Bis(2-cyanoacetamide) in Heterocyclic Synthesis: Synthesis of Some Bis[2-oxopyridine, 2-iminochromene, Chromeno[3,4-c]pyridine, Benzochromeno[3,4-c]pyridine] Derivatives
1Chemistry Department, Collage of Sciences and Arts, Northern Border University, Rafha, Saudi Arabia.
2Department of Pharmaceutical Chemistry, Collage of Pharmacy, Northern Border University, Rafha, Saudi Arabia.
Corresponding Author E-mail: hamdykhamees@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/400108
Article Received on : 01 Nov 2023
Article Accepted on : 18 Jan 2024
Article Published : 31 Jan 2024
Reviewed by: Dr. Mohamed Sayed Abd El-all El-Gaby
Second Review by: Dr. Gunawan
Final Approval by: Dr. Nenad Ignjatovic
N,N'-(methylenebis(1,4-phenylene))bis-(2-cyanoacetamide) was exploited as a precursor for synthesing some bis (benzylidene 5a-c, pyridines 7, 8, 10a,b, chromene 14, benzochromene 15) derivatives containing diphenyl-methylene spacer via the reaction with each of aromatic aldehydes, pentane-2,4-dione, acetaldehyde/ malononitrile, arylidene-malononitriles, ethyl cinnamates, 2-hydroxybenzaldehyde, and 2-hydroxy-1-naphthaldehyde). Bis(chromeno[3,4-c]pyridines 16&18) were synthesized via Michael's addition of malononitrile or ethyl cyanoacetate to bis(chromene) derivative. The newly prepared compound structures were established via ir, NMR spectroscopic data.
KEYWORDS:Bis[2-cyanoacetamide; chromeno[3,4-c]pyridines]; 2-cyanoacrylamides; 2-imino-chromenes; 2-pyridones
Download this article as:| Copy the following to cite this article: Thabet H. K, Imran M, Alaql S, Helal M. H. M. Bis(2-cyanoacetamide) in Heterocyclic Synthesis: Synthesis of Some Bis[2-oxopyridine, 2-iminochromene, Chromeno[3,4-c]pyridine, Benzochromeno[3,4-c]pyridine] Derivatives. Orient J Chem 2024;40(1). |
| Copy the following to cite this URL: Thabet H. K, Imran M, Alaql S, Helal M. H. M. Bis(2-cyanoacetamide) in Heterocyclic Synthesis: Synthesis of Some Bis[2-oxopyridine, 2-iminochromene, Chromeno[3,4-c]pyridine, Benzochromeno[3,4-c]pyridine] Derivatives. Orient J Chem 2024;40(1). Available from: https://bit.ly/3OqjM6Z |
Introduction
We have worked on a project for the past years that aims to provide new, straightforward methods for synthesizing several new heterocycles of biological importance using inexpensive, readily available starting intermediates in laboratories1-12. The synthesis of novel mono- and bis-heterocyclic derivatives, which are predicted to display a variety of biological activities, has drawn more interest in recent years13-20. The pyridine unit was found in structurally simple pharmaceutical agents like 2-pyridone ligand, isoniazid, milrinone, bupicomide, and SD-56021-27 (Figure 1). Functionalized chromenes are widely used to synthesize promising compounds in medicinal chemistry28-35. Wide-ranging pharmacological activity, such as antibacterial36, anti-inflammatory37, antimicrobial38, anti-proliferative39, hypotensive40, and antirheumatic41 properties, have been reported for derivatives of chromenopyridine. Many natural and pharmaceutical compounds contain fused chromenes like chromenopyridines. For example, chromeno[3,4-c]pyridine (C) may be an antipsychotic since it blocks D4 receptors36; schumaniophytine (A) and isoschumaniophytine (B) may be antiviral, central and autonomic system depressing42 (Figure 1). Furthermore, anti-inflammatory, antibacterial, antifungal, and antitubercular43-45 properties are demonstrated by chromeno[3,4–c]pyridines. In light of the aforementioned findings, we present here the synthesis of bis(benzylidene, pyridines, chromenes, and chromeno(3,4-pyridine) derivatives comprising a new diphenyl-methylene spacer using N,N’-(methylenebis(1,4-phenylene))bis(2-cyanoacetamide) as a suitable precursor.
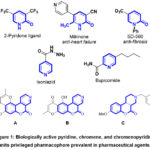 |
Figure 1: Biologically active pyridine, chromene, and chromenopyridine units privileged pharmacophore prevalent in pharmaceutical agents. |
Materials and Methods
General methods
Melting points were determined on the Cole-Parmer Digital Melting Point Apparatus instrument and are uncorrected. IR spectra (KBr) were measured on a Nicolet Summit FTIR iS50 spectrometer. NMR spectra (1H & 13C) were recorded in (DMSO–d6) on a Varian Gemini 500 (500 MHz for 1H and 125 MHz for 13C) spectrometer using TMS as a standard reference; chemical shifts are conveyed as δ units. The elemental analyses were performed on a Perkin-Elmer 240 microanalyzer, PE 2400 Series II CHNS/O Analyzer at the Microanalytical Center, Al-Azhar University, Cairo, Egypt.
N,N’-(Methylenebis(4,1-phenylene))bis(2-cyanoacetamide) (3).
A solution of 4,4′-methylenedianiline 1 (10 mmol, 1.98g), compound 2 (20 mmol, 3.26g) in benzene (40 ml) underwent reflxux for 2 hrs, then filtered while hot, and then recrystallized (Table 1).
Synthesis of benzylidene derivatives 5a-c:
The appropriate aromatic aldehyde (namely; 4-Cl, 4-F-, 4-OMe-benzaldehyde, 20 mmol, 2.82g, 2.48g, 2.72g, respectively) in EtOH (30 ml) containing pip. (0.5 mL), compound 3 (10 mmol, 3.32g) was added. The solution underwent refluxing for 6 hrs. The obtained product by refluxing was collected, filtered, and recrystallized to give 5a-c (Table 1).
Synthesis of 1,1′-(Methylenebis(4,1-phenylene))bis(4,6-dimethyl-2-oxo-1,2-dihydro-pyridine-3-carbonitrile) (7).
Compound 3 (10 mmol, 3.32g) and acetylacetone 6 (20 mmol, 2g) with piperidine (0.5 ml) was refluxed in an oil-bath for 2 hours and then cooled. The product that obtained, collected, and recrystallized (Table 1).
Synthesis of 1,1′-(Methylenebis(4,1-phenylene))bis(6-amino-4-methyl-2-oxo-1,2-dihydropyridine-3,5-dicarbonitrile) (8):
A solution of the 3 (10 mmol, 3.32g), acetaldehyde (20 mmol, 0.88g), malononitrile (20 mmol, 1.32g), and piperidine (0.5 ml) in EtOH (50 ml) was refluxed for 3 hrs. The product was recrystallized (Table 1).
Synthesis of bis(2-oxopyridone) derivatives 10a,b & 12a-c:
General procedure
Compound 3 (10 mmol, 3.32g), suitable cinnamonitrile 9a/9b (20 mmol, 3.36g, 3.78g, respectively) orethyl cinnamates 11a-c (20 mmol, 4.72g, 4.30g, 4.32g, respectively), and pip. (0.5 ml) in absolute ethanol (30 ml) was refluxed for 6 hours. The basic solid was collected and recrystallized to give 10a,b, 12a-c (Table 1).
Synthesis of 2-Iminochromene derivatives (14 and 15): General procedure
A solution of bis(cyanoacetamide) 3 (10 mmol, 3.32g) and the requested salicylaldehyde derivative (20 mmol, 2.44g, 3.44g, respectively), NH4OAc (3 g) in EtOH (40 ml) was refluxed for 3hrs. The product that obtained was filtered off while solution hot and recrystallized to give 14 and/or 15 (Table 1).
Synthesis of (benzo)chromeno[3,4-c]pyridines 16 and 18
General procedure: bis(chromene) derivative 14 (10 mmol, 5.41g), and malononitrile or ethyl cyanoacetate (20 mmol, 1.32g, 2.26g, respectively) and pip. (0.5 ml) in dioxan (40 mL) was refluxed for 3 hrs., The obtained solid was filtered off and recrystallized to give 16 and/or 18 respectively (Table 1).
Table 1: Physical data of the compounds
|
Compd. No. |
M.P. (oC) |
Yield (%) Cryst. Solvent |
Formula (Mol. Wt.) |
Elemental Analyses Calcd. /Found % |
||
|
C |
H |
N |
||||
|
3 |
233-35 |
93 (A) |
C19H16N4O2 (332.36) |
68.66 68.50 |
4.85 4.78 |
16.86 16.71 |
|
5a |
271-73 |
86 (C) |
C33H22Cl2N4O2 (577.47) |
68.64 68.49 |
3.84 3.72 |
9.70 9.56 |
|
5b |
267-69 |
81 (B) |
C33H22F2N4O2 (544.56) |
72.79 72.63 |
4.07 3.92 |
10.29 10.18 |
|
5c |
260-62 |
88 (C) |
C35H28N4O4 (568.63) |
73.93 73.80 |
4.96 4.80 |
9.85 9.72 |
|
7 |
278-280 |
79 (C) |
C29H24N4O2 (460.54) |
75.63 75.51 |
5.25 5.11 |
12.17 12.04 |
|
8 |
>300°C |
70 (B) |
C29H20N8O2 (512.53) |
67.96 67.82 |
3.93 3.72 |
21.86 21.69 |
|
10a |
292-94 |
73 (B) |
C41H28N8O2 (664.73) |
74.08 73.91 |
4.25 4.08 |
16.86 16.70 |
|
10b |
>300°C |
81 (B) |
C39H22Cl2N8O2 (705.56) |
66.39 66.22 |
3.14 3.05 |
15.88 15.61 |
|
12a |
>300°C |
71 (B) |
C43H32Cl2N6O6 (799.67) |
64.59 64.42 |
4.03 4.10 |
10.51 10.32 |
|
12b |
296-98 |
76 (B) |
C45H38N6O6 (758.84) |
71.23 71.08 |
5.05 4.92 |
11.08 10.96 |
|
12c |
263-65 |
68 (B) |
C45H38N6O8 (790.83) |
68.35 68.20 |
4.84 4.63 |
10.63 10.46 |
|
14 |
254-56 |
79 (B) |
C33H24N4O4 (540.58) |
73.32 73.19 |
4.48 4.31 |
10.36 10.24 |
|
15 |
277-79 |
81 (B) |
C41H28N4O4 (640.70) |
76.86 76.72 |
4.41 4.25 |
8.74 8.60 |
|
16 |
>300 |
62 (B/D) |
C39H24N8O4 (668.67) |
70.05 69.86 |
3.62 3.47 |
16.76 16.62 |
|
18 |
>300 |
67 (B/D) |
C43H34N6O8 (762.78) |
67.71 67.57 |
4.49 4.36 |
11.02 10.84 |
A = EtOH, B = dioxane, C = AcOH, D = DMF
Table 2: The characteristic spectroscopic data of the prepared compounds
|
Compd. No. |
IR |
1H-NMR |
13C-NMR |
|
3 |
3304 (NHstr), 3089 (=CHstr), 2956, 2923 (CH2str), 2259 (C≡Nstr), 1664 (C=Ostr), 1612 (C=Cstr) |
3.85 (2H, s, CH2), 3.87 (4H, s, 2CH2), 7.16 (4H, dd, ArH), 7.42 (4H, dd, ArH), 10.23 (2H, s, 2NH) |
27.1 (CH2), 62.4 (CH2), 116.5 (C≡N), 119.9, 129.5, 136.8, 137.4 (Ar-C), 161.3 (C=O) |
|
5a |
3327 (NHstr), 2922, 2851 (=CHstr), 2215 (C≡N), 1675 (C=O) |
3.92 (s, 2H, CH2), 7.22 (dd, 4H, ArH), 7.59 (dd, 4H, ArH), 7.66 (dd, 4H, ArH), 7.98 (dd, 4H, ArH), 8.25 (s, 2H, 2 benzylidene-H), 10.28 (s, 2H, 2NH) |
27.3, 108.6, 116.4, 121.3, 129.4, 129.9, 131.3, 132.1, 136.7, 137.5, 137.9, 149.8, 160.6 |
|
5b |
3320 (NHstr), 3034 (=CHstr), 2925 (CHstr) 2221 (C≡N), 1672 (C=O) |
3.87 (2H, s, CH2), 7.19 (4H, dd, ArH), 7.42 (4H, dd, ArH), 7.55 (4H, dd, ArH), 8.02 (4H, dd, ArH), 8.22 (2H, s, 2 benzylidene-H), 10.32 (2H, s, 2NH) |
27.3, 107.4, 117.0, 117.2, 121.2, 129.12, 129.15, 129.4, 133.31, 133.38, 136.7, 137.9, 150.0, 163.6, 165.6 |
|
5c |
3332 (NHstr), 3032 (=CHstr), 2933, 2840 (CHstr) 2216 (C≡N), 1676 (C=O) |
3.86 (6H, s, 2OCH3), 3.90 (2H, s, CH2), 7.17 (4H, dd, ArH), 7.21 (4H, dd, ArH), 7.57 (4H, dd, ArH), 8.00 (4H, dd, ArH), 8.19 (2H, s, 2 benzylidene-H), 10.23 (2H, s, 2NH) |
26.4, 56.1, 104.1, 115.4, 117.4, 121.2, 124.9, 129.4, 133.1, 136.9, 137.8, 150.8, 161.3, 163.2 |
|
7 |
3063 (=CHstr), 2955 (CHstr), 2222 (C≡Nstr), 1652 (C=Ostr) |
1.94 (6H, s, 2CH3), 2.37 (6H, s, 2CH3), 3.83 (2H, s, CH2), 6.42 (2H, s, pyridine-H5), 7.24 (4H, dd, ArH), 7.46 (4H, dd, ArH) |
20.6, 21.5, 26.6, 108.8, 115.9, 127.8, 129.7, 135.2, 136.2, 142.4, 152.3, 160.8 |
|
8 |
3422, 3394 (NH2), 2937, 2862 (CH aliph.), 2196 (2 C≡N), 1687 (C=O) |
2.38 (6H, s, 2CH3), 3.85 (2H, s, CH2), 7.27 (4H, dd, ArH), 7.55 (4H, dd, ArH), 7.95 (4H, s, 2NH2) |
22.0, 26.3, 76.2, 88.3, 116.2, 116.8, 126.6, 129.9, 129.4, 131.7, 132.0, 135.3, 145.1, 157.7, 159.1, 160.1 |
|
10a |
3282, 3199 (NH2str), 3090 (=CHstr), 2220 (C≡N), 1679 (C=O) |
2.39 (6H, s, 2CH3), 3.86 (2H, s, CH2), 7.33 (4H, dd, ArH), 7.37 (4H, dd, ArH), 7.43 (4H, dd, ArH), 7.52 (4H, dd, ArH), 7.83 (4H, hump, 2NH2) |
19.0, 26.9, 75.3, 88.2, 116.1, 116.8, 126.1, 126.6, 129.0, 129.4, 130.2, 131.7, 134.3, 134.5, 143.5, 157.5, 159.4, 161.0 |
|
10b |
3324, 3204 (NH2str), 3055 (=CHstr), 2970 (CHstr) 2229 (C≡N), 1677 (C=O) |
3.87 (2H, s, CH2), 7.32 (4H, dd, ArH), 7.54 (4H, dd, ArH), 7.58 (4H, dd, ArH), 7.67 (4H, dd, ArH), 7.93 (4H, hump, 2NH2) |
27.4, 75.8, 88.5, 116.1, 116.8, 129.0, 129.4, 130.4, 131.4, 132.3, 134.0, 135.7, 142.8, 157.7, 160.0, 160.6 |
|
12a |
3378, 3292 (NH2str), 2970, 2881 (CHstr) 2214 (C≡N), 1742, 1673 (C=O) |
1.29 (6H, t, 2CH3), 3.90 (2H, s, CH2), 4.33 (4H, q, 2CH2), 7.22 (4H, d, ArH), 7.43 (4H, d, ArH), 7.55 (4H, d, ArH), 7.68 (4H, d, ArH), 8.26 (4H, s, 2NH2) |
16.4, 26.3, 56.4, 75.9, 88.2, 116.9, 126.1, 126.6, 128.9, 129.4, 130.2, 130.9, 131.1, 131.7, 134.4, 135.2, 143.4, 145.1, 157.7, 159.1, 160.9, 176.7 |
|
12b |
3362, 3268 (NH2str), 2210 (C≡N), 1730, 1669 (C=O) |
1.46 (6H, t, 2CH3), 2.14 (s, 6H, 2CH3), 3.88 (2H, s, CH2), 4.38 (4H, q, 2CH2), 7.32 (4H, dd, ArH), 7.58 (4H, dd, ArH), 8.05 (4H, dd, ArH), 8.18 (4H, dd, ArH), 8.52 (4H, s, 2NH2) |
18.7, 22.4, 29.2, 56.1, 75.1, 87.9, 115.8, 125.8, 126.3, 128.7, 129.2, 129.9, 131.5, 132.0, 134.3, 144.8, 157.2, 159.1, 160.7, 173.8 |
|
12c |
3410, 3357 (NH2str), 2211 (C≡N), 1725, 1676 (C=O) |
1.25 (6H,t, 2CH3), 3.83 (6H, s, 2OCH3), 3.87 (2H, 2, CH2), 4.45 (4H, q, 2CH2), 7.15 (4H, d, ArH), 7.41 (4H, d, ArH), 7.52 (4H, d, ArH), 7.64 (4H, d, ArH), 7.98 (4H, s, 2NH2) |
21.3, 27.1, 58.7, 66.8, 75.7, 88.4, 116.9, 126.1, 126.6, 128.7, 128.9, 129.4, 130.2, 131.4, 131.7, 134.5, 143.4, 145.1, 157.7, 159.1, 160.0, 175.4 |
|
14 |
3297 (NH), 1678 (C=O) |
3.88 (2H, s, CH2), 7.15 (4H, d, ArH), 7.19 (2H, d, ArH), 7.25 (2H, t, ArH), 7.55 (2H, t, ArH), 7.66 (4H, dd, ArH), 7.78 (2H, d, ArH), 8.52 (2H, s, 2 chromene-H4), 9.22 (2H, s, 2C=NH), 12.78 (2H, s, 2 CONH) |
27.8, 115.7, 118.4, 120.0, 121.4, 124.2, 130.1, 133.3, 134.6, 141.6, 149.8, 155.7, 157.4, 163.5 |
|
15 |
3230 (NH), 1671 (C=O) |
3.87 (2H, s, CH2), 6.97 (2H, d, ArH), 7.20 (4H, dd, ArH), 7.45 (4H, dd, ArH), 7.61 (2H, t, ArH), 7.76 (2H, t, ArH), 7.91 (2H, d, ArH), 8.05 (2H, d, ArH), 8.19 (2H, d, ArH), 8.47 (2H, t, ArH), 9.20 (2H, s, 2 benzochromene-H4), 9.62 (2H, s, 2C=NH), 12.80 (2H, s, 2 CONH) |
26.7, 116.2, 119.6, 122.14, 122.17, 124.9, 125.6, 127.3, 128.9, 129.2, 129.8, 130.3, 134.1, 134.5, 137.1, 138.8, 153.8, 156.3, 163.7 |
|
16 |
3421, 3351, 3192 (NH2/ NH), 2226 (C≡N), 1680 (C=O) |
3.86 (2H, s, CH2), 6.98 (2H, s, NH2), 7.20 (4H, d, ArH), 7.45 (4H, d, ArH), 7.52 (2H, t, ArH), 7.61 (2H, d, ArH), 7.76 (2H, t, ArH), 7.89 (2H, d, ArH), 9.79 (2H, s, 2NH) |
26.6, 75.2, 110.4, 116.4, 126.6, 127.6, 128.5, 130.8, 135.7, 153.2, 157.9, 162.3, 162.4 |
|
18 |
3435, 3329, 3241 (NH2/ NH), 2218 (C≡N), 1679, 1734 (C=O) |
1.33 (3H, t, CH3), 3.88 (2H, s, CH2), 4.25 (2H, d, CH2), 6.92 (4H, s, 2NH2), 7.25 (4H, d, ArH), 7.47 (4H, d, ArH), 7.59 (2H, t, ArH), 7.72 (2H, d, ArH), 7.77 (2H, t, ArH), 7.92 (2H, d, ArH), 9.38 (2H, s, 2NH) |
27.3, 79.6, 112.3, 116.2, 125.4, 127.8, 129.9, 131.9, 137.8, 150.2, 154.4, 159.2, 163.4, 164.2, 167.6 |
Results and Discussion
Cyanoacetamide derivatives are versatile synthetic intermediates in organic synthesis due to their accessibility and their tendency to undergo nucleophilic additions. Also, cyanoacetamide derivatives are the key precursor for many drugs having a broad spectrum in various branches of medicine chemistry, because of their multiplicity uses in the drug industry46-49.
The crucial intermediate, bis(cyanoacetamide) 3 containing methylene bridge was obtained in quantitative yield (93%) by the reaction of 4,4′-methylenedianiline 1 with two-equivalents of cyanoacetylating agent 2 in refluxing benzene (Scheme 1). IR spectrum of key intermediate 3 displayed bands at 3304 cm-1 due to NH group, 2259 cm-1 due to C≡N group, 1664 cm-1 for the C=O group. Moreover, the 1HNMR spectrum exposed a singlet at 3.85 ppm for two CH2 protons, a singlet at 3.87 for methylene bridge protons, two doublets at 7.16, 7.42 for Ar-protons, and a singlet at 10.23 ppm assigned to amino protons; 13C NMR exhibited signals at δC: 27.1 ppm assigned to CH2-bridge, 62.4 ppm assigned to (CH2C≡N), 116.5 ppm (C≡N), 161.3 ppm assigned to (C=O), and aromatic-c’s appeared at 119.9-137.4 ppm. The reaction of 3 with some electrophiles was investigated. Thus, bis(benzylidene) derivatives 5a-c were gained in great yield via condensation of 3 with aromatic aldehydes 4a-c (namely, 4-Cl, 4-F, 4-OMe-benzaldehye) in 1:2 molar ratio in alcoholic-piperidine at reflux conditions (Scheme 1). Assignment of 5a-c was long-established based on their spectral records. IR spectrum of compounds 5a-c demonstrated the characteristic bands for the imino group at 3327 cm-1, for the C≡N group at 2215 cm-1, and C=O at 1675 cm-1. 1HNMR spectrum (DMSO-d6) of 5a exposed three singlets at 3.92, 8.25 and 10.28 ppm for CH2, two benzylidene-H and CONH, respectively, and the ArH in the spectrum appeared at 7.22-7.98 ppm. 13C NMR of compound 5a exhibited signals at δC: 27.38 ppm assigned to CH2-bridge, 108.62 =C-C≡N, 116.45 ppm for carbonitrile groups, 149.81 ppm (benzylidene-C), 160.63 ppm for carbonyl groups, and the aromatic C’s was found in the spectrum at 121.38-137.99 ppm, for more details of characterization of compounds 5a-c; see experimental section.
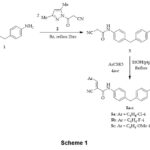 |
Scheme 1 |
Cyclocondensation of compound 3 with pentane-2,4-dione 6 afforded bis(4,6-dimethyl-2-pyridone) derivative 7, via heterocyclization of the intermediate A by elimination of two H2O molecules. Refluxing of bis(cyano-acetamide) derivative 3 with acetaldehyde, and malononitrile gave pyridine derivative 8, Scheme 2. IR spectrum of 7 presented the characteristic bands at 2222 and 1652 cm-1 for C≡N & C=O groups, respectively. Furthermore, the 1HNMR of compound 7 naked two singlets at 1.94 and 2.37 ppm attributed to four methyl protons, 3.83 ppm for methylene-bridge protons, singlet at 6.42 ppm for two pyridine-H5, and aromatic protons appeared as two doublets at 7.24 and 7.46 ppm. 13CNMR spectrum this compound exhibited signals at 20.6, 21.5 ppm for two methyl-c’s, signal at 26.6 ppm for methylene-C, signal at 115.9 for two C≡N, the signal at 152.3 ppm for C-4, the signal at 160.8 ppm for C-2, and signals from 108.8-142.4 ppm for aromatic-c’s. IR spectrum of pyridine derivative 8 revealed characteristic bands at 3422, 3394 cm-1 for amino group, at 2937, 2862 cm-1 for aliphatic-CH, at 2196 cm-1 for C≡N group, and 1687 cm-1 for C=O group. 1HNMR spectrum of 8 exposed a singlet at 2.38 ppm for two CH3 protons, 3.85 ppm for methylene bridge protons, two doublets at 7.27 and 7.55 ppm for Ar-H, singlet at 7.95 ppm attributed to two amino protons. 13CNMR spectrum of 8 demonstrated signals at 22.0 ppm for two methyl-c’s, a signal at 26.3 ppm for methylene-C, two signals at 76.2, and 88.3 ppm assigned to C-5, and C-3, respectively, signals at 116.2, 116.8 ppm attributed to two C≡N, signals at 157.7, 159.1, 160.1 ppm attributed to C-2, C-6, and C-4, respectively, besides aromatic signals were found in the spectrum at 126.6-145.1 ppm.
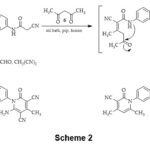 |
Scheme 2 Click here to View Scheme |
Compound 3 underwent the reaction with arylidenemalononitriles 9a,b (in 1: 2 molar ratio) in refluxing ethanolic-piperidine and gave bis(3,5-dicyano-2-pyridone) derivatives 10a,b, while its reaction with ethyl cinnamates 11a,b afforded bis(pyridine) derivatives 12a-c and the other possible structure 13a-c was excluded upon the spectral data, Scheme 3. IR of 10a exhibited a strong band at 3282 and 3199 cm−1, attributed to the amino groups, besides the existence of a strong bands at 2220 and 1679 cm−1 attributed to carbonitrile & carbonyl functional groups, respectively. The 1H NMR spectrum displayed two singlets at 2.39, 3.86 ppm for two methyl and methylene protons, four doublets at 7.33, 7.37, 7.43, 7.52 ppm for aromatic protons, hump at 7.83 ppm corresponding to two NH2 protons. 13CNMR spectrum of 10a displayed signals at 19.0 ppm for two methyl-c’s, a signal at 26.9 ppm for CH2, two signals at 75.3, 88.2 ppm assigned to C-5, C-3, respectively, two signals at 116.1, 116.8 ppm attributed to two C≡N, three signals at 157.5, 159.4, 161.0 ppm attributed to C-2, C-6, C-4, correspondingly, beside and aromatic signals at 126.1-143.5 ppm. The structure of 10b was entirely consistent with the elemental analysis and spectroscopic information. (see. Experimental section). IR spectrum of 12a showed intense stretching band at 3378, 3292 cm-1 for NH2 groups, 2214 cm-1 for C≡N groups, and 1673, 1742 cm-1 for each pyridine-2-one and ester functional groups, respectively. Its 1HNMR spectrum revealed signals at 1.29 ppm for two ester-CH3 protons (triplet), 3.90 ppm for methylene protons (singlet), 4.33 ppm for two ester-CH2 protons (quartet), 8.26 corresponding to two amino protons (singlet), and the Ar-H appeared at 7.22-7.68 ppm. The 13CNMR spectrum of compound 12a showed peaks at 16.4 ppm for CH3-ester residue, 26.36 ppm for CH2, 56.4 ppm for CH2-ester residue, two peaks at 75.9, 88.2 ppm assigned to C-5, C-3, respectively, peak at 116.9 ppm for C≡N, three signals at 157.7, 159.1, 160.9 ppm assigned to C-2, C-6, C-4, respectively, a signal at 176.7 assigned to ester-carbonyl group, and the aromatic signals were found in the spectrum at 126.1-145.1 ppm. The IR, 1HNMR, and 13CNMR spectra of derivatives 12b,c were per their structure.
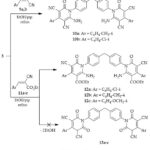 |
Scheme 3 Click here to View Scheme |
The formation of compounds 10a,b, and 12a-c was supposed to progress most likely via the initial addition of activated CH2 in compound 3 to arylidene-malononitriles 9a,b and/or ethyl cinnamates 11a-c forming the adduct B. The proton transfer results in the transformation of B into C. Isomerization of C to D and subsequent oxidation via loss of two hydrogen molecules resulted in the creation of the final products 10a,b, and 12a-c, Scheme 4.
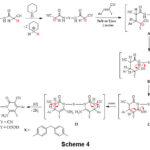 |
Scheme 4 Click here to View Scheme |
Refluxing of compound 3 with 2-hydroxybenzaldehyde in ethanolic-NH4OAc medium gave bis(2-iminochromene) derivative 14. Analogy, bis(benzo[f]chromeno) derivative 15 was obtainedbyheating compound 3 with 2-hydroxynaphthalene-1-carbaldehyde, scheme 5. The lack of frequency of the C≡N in the IR of each compounds 14,15, indicates the formation of the products. Also, the structure of 14 was reinforced by the 1HNMR spectrum which presented singlet at 8.52 ppm for two chromene-H4, two singlets at 9.22, 12.78 ppm for C=NH and CONH groups, respectively, and the aromatic protons emerged as multiplets at 7.15-7.78 ppm. 1HNMR spectrum of 15 which showed three singlets at 9.20, 9.62 and 12.80 ppm for benzo chromene-H4, C=NH and CONH protons, respectively, beside aromatic protons at 6.97-8.47 ppm.
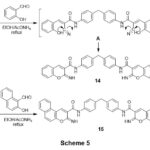 |
Scheme 5 Click here to View scheme |
The corresponding bis(2-amino-4H-chromeno[3,4-c]pyridine) derivative 16 was gainedviarefluxing of bis(iminochromene) derivative 14 with malononitrile in dioxan containing piperidine as a catalyst, Scheme 5. Analogy, reaction of 14 with ethyl cyano-acetate gave chromenopyridine derivative 18 not 17 grounded on the spectral data. The presence of strong absorption bands for (C≡N) indicates the formation of compounds 16&18. Further support for the compounds is the lack of chromene-H4 in their 1H NMR spectra and the existence of peaks for C≡N and ester functional groups in their 13C NMR spectra.
The reaction pathway for the creation of compound 16&18 was assumed to continue via Michael addition of the activated CH2 in dicyanomethane or ethylcyanoacetate to deficient double bond of 14 to provide adducts A and A’ followed by addition of NH2 group to carbonitrile group, tautomerization then oxidation by loss of two hydrogen molecules, Scheme 6.
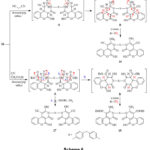 |
Scheme 6 |
Conclusion
A facile and suitable preparations of some distinctive bis (benzylidenes, pyridines, chromene, benzochromene, chromeno[3,4-c]-pyridines) containing diphenyl-methylene spacer hve been illustrated. Utilizing elemental analysis and 1H&13C-NMR, the specified compounds’ chemical structures were determined.
Acknowledgement
The authors would like to express their deep gratitude’s to the Northern Border University, Kingdom of Saudi Arabia for providing financial support of this research (project 2022-11-1922).
Conflicts of interest
There are no conflicts of interest.
Funding Sources
There are no funding sources
References
- Hessein, S.A.; El-Sharief, M.A.M.Sh.; Abbas, S.Y.; Thabet, H.Kh.; Ammar, Y. A. Croat. Chem. Acta, 2016, 89, 91–100.
CrossRef - Ammar, Y.A.; Al-Sehemi, A.G.; Ali, M.M.; Mohamed, Y.A.; Thabet, H.Kh.; El-Gaby, M.S.A. Heteroletters, 2015, 5, 157-167.
- Ammar, Y.A.; El-Gaby, Salem, M. A. Arabian Journal of Chemistry, 2014, 179, 615-622.
CrossRef - Ragab, A.; Fouad, S. A.; Abu Ali, O. A.; Ahmed, E. M.; Ali, A. M.; Askar, A. A.; Ammar, Y. A. Antibiotics (Basel), 2021, 10(2), 162-193.
CrossRef - Salem, M. A., Abbas, S. Y.; El-Sharief, M. A.M. Sh.; Alzahrani, A. Y.; Helal, M. H.; H. Kh. Thabet, Synthetic Communications, 2021, 51, 3325-3331.
CrossRef - Thabet, H.Kh. Oriental Journal of Chemistry, 2020,36, 320-326.
- Ubeid, M.T.; Thabet, H.Kh.; El-Feky, S.A. Heterocyclic Communications, 2016,22, 43-47.
CrossRef - Thabet, H. Kh.; Al-Hossainy, A.F.; Imran M.Optical Materials, 2020, 105, 109915.
- Alam, Md J.; Alam, O.; Naim, M.J.; Nawaz, F.; Manaithiya, A.; Imran, M.; Thabet, H.Kh.; Alshehri, S.; Ghoneim, M. M.; Alam, P.; Shakeel, F. Molecules, 2022, 27, 8708-8781.
CrossRef - Khan, A.; Diwan, A.; Thabet, H.Kh.; Imran, M. Drug Development Research, 2020,81, 573-584.
CrossRef - Khan, A.; Diwan, A.; Thabet, H.Kh.; Imran, M.; Bakht, Md. A. Molecules, 2020, 25, 2002-2021.
CrossRef - El-Feky, S.A.; Thabet, H.Kh.; Ubeid, M.T. Journal of Fluorine Chemistry, 2014, 161, 87-94.
CrossRef - Zimmermann, L.A.; de Moraes, M.H.; da Rosa, R.; de Melo, E.B.; Paula, F.R.; Schenkel, E.P.; Steindel, M.; Bernardes, L.S.C. Bioorganic & Medicinal Chemistry, 2018, 26, 4850–4862.
CrossRef - Larghi, E.L.; Bruneau, A.; Sauvage,F.; Alami, M.; Gauduchon, J.V.; Messaoudi, S. Molecules, 2022, 27, 412-425.
CrossRef - Vasilenko, D.A.; Sadovnikov, K.S.; Sedenkova, K.N.; Karlov, D.S.; Radchenko, E.V.; Grishin, Y.K.; Rybakov, V. B.; Kuznetsova, T.S.; Zamoyski, V.L.; Grigoriev, V. V.; Palyulin, V.A.; Averina, E.B. Molecules, 2021, 26, 6411-6426.
CrossRef - Popov, A. B.; Vianelo, R.; Grbčić, P.; Sedić,M.; Pavelić, S. K.; Pavelić,K.; Malić, S. R. Molecules, 2021, 26, 3334-3360.
CrossRef - Stack, D.L.; Masuda, J.D. Molbank, 2017, 2017, M962.
CrossRef - Kour, J.; Khajuria, P.; Verma, P.K.; Kapoor, N.; Kumar, A.; Sawant, S.D. ACS Omega, 2022, 7, 15, 13000–13009.
CrossRef - E. M.; Eid, H.M.E.; Hassaneen, I.A.; Abdelhamid, Elwahy, A.H.M. Journal of Heterocyclic Chemistry, 2020, 57, 2243-2255.
CrossRef - Al-Jumaili, M.H.A.; Hamad, A.A.; Hashem, H.E.; Hussein, A.D.; Muhaidi, M.J.; Ahmed, M.A.; Albanaa, A.H.A.; Siddique, F.; Bakr, E.A. Journal of Molecular Structure, 2023, 1271, 133970.
CrossRef - Hwang, J.; Strange, N.; Phillips, M.J.A.; Krause, A.L.; Heywood, A.; Gamble, A.B.; Huston, W.M.; Tyndall, J.D.A. Eur. J. Med. Chem., 2021, 224, 113692.
CrossRef - Parraguez, M.F.; Miranda, C.A.; Cho, Y.H.; Mahana, H. P.; Garrido, C.G.; Chung, H.; Faúnde, M.; Mahana, D.P. Int. J. Mmol. Sci. 2021, 22, 11212.
CrossRef - Boraei, A.T.A.; Eltamany, E.H.; Ali, I.A.I.; Gebriel, S. M.; Nafie, M.S. Bioorg. Chem. 2021, 111, 104877.
CrossRef - Shang, Y.; Wu, C.; Gao, Q.; Liu, C.; Li, L. Zhang, X.; Cheng, H.G.; Liu, S.; Zhou,Q. Nat. Commun. 2021, 12, 2988.
CrossRef - Fioravanti, R.; Stazi, G.; Zwergel, C.; Valente, S.; Mai, Chem. Rec., 2018, 12, 1818–1832.
CrossRef - Bera, M. K.; Domínguez, M. ; Hommes, P. ; Reissig, H. U. Beilstein J. Org. Chem. 2014, 10, 394–404.
CrossRef - Elinson, M. N.; Vereshchagin, A. N.; Bobrovsky, S. I.; Nasybullin, R. F.; Ilovaisky, A. I.; Merkulova V. M. Comptes Rendus Chimie, 2016, 19, 293-298.
CrossRef - Maleki, A.; Azizi, M.; Emdadi, Z. Green Chemistry Letters and Reviews, 2018,11, 573-582.
CrossRef - Song, X.; Gao, C.; Zhang, X.; Fan, X. J. Org. Chem. 2018, 83, 24, 15256–15267.
CrossRef - Koz, G.; Koz,Ö. Z. Naturforsch., 2017, 72, 647–653.
CrossRef - Neo, A.G.; Castellano, T.G.; Marcos, C.F. Arkivoc 2017, part iii, 21-31.
CrossRef - Raj, V.; Lee, J. Frontiers in Chemistry, 2020, 8, Article 623.
CrossRef - Ballarotto,M.; Solinasa, M.; Temperini, A. Org. Biommol. Chem., 2021, 19, 10359-10375.
CrossRef - Song, Z.; Jia, Y.; Zhang, D.; Wang, D. European Journal of Oragnic Chemistry, 2021, 2021, 1942-1948.
CrossRef - Rajput, D.; Jan, G.; Karuppasamy, M.; Bhuvanesh, N.; Nagarajan, S.; Maheswari, C. U.; Menéndez, J. C.; Sridharan, V. J. Org. Chem., 2023, 88, 11778–11792.
CrossRef - Douka, M.D.; Litinas, K.E. Molecules 2022, 27, 7256-7347.
CrossRef - Alizadeh, A.; Bayat, F.; Zhu, Z. Research on Chemical Intermediates, 2016, 42, 5927–5936.
CrossRef - Mohamed, Kh.S.; Elbialy, E.E. Heterocycles, 2020, 100, 1035-1047.
CrossRef - Banerjee, S.; Wang, J.; Pfeffer, S.; Ma, D.; Pfeffer, L.M.; Patil, SA.; Li, W.; Miller, D.D. Design, Molecules, 2015, 20, 17152–17165.
CrossRef - Kibou, Z.; Villemin, D.; Lohier, J. F.; Cheikh, N.; Bar, N.; Braham, N. C. Tetrahedron, 2016, 72, 1653-1661.
CrossRef - Gajurel, S.; Sarkar, R.; Sarkar, F. K.; Kyndiah, L.; Pal, K. ACS Omega. 2022, 7, 48087–48099.
CrossRef - Mekky, A.E.M.; Sanad, S.M.H. Synthetic Communications, 2019, 49, 1385–1395.
CrossRef - Adolfsson, D.E.; Tyagi, M.; Singh, P.; Deuschmann, A.; Ådén, J.; Gharibyan, A.L.; Jayaweera, S.W.; Lindgren, A.E.G.; Olofsson, A.; Almqvist, F. J. Org. Chem. 2020, 85, 14174–14189.
CrossRef - Hu, Z.; Wang, C.; Sitkoff, D.; Cheadle, N. L.; Xu, S.; Muckelbauer, J. K.; Adam, L. P.; Wexler, R. R.; Quan, M. L. Bioorg. & Med. Chem. Lett., 2020, 30, 127474
CrossRef - Behbehani, H.; Dawood, K.M.; Aryan, F.A.; Ibrahim, H.M. ACS Omega, 2021, 6(49), 34065–34074.
CrossRef - Ghozlan, S. A. S.; Abdelmoniem, A. M.; Ramadan, M. A.; Abdelwahab, H. M.; Abdelrahman, M. G. M.; Abdelhamid, I. A. Arkivoc, 2020, part i, 297-399.
CrossRef - Emam, D. R.; Soliman, N. N.; Fadda, A. A.; Bayoumy, N. M.; Journal of Molecular Structure, 2024, 1297 (Part 2), 137009.
CrossRef - Hamed, E. O.; Assy, M. G.; Shalaby, A. M.; Sayed, R. E. Russian Journal of Organic Chemistry, 2020, 56, 2005–2013.
CrossRef - Fadda, A. A.; Ghanem, R. A.; Gaffer, H. E.; Waly, M. M.; Tawfik, E. H. Polycyclic Aromatic Compounds, 2023, 43 (4), 3429-3449.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.










