Phytochemical Screening and Antihyperglycemic Effects of Stevia rebaudiana Leaves Extract on Glucose Loaded Rats
Sharmin Jamal1† , Suman Barua1†,*
, Suman Barua1†,* , Abhijit Barua1
, Abhijit Barua1 , A. J. M. Morshed2 Rasheda Akter2
, A. J. M. Morshed2 Rasheda Akter2 and Shireen Akhter3
and Shireen Akhter3
1Department of Applied Chemistry and Chemical Engineering, University of Chittagong, Chittagong 4331, Bangladesh.
2Bangladesh Council of Scientific and Industrial Research, Chattogram-4220, Bangladesh.
3Chattogram Veterinary and Animal Sciences University, Chattogram, Bangladesh.
Corresponding Author E-mail: sumanbarua@cu.ac.bd
DOI : http://dx.doi.org/10.13005/ojc/400109
Article Received on : 17 Nov 2023
Article Accepted on : 29 Jan 2024
Article Published : 14 Feb 2024
Reviewed by: Dr. Sai Smitha
Second Review by: Dr. Sanjay kumar
Final Approval by: Dr. Charnjeet Kaur
This study focused on Stevia rebaudiana, a plant known for its sweet taste and unique medicinal properties in managing diabetes complications. The research aimed to evaluate the antihyperglycemic potential of crude ethanolic and aqueous extracts from Stevia rebaudiana leaves, utilizing the Oral Glucose Tolerance Test (OGTT) on albino rats subjected to glucose loading. Additionally, a thorough phytochemical analysis was conducted to identify essential secondary metabolites present in the extracts. The study involved five groups, each comprising equal number of male Wistar albino rats. Groups II, III, IV, and V received an oral solution of 8 gm/kg glucose. Group IV was administered a 2 gm/kg ethanolic extract, while Group V received a 2 gm/kg aqueous extract. Blood glucose levels (BGL) were monitored at specified intervals of 30, 60, 90, and 120 minutes. Phytochemical screening confirmed the presence of various phytoconstituents in the extracts. The ethanolic extract demonstrated a 39.49% reduction in blood glucose levels, and the aqueous extract exhibited a 35.39% reduction. Both extracts from Stevia rebaudiana leaves displayed significant antihyperglycemic effects in glucose-loaded rats after 120 minutes.
KEYWORDS:Antihyperglycemic; Extracts; Phytochemical Test; Stevia Rebaudiana
Download this article as:| Copy the following to cite this article: Jamal S, Barua S, Barua A, Morshed A. J. M, Akter R, Akhter S. Phytochemical Screening and Antihyperglycemic Effects of Stevia rebaudiana Leaves Extract on Glucose Loaded Rats. Orient J Chem 2024;40(1). |
| Copy the following to cite this URL: Jamal S, Barua S, Barua A, Morshed A. J. M, Akter R, Akhter S. Phytochemical Screening and Antihyperglycemic Effects of Stevia rebaudiana Leaves Extract on Glucose Loaded Rats. Orient J Chem 2024;40(1). Available from: https://bit.ly/49eDmeD |
Introduction
Diabetes mellitus is a non-communicable ailment marked by an impairment in the body’s capacity to control blood sugar levels. This disorder occurs when the body either lacks the production of sufficient insulin or is unable to efficiently utilize the insulin it generates, often due to flaws in insulin secretion or dysfunction of cellular receptors. Insulin, a crucial metabolic hormone, is typically synthesized by the β-cells located in the islets of Langerhans within the pancreas. Its primary function is to enable the entry of glucose into cells by binding to specific cellular receptors.
In contrast to type 1 diabetes, where insulin production is entirely absent, type 2 diabetes is distinguished by the existence of insulin production; however, the body struggles to utilize it effectively. This dysfunction leads to the accumulation of excess glucose in the bloodstream, causing hyperglycaemias 1-3. Alarming statistics from 2019 estimated that approximately 463 million people worldwide were affected by diabetes. Projections indicated that this number could skyrocket to 700 million by 2045, underscoring the global urgency of addressing this health issue 1. Throughout history, medicinal plants have played a crucial role in traditional medicine across diverse cultures. These plants have been relied upon as natural sources of remedies for various ailments, including diabetes5. Among the numerous plant species used in the treatment of diabetes, Stevia rebaudiana stands out as one of them being used traditionally6. About two hundred species of herbs and shrubs belong to the genus Stevia, which is part of the sunflower family (Asteraceae). Stevia belongs to Asteraceae family and has too many species, among them S. rebaudiana Bertoni is the sweetest of all 7,8. Steviol glycosides, make up most stevia’s ingredients and give it its sweetness. The main diterpene glycosides in stevia are stevioside, steviolbioside, rebaudioside A, B, C, D, E, F and dulcoside A, Among them stevioside gives 200-250 times more sweetness than any other table sugar9-12. It is said that stevioside is the responsible antidiabetic agent in Stevia rebaudiana 13. However, a variety of factors, prominent among them environmental factors, have an impact on the quantity and quality of these chemical metabolites in plants. This review provides an overview of plant-produced chemical compounds with medicinal properties and how their production is affected by different environmental factors. Environmental factors (Such as light, elements in soil, temperature, moisture, etc.) secondary metabolites, active substances and their concentrations in the plant can vary from one region to another 14,15. Other than stevia there are also many plant species that are used as antidiabetic medicines traditionally, but the specificity of stevia is that it is not only used to treat diabetes but also used as non-caloric sugar substitute 16. According to some research it has also properties of antihypertensive, anticancer, antioxidant, etc. As it contains many phytoconstituents 17. Consequently, this research endeavours to evaluate the antihyperglycemic effects of Stevia rebaudiana in rats and to elucidate the presence of valuable phytoconstituents within its extracts.
Materials and methods
Plant materials
The fresh leaves of Stevia rebaudiana were collected from the Bangladesh Council of Scientific and Industrial Research (BCSIR), Chattogram. The leaves were washed properly and dried in the mild heat of the sun. The dried leaves were ground to a fine powder with the help of a grinder. Powders were freshly made to prepare the aqueous and ethanolic extract.
Preparation of extracts
Ethanolic extract
For the preparation of ethanolic extract 60grams of freshly prepared stevia powder was mixed with 1000 ml of 99% ethanol. The mixtures were kept in a round bottle flaskfor two days. Then it was filtered with Whatman filter paper. Filtrate was fed into a cyclone separator where the solvent evaporates and made the filtrate more concentrated. Then it was kept in an oven below 40°C until the extract became thick 18.
Aqueous extract
60grams of freshly prepared stevia powder was mixed with 800 ml water, filtered, and then fed into a cyclone separator. The concentrated filtrate was kept in an oven below 40°C to get a thick aqueous extract 19.
Phytochemical screening
The crude aqueous and ethanolic extracts were screened to find out the presence of phytoconstituents such as alkaloids, steroidal compounds, phenolic compounds, flavonoids, saponins and tannins. Standard procedures were followed to perform phytochemical screening.
Identification of alkaloids (Dragendroff’s test)
100 mg of both ethanolic and aqueous extract was dissolved in dilute hydrochloric acid. The solution was clarified by filtration. Filtrate was assessed with Dragendroff’s reagents (Bismuth nitrate, Hydrochloric acid, Potassium iodide). If any orange-red precipitate is observed, the treated solutions are expected to contain alkaloids.
Identification of steroidal compounds (Salkowski’s test)
0.5 grams of the ethanolic and aqueous extracts were dissolved in 2 ml of chloroform in a test tube to identify steroidal chemicals. To create a lower layer, concentrated sulphuric acid was carefully applied to the test tube’s wall. The presence of a steroid ring was confirmed by the reddish-brown hue at the interface.
Identification of phenolic compounds (Ferric chloride test)
Distilled water was mixed separately with 0.5 grams of both extracts. To identify phenolic compounds, three drops of a freshly made combination of 1 ml of 1% ferric chloride and 1 ml of potassium ferro cyanide were then added. The presence of phenolic compounds was demonstrated by the formation of bluish-green colour.
Identification of flavonoids
Free flavonoids test
A mixture of 0.5 grams of each extract blended with five millilitres distilled water and five millilitres of ethyl acetate was used to create the solution. It was shaken and left to settle. The organic layer was next checked for the generation of yellow colour, which indicated the existence of free flavonoids.
Lead acetate test
A 10% lead acetate solution was added to a 0.5 grams solution of the ethanolic and aqueous extract in distilled water. If a yellow precipitate forms, flavonoids have been confirmed as present.
Reaction with sodium hydroxide
0.5 grams of each extract were dissolved in distilled water, and then a diluted sodium hydroxide solution was added. The appearanceof yellow colour, which is thought to be a favourable reaction for flavonoids, was assessed in the mixture.
Identification of saponins (Froth test)
In two separate test tubes,0.5 grams of each extract were dissolved in 10 cubic centimetresof distilled water. After aggressively shaking the test tubes for around 30 seconds with a stopper, they were left to stand for 30 minutes and were kept under observation in vertical position. After 30 minutes, if a honeycomb like froth is still visible above the surface, saponins are thought to be present in the sample.
Identification of tannins (Ferric chloride test)
The tannin test can be confirmed by performing ferric chloride, formaldehyde, and modified iron complex tests. In this research work ferric chloride test was done. 0.5 grams of both extracts were taken in different test tubes and then mixed with distilled water. A 10% ferric chloride solution was added after the solution was filtered. This was seen as the colour changed to bluish black.
Acute toxicity test
The study followed a well-structured experimental design by using two diverse types of stevia extracts (ethanolic and aqueous) and three different dosage levels to assess acute toxicity. The use of thirty mice, divided equally between the two extract groups, ensures a sufficiently large sample size to draw meaningful conclusions from the experiment. The 14-day observation period is suitable for assessing acute toxicity, as it allows for the detection of any immediate adverse effects. Three different dosage levels 4 gm/kg, 2 gm/kg, and 1 gm/kg were administered, and the mice were closely monitored for any signs of mortality, significant behavioural changes, or toxic effects throughout the observation period [25]. The highest dose of 4 gm/kg is particularly important as it represents a significant dose.
Experimental animal
Thirty male Wistar albino rats, each weighing an average of 200 g, were purchased from the BCSIR Laboratories’ animal house in Chattogram. For the studies, the animals were housed in cages with conventional laboratory settings (temperature 24°C, relative humidity 55%). The animals received conventional food and unlimited access to water. The average diet had the following ingredients: wheat (40–32%), wheat bran (8–28%), dry fish powder (8–10%), oil cake (10–19%), pens (10–19%), milk powder (4%), soy oil (1%), rice powder (5%), salt (1%), molasses (1%), minerals (1.01%), and vitamins (1%) 20.
Experimental protocol
To perform the work 30male Wistar albino rats were divided into five experimental groups which were marked as Group I to V. Six rats were taken into each group. Group I, Group II and Group III were named normal control, diabetic control, and positive control group, respectively. Group IV and Group V were recognized as treated groups for ethanolic extract and aqueous extract of Stevia rebaudiana leaves, respectively.
Feeding and checking BGL
In this research work evaluation of the antihyperglycemic activity of stevia ethanolic and aqueous extracts were done by OGTT (Oral Glucose Tolerance Test). The rats were fasted for 16 hours. The fasting BGL of all the rats in the groups were recorded using a glucometer 21,22. Blood was collected from the tip of the tail. For the rats of the normal control group (Group I), 2 ml of distilled water was supplied. Rats of Group II, Group III, Group IV, and Group V were administered 8 gm/kg (body weight) glucose solution orally. The BGLs of all the rats were recorded again, after 30 minutes of glucose administration. Glucose-loaded rats of the positive control group, Group III was administered orally a reference antidiabetic drug named glibenclamide at a dose of 4 mg/kg. The ethanolic and the aqueous extract were administered orally at the dose of 2 gm/kg (body weight) on glucose-loaded rats of Group IV (ethanolic extract treated) and Group V (aqueous extract treated). Again, the BGLs of the rats were recorded after 30, 60, 90 and 120 minutes23.
Results and discussions
Yield of plant extracts
In the process of obtaining extracts from dried leaf powder, we initially utilized 60grams of the powder for both the aqueous and ethanolic extraction methods. The outcome of these procedures revealed intriguing differences in the yields of the respective extracts. A total of 26.4 grams of aqueous extract was obtained. On the other hand, when employing the ethanolic extraction method with the same initial quantity of 60grams of dried leaf powder, the resulting extract weighed 32.4 grams. This suggests that the ethanolic solvent was able to extract a larger quantity of soluble compounds from the leaf powder compared to the aqueous solvent. To express these findings in terms of yield, we can calculate the yield percentages for both the ethanolic and aqueous extracts. The yield for the ethanolic extract is found by dividing the final extract weight (32.4 grams) by the initial dried leaf powder weight (60 grams), which results in a yield of 54%. In contrast, the yield for the aqueous extract is calculated in the same manner, yielding a lower percentage of 44%. These divergent yields highlight the varying abilities of the two solvents to extract compounds from the leaf powder. The higher yield obtained with the ethanolic extraction method suggests that it was more efficient at extracting soluble compounds from the plant material compared to the aqueous extraction method, which yielded a lower percentage of extract from the same starting material. This information could be significant in selecting the most suitable extraction method for specific research or industrial applications.
Phytochemical group test
A comprehensive phytochemical analysis was conducted on aqueous and ethanolic extracts of Stevia rebaudiana leaves to investigate the presence of secondary metabolites. The findings from the phytochemical group tests have been documented in Table 1. It is noteworthy that there are notable similarities between these results and those reported in references 24,25.
The phytochemical examination of Stevia rebaudiana leaves revealed the presence of several important secondary metabolites, including alkaloids, steroidal compounds, phenolic compounds, flavonoids, and saponins. These compounds were found in both the aqueous and ethanolic extracts, indicating their robust presence in this plant species. Presence of flavonoids wasin trace amount for aqueous extract. However, it is worth mentioning that the presence of tannins was only detected in trace amounts in both extracts which was different from the reference24 except for aqueous extract in potassium dichromate. This study’s results not only confirm the presence of these secondary metabolites in Stevia rebaudiana but also align with previous research findings, further validating the chemical composition of this valuable plant species. The presence of these phytochemicals underscores the potential medicinal and nutritional significance of Stevia rebaudiana leaves, making them a subject of interest for various applications in the food and pharmaceutical industries. Further research and exploration of these compounds may uncover their specific health benefits and potential therapeutic uses.
The phytochemical observations of Stevia rebaudiana leaves revealed the presence of several important secondary metabolite groups in both the aqueous and ethanolic extracts. These included alkaloids, steroidal compounds, phenolic compounds, flavonoids, and saponins. Importantly, these metabolites were found to be present in at least one of the two extracts, if not in both. Alkaloids, which are known for their diverse pharmacological activities, were detected in both extracts. Steroidal compounds, which often play significant roles in various physiological processes, were also found in both extracts. Phenolic compounds, known for their antioxidant properties and potential health benefits, were present in both extracts as well. Flavonoids, another group of compounds with recognized health-promoting properties, were identified in both the aqueous and ethanolic extracts. Saponins, which have been associated with various biological activities, were likewise detected in both extracts, indicating the potential utility of Stevia rebaudiana in traditional medicine and other applications. Interestingly, while these secondary metabolites were found in appreciable amounts in both extracts, the presence of tannins was observed only in trace amounts in both the aqueous and ethanolic extracts. Tannins are known for their astringent properties and are often associated with certain health benefits, including anti-inflammatory effects. The limited presence of tannins in the extracts suggests that Stevia rebaudiana may not be a significant source of these compounds, at least under the conditions of this study. The phytochemical analysis of Stevia rebaudiana leaves revealed the presence of several valuable secondary metabolite groups, including alkaloids, steroidal compounds, phenolic compounds, flavonoids, and saponins. These findings align with existing references and underscore the potential utility of Stevia rebaudiana in various applications, particularly in traditional medicine and the development of health-promoting products. Additionally, the limited presence of tannins highlights the specificity of the secondary metabolites present in this plant species.
Table 1: Results of Phytochemical group test of the extracts of Stevia rebaudiana leaves.
|
Name of the test |
Reagents |
Aqueous extract |
Ethanolic extract |
|
Test for alkaloids |
Dragendorff’s |
++ |
+++ |
|
Test for steroidal compound |
Chloroform and concentrated sulphuric acid |
+++ |
++ |
|
Test for phenolic compound |
Ferric chloride and potassium ferrocyanide |
+++ |
+++ |
|
Test for flavonoids |
NaOH |
++ |
|
|
Test for saponins |
Froth test |
+++ |
++ |
|
Test for tannins |
Ferric chloride |
+++ indicates strongly positive, ++ moderately positive and indicates trace amount, respectively.
Acute toxicity test
Remarkably, none of the mice experienced mortality, abnormal behaviour such as convulsions, or diarrhoea, even when administered the highest dosage of 4 gm/kg.
The results are clear and concise, indicating that neither the ethanolic nor the aqueous stevia extracts caused mortality, abnormal behaviour, or diarrhoea at any of the tested dosage levels. The study’s findings suggest that both ethanolic and aqueous stevia extracts are well-tolerated by Swiss albino mice at the tested acute dosage levels. This information is valuable for assessing the safety of these extracts for potential human consumption. It is important to note that results in mice may not directly translate to human responses. Further research, including chronic toxicity studies and human trials, may be needed to establish safety for human consumption fully. The study demonstrates that ethanolic and aqueous stevia extracts, even at relatively high doses, do not exhibit acute toxicity or adverse effects in Swiss albino mice. This information contributes to the understanding of the safety profile of these extracts and may inform their potential use in various applications, including food and beverage products.
Effect of stevia extracts by OGTT on glucose-loaded rats
In this study, we have documented the outcomes of an Oral Glucose Tolerance Test (OGTT) using two different extracts, namely, the ethanolic extract and the aqueous extract of Stevia rebaudiana leaves, on Wistar albino rats. The summarized results are presented in Table 2. During the initial observations, fasting blood glucose levels were measured for various groups, including normal, diabetic, positive control, ethanolic extract-treated, and aqueous extract-treated rats. The respective BGLwere recorded as follows: 4.18 mmol/L for normal rats, 3.22 mmol/L for diabetic rats, 3.78 mmol/L for positive control rats, 3.8 mmol/L for ethanolic extract-treated rats, and 3.76 mmol/L for aqueous extract-treated rats. Subsequently, after the administration of glucose, the BGL of the diabetic, positive control, ethanolic extract-treated, and aqueous extract-treated rats increased. These values were measured as 8.06 mmol/L, 9.44 mmol/L, 7.9 mmol/L, and 8.08 mmol/L, respectively. Following this, the study involved three distinct groups: Group III received glibenclamide (4 mg/kg) orally, Group IV received ethanolic extract (2 gm/kg), and Group V received aqueous extract (2 gm/kg). After 30 minutes, their blood glucose levels were recorded, with the respective BGLs for these groups being 6.1 mmol/L, 7.16 mmol/L, and 7.5 mmol/L. Further measurements were taken at 30-minute intervals up to 120 minutes. After 120 minutes, the blood glucose levels for each group were as follows: Group I – 4.08 mmol/L, Group II (diabetic control) – 7.1 mmol/L, Group III (positive control) – 3.9 mmol/L, Group IV (ethanolic extract-treated) – 4.78 mmol/L, and Group V (aqueous extract-treated) – 5.22 mmol/L. To analyse the effectiveness of the treatments, the percentage decrease in blood glucose levels compared to the baseline (0 minutes) for Group III, Group IV, and Group V was graphically represented in Figure 1. Notably, at the 120-minute mark, the positive control (Glibenclamide) lowered the BGL by 58.68%, Stevia rebaudiana ethanolic extract reduced it by 39.49%, and Stevia rebaudiana aqueous extract reduced it by 35.39%. Figure 2 illustrates the percentage decrease in blood glucose levels compared to the diabetic control group. At 120 minutes, compared to the diabetic control group, the positive control (Group III) lowered blood glucose levels by 45.07%, Stevia rebaudiana ethanolic extract (Group IV) reduced BGL by 32.67%, and aqueous extract (Group V) lowered BGL by 26.47%. These findings suggest that both the ethanolic and aqueous extracts of Stevia rebaudiana leaves possess potential hypoglycaemic properties, albeit with varying degrees of effectiveness compared to the positive control (Glibenclamide) in this experimental model. It is important to mention that even if stevia does not reduce blood glucose significantly at least it does not increase blood glucose after consumption despite being sweet; also it does not have excessive hypoglycaemic effect which is also fatal to any living being. Further research and investigation are warranted to elucidate the mechanisms and potential therapeutic applications of these extracts in managing diabetes.
Table 2: Effects of ethanol and aqueous extracts of Stevia rebaudiana on OGTT
|
Group |
Treatment |
Mean Blood Glucose concentration (mmol/L) |
|||||
|
fasting BGL |
BGL after 30 min Glucose feeding |
BGL after extract administration |
|||||
|
0 min |
30 min |
60 min |
90 min |
120 min |
|||
|
Group I |
Normal control (Distilled water 2ml/ rat) |
4.18 0.89 |
4.56 |
4.88 0.92 |
5.12 1.01 |
4.7 0.91 |
4.08 0.96 |
|
Group II |
Diabetic control (Glucose+ distilled water) |
3.22 0.53 |
8.06 |
7.74 0.15 |
7.58 0.15 |
7.36 0.21 |
7.1 0.20 |
|
Group III |
Positive control (Glucose + Glibenclamide) |
3.78 0.37 |
9.44 |
6.1 0.41 |
5.92 0.33 |
5.02 0.11 |
3.9 0.12 |
|
Group IV |
Sample treated (Glucose + Ethanol Extract) |
3.8 0.79 |
7.9 |
7.16 0.09 |
6.44 0.38 |
5.9 0.27 |
4.78 0.66 |
|
Group V |
Sample treated (Glucose + Aqueous Extract) |
3.76 0.17 |
8.08 |
7.5 0.07 |
6.98 0.24 |
6.14 0.40 |
5.22 0.23 |
** Values are given as mean ± standard deviation for groups of five rats. Values are statistically significant at * p< 0.05.
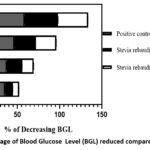 |
Figure 1: Percentage of Blood Glucose Level (BGL) reduced compared to zero minute. |
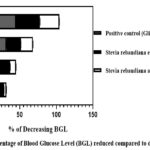 |
Figure 2: Percentage of Blood Glucose Level (BGL) reduced compared to diabetic control |
Conclusion
There are plenty of medicinal plants all around the world. Some plants can treat one specific disease or two or more. Some plants can be used as a medicine for diabetes, Stevia rebaudiana is one of them. The specialty of this plant is that it tastes sweet and can be taken as a daily diet rather than any other antihyperglycemic plant. The main objective of the present study is to assess the antihyperglycemic activity of ethanol and aqueous extracts of Stevia rebaudiana plants. In this investigation, it was found that the reference drug, ethanol, and aqueous extract all reduced blood glucose levels after 120 minutes though the reference drug showed better results. Reference drug, ethanolic and aqueous extract lowered blood glucose by 58.68%, 39.49%, and 35.39% respectively in glucose loaded rats. The existence of secondary metabolites such as alkaloids, flavonoids, phenols, saponins, tannins, and sterol compounds may preface the scope for treating other diseases. Again, both the extracts did not show any toxicity or side effects. Further investigations on streptozotocin induced diabetic rats may fetch a prosperous outcome. In the future by isolating the specific antidiabetic constituent, a natural medicine and a sugar substitute can be developed. In conclusion, our exploration into the world of medicinal plants has brought forth the noteworthy potential of Stevia rebaudiana as a remedy for diabetes. What sets this plant apart is its inherent sweetness, which allows it to be seamlessly incorporated into daily diets, setting it apart from other antihyperglycemic botanicals. The primary objective of our study was to evaluate the antihyperglycemic properties of both ethanol and aqueous extracts derived from Stevia rebaudiana. Our investigation revealed that, after a 120-minute interval, both the reference drug and the extracts exhibited the ability to significantly lower blood glucose levels. Notably, the reference drug outperformed the ethanolic and aqueous extracts, reducing blood glucose levels by 58.68%, 39.49%, and 35.39%, respectively, in glucose-loaded rats. Moreover, the presence of various secondary metabolites, including alkaloids, flavonoids, phenols, saponins, tannins, and sterol compounds in these extracts’ hints at a broader potential for treating various other ailments. It is worth highlighting that neither of the extracts exhibited any signs of toxicity or adverse effects during our investigations. Further research involving streptozotocin-induced diabetic rats may yield promising outcomes. There’s potential for isolating specific antidiabetic constituents from Stevia rebaudiana, which could pave the way for the development of natural medicines and sugar substitutes in the future. This exciting avenue of exploration underscores the diverse and promising nature of medicinal plants and their potential to improve human health and well-being.
Acknowledgement
The research has partially been supported by Research Cell of the University of Chittagong, Bangladesh. No: 6751/CU Research Cell/2019 and 241/2023-24/1st invite/17/2023
Author contributions
Sharmin Jamal formal analysis, investigation, data curation, writing original draft, Suman Barua conceptualization, methodology, formal analysis, investigation, data curation, writing original draft, project administration, funding management and supervision, Abhijit Barua formal analysis, investigation, data curation, A. J. M. Morshed, Rasheda Akter, andShireen Akhter formal analysis, investigation, data curation.
Conflict of interest
Authors declare no financial and nonfinancial conflict of interest.
References
- Mealey, B.L. and T.W. Oates. 2006. 77(8): p. 1289-1303.
CrossRef - Organization, W.H., 2013.
- Association, A.D. Diabetes Care, 2014. 37(Supplement_1): p. S81-S90.
CrossRef - Saeedi, P., I. Petersohn, P. Salpea, B. Malanda, S. Karuranga, N. Unwin, S. Colagiuri, L. Guariguata, A.A. Motala, K. Ogurtsova, J.E. Shaw, D. Bright, and R. Williams. Diabetes Research and Clinical Practice, 2019. 157: p. 107843.
CrossRef - Ignacimuthu, S., M. Ayyanar, and S. Sivaraman K. Journal of Ethnobiology and Ethnomedicine, 2006. 2(1): p. 25.
CrossRef - Gregersen, S., P.B. Jeppesen, J.J. Holst, and K. Hermansen. Metabolism, 2004. 53(1): p. 73-76.
CrossRef - Kinghorn, A.D., N.P.D. Nanayakkara, D.D. Soejarto, P.J. Medon, and S. Kamath. Journal of Chromatography A, 1982. 237(3): p. 478-483.
CrossRef - Soejarto, D.D., C.M. Compadre, P.J. Medon, S.K. Kamath, and A.D. Kinghorn. Economic Botany, 1983. 37(1): p. 71-79.
CrossRef - Kolb, N., J.L. Herrera, D.J. Ferreyra, and R.F. Uliana. Journal of Agricultural and Food Chemistry, 2001. 49(10): p. 4538-4541.
CrossRef - A. Esmat Abou-Arab, A.A.A.-A.a.M.F.A.-S. African Journal of Food Science, 2010. 4: p. 269- 281.
- Brahmachari, G., L.C. Mandal, R. Roy, S. Mondal, and A.K. Brahmachari. 2011. 344(1): p. 5-19.
CrossRef - Lemus-Mondaca, R., A. Vega-Gálvez, L. Zura-Bravo, and K. Ah-Hen. Food Chemistry, 2012. 132(3): p. 1121-1132.
CrossRef - Chatsudthipong, V. and C. Muanprasat. Pharmacology & Therapeutics, 2009. 121(1): p. 41-54.
CrossRef - Ncube, B., J.F. Finnie, and J. Van Staden. South African Journal of Botany, 2012. 82: p. 11-20.
CrossRef - Liu, W., D. Yin, N. Li, X. Hou, D. Wang, D. Li, and J. Liu. Scientific Reports, 2016. 6(1): p. 28591.
- Stoyanova, S., J. Geuns, É. Hideg, and W. Van Den Ende. International Journal of Food Sciences and Nutrition, 2011. 62(3): p. 207-214.
CrossRef - Ferrazzano, G.F., T. Cantile, B. Alcidi, M. Coda, A. Ingenito, A. Zarrelli, G. Di Fabio, and A.J.M. Pollio. 2016. 21(1): p. 38.
CrossRef - Kujur, R.S., V. Singh, M. Ram, H.N. Yadava, K.K. Singh, S. Kumari, and B.K. Roy. Pharmacognosy Journal, 2010. 2(14): p. 27-32.
CrossRef - Hymete, A. 1986: p. 54-67.
- Ragavan, B. and S. Krishnakumari. Indian Journal of Clinical Biochemistry, 2006. 21(2): p. 123-128.
CrossRef - Du Vigneaud, V. and W.G.J.T.J.o.B.C. Karr. 1926. 66: p. 281-300.
CrossRef - S., S., M. A., M. P., and B. V.K. Romanian Biotechnological Letters, 2011. 16(3): p. 6187-6199.
- Islam, M.A., M.A. Akhtar, M. Khan, M.S. Hossain, A. Alam, M.I. Ibne-Wahed, M.S. Amran, B.M. Rahman, and M. Ahmed. Pakistan Journal of Pharmaceutical Sciences, 2009. 22(4): p. 402-404.
- Md. Moniruzzaman Sohag Howlader, S.R.A., , Khadizatul Kubra, Md. Khairul Hassan Bhuiyan. Asian J. Med. Biol. Res 2016. 2(1): p. 121-130.
CrossRef - Pariyani, R., I. Safinar Ismail, A.A. Azam, F. Abas, K. Shaari, and M.R. Sulaiman. BioMed Research International, 2015. 2015: p. 742420.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









