
+ |
+ |
|
3 |
Steroids |
– |
+ |
– |
– |
– |
|
4 |
Alkaloids |
+ |
+ |
+ |
+ |
+ |
|
5 |
Phenols |
+ |
– |
+ |
– |
– |
|
6 |
Tannins |
+ |
+ |
+ |
– |
– |
|
7 |
Glycosides |
+ |
+ |
– |
+ |
+ |
|
8 |
Terpenoids |
+ |
+ |
+ |
+ |
+ |
The presence of saponins, flavanoids, steroids, alkaloids, phenols, Tannins, glycosides, and terpenoids indicates high medicinal value. The phytochemical analysis of all the plants indicate that all the selected plants have medicinal value and strong potential to be valuable treatments for improving human health. Using chromatographic and spectroscopic methods, the current work leads to future research in the separation and identification of the active compound from the selected plants. Considering the presence of maximum phytochemicals in ethanol and hexane extracts, the following FTIR analysis was done for ethanol and hexane extracts only.
GCMS Analysis
The GCMS result show no presence of any heterocyclic compounds as in case of alkaloids and carbohydrates. The presence of – O – linkage in the different structures obtained indicates the presence of glycosides. None of the extracts contain steroids and Tannins. The presence of alkaloids is confirmed in mentha plant by the presence of amines.
The GCMS study for all the plant extracts except the Ethanol extract of Ocimum shows the presence of long chain hydrocarbons indicating the presence of higher fatty acids and higher esters.
The GCMS chromatograms obtained for ethanol and hexane extracts of Ocimum and Mentha plant are shown in the following tables respectively.
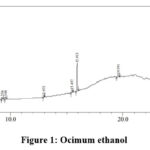 |
Figure 1: Ocimum ethanol. |
Table 4: Ocimum ethanol extract analysis
|
Sr.no. |
Name |
Molecular Weight |
R.Time |
|
1 |
Benzyl 2 chloroethyl sulfone; Idomethyl benzene |
218 |
12.973 |
|
2 |
2-methoxy-3-(2-propenyl)-phenol; Eugenol |
164 |
15.457 |
|
3 |
Methyl eugenol |
178 |
15.914 |
|
4 |
Caryophyllene oxide |
220 |
19.591 |
|
5 |
Neophytadiene; 3,7,11,15-tetramethyl-2-hexadecan-1-ol; phytol acetate |
278, 296, 338 |
23.931 |
The peak at 15.457 minutes indicates the presence phenolic compound, flavonoids and the glycosidic linkage. The presence of flavonoids is further confirmed by the peak obtained at 15.914 minutes. The peaks obtained at 19.591 and 23.931 minutes indicate the presences of terpenoids and saponins.
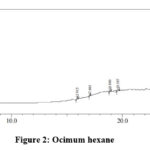 |
Figure 2: Ocimum hexane |
Table 5: Ocimum hexane extract analysis
|
Sr.no. |
Name |
Molecular Weight |
R.Time |
|
1 |
Methyl eugenol |
178 |
15.916 |
|
2 |
Bicyclo[3.1.1]hept-3-en-2-one,4,6,6-trimethyl; 4-hydroxy-2,6,6-trimethylcyclohex-1-ene carbaldehyde |
150, 168 |
17.061 |
|
3 |
Cyclopropane-1-chloro-2,2-dimethyl-3-(3,3-dimethyl-1-butynl); 1-cyclohex-1-enyl-1-phenyl ethanol |
184, 202 |
18.86 |
|
4 |
Caryophyllene oxide; Methyl (z)-5,11,14,17-eicosateraenoate |
220, 318 |
19.588 |
|
5 |
Neophytadiene; 3,7,11,15-tetramethyl-2-hexadecan-1-ol; phytol acetate |
278, 296, 338 |
23.934 |
|
6 |
Neophytadiene; 3,7,11,15-tetramethyl-2-hexadecan-1-ol; phytol acetate |
278, 296, 338 |
25.11 |
|
7 |
Ethyl 13-methyl-tetradecanoate; Hexadecanoic acid ethyl ester |
270, 284 |
28.162 |
|
8 |
Nonadecene; 9-Tricosene(z); Behenic alcohol |
266, 322, 326 |
28.681 |
The peaks obtained at 15.916 and 17.061 minutes clearly indicates the presence of flavonoids. The peak with molecular weight 168 indicates the presence of phenolic compound. The peaks at run time 19.588 and 23.934 shows the presence of terpenoids and saponins. The presence of glycosidic linkages can be confirmed from peaks at 15.916, 19.588 and 28.162.
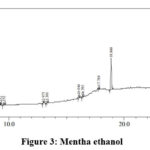 |
Figure 3: Mentha ethanol. |
Table 6 : Mentha ethanol extract analysis
|
Sr.no. |
Name |
Molecular Weight |
R.Time |
|
1 |
Propanamide, 2-methoxy-N-methyl-; 2,2-diethoxy-propane |
117, 132 |
5.043 |
|
2 |
2,2-dimethyl cyclopropoane carboxylic acid, cyanomethyl ester; 2-Butenamide, N-(aminocarbonyl)-2-ethyl-(z)-; Cyclopropane carboxylic acid, 3-formyl-2,2-diethyl-, ethyl ester |
153, 156, 170 |
9.552 |
|
3 |
Benzyl 2 chloroethyl sulfone |
218 |
12.971 |
|
4 |
8,9-dehydrothymol; Propanal, 2-methyl-3-phenyl-; Benzene, 1-methoxy-2-(1-methylethenyl)- |
148 |
13.301 |
|
5 |
Cyclopentene, 1-isopropyl-2,3-dimethyl-; Bicyclo[3.2.1]octan-3-one, 6-(2-hydroxyethyl)-endo- |
138, 168 |
16.046 |
|
6 |
2-cyclopentene-1-one, 2-(2-butenyl)-4-hydroxy-3-methyl-(z)- |
166 |
16.394 |
|
7 |
Propanoic acid, 2-bromo-ethyl ester; 2-Octene-1-ol, 7-ethoxyl-3,7-dimethy-(E)-; 5,6-dihydroxy-5-isopropyl-6-methyl-hept-3-en-2-one |
166, 200 |
17.789 |
|
8 |
Cyclopropane-1-chloro-2,2-dimethyl-3-(3,3-dimethyl-1-butynl); Myrtene acid chloride |
184 |
18.86 |
|
9 |
Neophytadiene; 3,7,11,15-tetramethyl-2-hexadecan-1-ol; phytol acetate |
278, 296, 338 |
23.927 |
|
10 |
Neophytadiene; 3,7,11,15-tetramethyl-2-hexadecan-1-ol; phytol acetate |
278, 296, 338 |
25.105 |
|
11 |
Ethyl 13-methyl-tetradecanoate; Hexadecanoic acid ethyl ester |
270, 284 |
28.149 |
|
12 |
Nonadecene; n-Nonadecanol-1; 9-Tricosene(z) |
266, 284, 322 |
28.67 |
The peaks at 5.043 and 9.552 minutes shows the presence of alkaloid whereas peak at 3.301 minutes indicate the presence of phenol. The presence of glycosidic linkages can be seen in the peaks obtained at run time 5.043, 13.301, 17.789 and 28.149 minutes. Flavanoids are obtained at 13.301, 16.394 and 17.789 minutes. The presence of terpenes is clearly indicated by the peaks at 13.301, 16.046, 18.86 and 25.105 minutes. The peak obtained at 28.67 minutes shows the presence for long chain hydrocarbons.
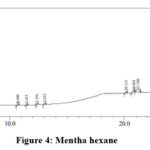 |
Figure 4: Mentha hexane |
Table 7: Mentha hexane extract analysis
|
Sr.no. |
Name |
Molecular Weight |
R.Time |
|
1 |
1-Undecene, 4-methyl-; Decane, 3,7-dimethyl-; Undecane, 4,7-dimethyl |
168, 170, 184 |
10.688 |
|
2 |
Octane, 6-ethyl-2-methyl-; Dodecane, 2,6,11-trimethyl-; Sulfurous acid, nonyl-2-propyl ester |
156, 212, 250 |
11.458 |
|
3 |
Benzenamine, 2,5-dimethyl- |
121 |
12.354 |
|
4 |
Azulene, Naphthalene |
128 |
13.042 |
|
5 |
Ar-Tumerone; Cinnamyl angelate, E- |
216 |
20.125 |
|
6 |
n-propyl, 9,12-hexadocadienoate; Linolcic acid ethyl ester |
294, 308 |
20.833 |
|
7 |
9,12,15-Octadecatrienoic acid ethyl ester |
306 |
21.188 |
|
8 |
Ethyl 13 methyl-tetradecanoate; Octadecanoic acid, 17-methyl-methyl ester |
270, 312 |
22.833 |
|
9 |
Neophytadiene; 3,7,11,15-tetramethyl-2-hexadecan-1-ol; phytol acetate |
278, 296, 338 |
23.917 |
|
10 |
Nonadecene; Pentacos-1-ene |
266, 350 |
28.667 |
From the above table it is found that the peaks at 10.688, 11.458 and 23.917 minutes confirms the presence for terpenoids and saponins. The peaks at 20.125, 20.833, 21.188 and 22.833 minutes indicate the presence of glycosidic linkages. The alkaloid is shown by the peak at 12.354 minutes. The peak obtained at 28.667 minutes shows the presence for long chain hydrocarbons.
Conclusion
The phytochemicals that medicinal plants generate may end up being effective therapeutic agents for promoting human health. More and more, research on the phytochemical composition of plants is being used as the initial step in the search for powerful medications. The current research sets the door for future research utilizing the GCMS analysis to isolate and identify the active chemical from the selected plants. The two plants Ocimum sanctum and Mentha arvensis have very strong medicinal potential.
Acknowledgement
Authors are thankful to Department of Chemistry, Holker Science College, Indore for providing the place and necessary equipment to carry out the research work.
Conflict of Interest
The authors show no conflict of interest.
References
- Hanumanthaiah P., Hazaratali P., Chebte A., Haile A. and Belachew G. T., Tulsi (Ocimum sanctum) – a myriad medicinal plant, secrets behind the innumerable benefits. Arabian Journal of Medicinal & Aromatic Plants, 2020, 1, 105-127.
- Bhoora H. S.G., Srivastava N. G., Gargi B, Joshi M., Comparative study of antibacterial assay of Mentha piperita (in vivo and in vitro cultured) leaves extract on enveloped human pathogenic bacteria and its phytochemical screening. J Pharmacogn Phytochem, 2020, 9, 15-19.
CrossRef - Sharma M., Gautam D., Phytoconstituents and medicinal value of Mentha piperita. Modern Phytomorphology 2022, 15, 156–160.
- Bodalska A., Kowalczyk A., Włodarczyk M., Fecka I., Analysis of polyphenolic composition of a herbal medicinal product-peppermint tincture. Molecules 2020, 25, 69. https://doi.org/10.3390/ molecules25010069
CrossRef - Miya M.S., Timilsina S., Chhetri A., Ethnomedicinal uses of plants by major ethnic groups of hilly districts in Nepal. J Med Bot, 2020, 4, 24-37.
CrossRef - Poudel B., Bhandari J., Poudel A., Gautam D., Ethnomedicinaluse of common garden species in Arghakhanchi district, Western Nepal. Asian J Pharmacognocy, 2021, 4, 31-65.
- Pariyar D, Miya M.S, Adhikari A., Traditional uses of locally available medicinal plants in Bardiya district, Nepal. J Med Herbs, 2021, 12, 85-92. https://dx.doi.org/10.30495/medherb.2021.684660
- Mahendran G., and Rahman L. U., Ethnomedicinal, phytochemical and pharmacological updates on Peppermint (Mentha × piperita L.)-A review, Phytotherapy Research, 2020, 34, 2088–2139.
CrossRef - Jaber H., Oubihi A., Ouryemchi I., Chemical composition and antibacterial activities of eight plant essential oils from Morocco against Escherichia coli strains isolated from different Turkey organs, Biochemistry Research International, 2021, 20.
CrossRef - Eftekhari A., Khusro A., Ahmadian E., Dizaj S. M., Hasanzadeh A., Cucchiarini M., Phytochemical and nutra-pharmaceutical attributes of Mentha spp.: A comprehensive review. Arabian Journal of Chemistry, 2021, 14, 103106.
CrossRef - Agidew M. G., Phytochemical analysis of some selected traditional medicinal plants in Ethiopia. Agidew Bulletin of the National Research Centre, 2022, 46, 87 https://doi.org/10.1186/s42269-022-00770-8
CrossRef - Aung E.E, Kristanti A.N, Aminah N.S, Takaya Y, Ramadhan R., Plant description, phytochemical constituents and bioactivities of Syzygium genus: a review. Open Chem 2020, 18:1256–1281.
CrossRef - Kebede T, Gadisa E, Tufa A., Antimicrobial activities evaluation and phytochemical screening of some selected medicinal plants: a possible alternative in the treatment of multidrug-resistant microbes. (2021a) https://doi. org/10.1371/journal.pone.0249253.
CrossRef - Taye K, Eshetu G, Abreham T., Antimicrobial activities evaluation and phytochemical screening of some selected medicinal plants: a possible alternative in the treatment of multidrug-resistant microbes. (2021b) https://doi. org/10.1371/journal.pone.0249253.

This work is licensed under a Creative Commons Attribution 4.0 International License.










