An Overview on Free Radicals and Role of Antioxidants in The Management of Cancer
1School of Pharmaceutical Sciences, Shri Guru Ram Rai University, Patel Nagar, Dehradun, Uttarakhand, India.
2Department of Pharmaceutical Sciences, Faculty of Technology, Sir J.C.Bose Technical Campus Bhimtal, Kumaun University, Nainital-263136, Uttarakhand, India.
Corresponding Author E-mail: sushmitauni2015@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/400116
Article Received on : 26 Oct 2023
Article Accepted on : 22 Jan 2024
Article Published : 29 Jan 2024
Reviewed by: Dr. Ammar Kubba
Second Review by: Dr Sushama Kadam
Final Approval by: Dr. Murat HATİPOĞLU
Many diseases are associated with free radicals and oxidative stress, which result from reactive oxygen and nitrogen species. These high ROS levels affect various metabolic and signalling mechanisms leading to changes in physiological processes and the emergence of illnesses like cancer. In addition to dietary, mammalian cells have natural ROS scavenging mechanisms that includes enzymatic and non-enzymatic antioxidants. This review discuss the free radicals with relation to cancer development, as well as the role of antioxidants in the immune defence mechanism against free radicals. The review also explores various approaches for manipulating antioxidants and free radicals in the prevention and management of cancer, such as gene therapy, genetically engineered plants with higher antioxidant levels, artificial antioxidant enzymes, novel biomolecules, and antioxidant-rich foods. Future applications of these approaches are also discussed.
KEYWORDS:Antioxidant; Cancer; Free radicals; Oxidative stress
Download this article as:| Copy the following to cite this article: Uniyal S, Kumar N, Joshi B. C. An Overview on Free Radicals and Role of Antioxidants in The Management of Cancer. Orient J Chem 2024;40(1). |
| Copy the following to cite this URL: Uniyal S, Kumar N, Joshi B. C. An Overview on Free Radicals and Role of Antioxidants in The Management of Cancer. Orient J Chem 2024;40(1). Available from: https://bit.ly/3w0kUaO |
Introduction
Cancer has a substantial morbidity and mortality rate and is a very diversified disease. Despite intensive study and significant efforts to create targeted medicines, it remains a serious illness with a poor prognosis and high fatality rates. Numerous investigations have shown that modifications in the redox balance and dysregulation of redox signaling are distinctive features of cancer growth and therapeutic resistance. High quantities of reactive oxygen species (ROS) are consistently present in cancer cells due to oncogenic transformation, genetic alterations, metabolic changes, and tumor microenvironments. Based on recent studies, cancer cells have activated antioxidant pathways as a coping mechanism for high ROS levels. Thus, focusing on the redox pathways and ROS signaling pathways linked to the development of cancer offers a viable approach to cancer prevention 1. Reactive oxygen species (ROS) are generated as a normal by-product of metabolic processes. Because of their molecular structure, which includes unpaired electrons, ROS exist as free radicals, ions, and molecules with high reactivity 2. Nitrogen-free radicals such as hydroxyl radicals (•OH), peroxyl radicals (ROO•), superoxide (O2•-), organic radicals (R•), alkoxyl radicals (RO•), disulfides (RSSR), thiyl peroxyl radicals (RSOO•), sulfonyl radicals (ROS•), and thiyl radicals (RS•) are all considered reactive oxygen species (ROS). Additional types of reactive oxygen species (ROS) include singlet oxygen (O2•), hydrogen peroxide (H2O2), organic hydroperoxides (ROOH), ozone/trioxygen (O3), hypochloride (HOCl), nitrosoperoxycarbonate anion (O=NOOCO2−), nitrocarbonate anion (ON2OCO2−), peroxynitrite (OONO−), nitronium (NO2+), dinitrogen dioxide (N2O2), exceptionally reactive lipids, and carbonyl compounds derived from carbohydrates. The generation of ROS is strictly controlled in healthy cells and is crucial for the signaling of immunological response, autophagy, inflammation, cell division, and stress response. However, excessive production of ROS can result in cytotoxicity and oxidative stress, which can cause cellular malfunction and aid in the development of a number of diseases, including cancer. 3. The main goal of this review study’s main goal is to provide updated information on the connection between free radicals and the onset of cancer, as well as the function of antioxidants in the immune system’s defense mechanism against free radicals. Additionally, a discussion onvarious approaches for manipulating antioxidants and free radicals in the prevention and management of cancer is also included.
Methodology
The bibliographic search was analysed from worldwide established data sources like Pubmed, Google Scholar, ScienceDirect, SpringerLink and references from relevant articles. The terms included in the search were “cancer”, “free radicals”, “oxidative stress” and “antioxidants”.
Sources of free radicals
ROS can be produced in cells through a variety of chemical processes, including enzymatic reactions, toxic compound exposure, tobacco smoke, ultraviolet and ionizing radiation, and other environmental factors.4. Mitochondria’s electron transport chains are the most significant source of superoxide anions (O2•–) in aerobic cells. During metabolic processes 1-5% of oxygen escapes as free radicals from mitochondria. Oxidative reactions catalysed by enzymes such as cytochrome P450, cyclooxygenases, lipoxygenases, dehydrogenases, and peroxidases can generate free radicals 5,6. Other possible locations for the synthesis of O2•– and hydrogen peroxide (H2O2) include Fe-S proteins and NADH dehydrogenases. When xanthine is converted to uric acid, xanthine oxidase generates superoxide anions7,8, but neutrophil plasma membrane NAD(P)H oxidase produces O2•–either inside the membrane or on its exterior. When H2O2 comes into contact with some transition metal ion chelates, especially ferrous iron (Fe2+) and cuprous copper (Cu+), hydroxyl radicals may be produced.9.
Cellular formation of reactive oxygen species
ROS are created as a consequence of the oxygen that is consumed during aerobic cellular metabolism. Since mitochondrial respiration uses up to 80% of the oxygen used in oxidative phosphorylation, it is the intrinsic source of ROS production. On the inner membrane of mitochondria, the electron transport chain (ETC), which is made up of five complexes (I–IV) and ATP synthase, is in charge of producing most of the ATP required for cellular energy. However, the ETC is also a major source of ROS production, as electrons leak from the chain and react with oxygen to form superoxide radicals and other ROS. Even though ROS can be crucial for cellular signaling and defense against infections, excessive ROS production can cause oxidative stress and harm to DNA, lipids, and proteins in cells. 10,11.
Reactive oxygen species (ROS) are mostly produced by mitochondria during cellular respiration when electrons are transferred to the terminal electron acceptor of molecular oxygen via the electron transport chain (ETC). Superoxide radicals are produced when electrons react with O2, with complexes I, II, and III contributing the most to redox signalling. The primary source of these radicals is the intermembrane space within mitochondria, and they have the ability to enter the cytoplasm via the mitochondrial permeability transition pore. Superoxide dismutase catalyses the conversion of superoxide to hydrogen peroxide, which can diffuse readily as a secondary messenger. Peroxisomes also generate ROS through xanthine oxidase. Endogenous and exogenous metabolites, drugs, and immune responses can also lead to ROS production. The enzyme NADPH oxidases (NOXs) can also produce ROS as part of the oxidative burst during inflammatory reactions 12.
Free radicals interfere in carcinogenesis
A single cell clone is subject to a cumulative effect of multiple events during the complex process of cancer development. It is well-established that free radicals play a part in the development and spread of cancer. The general model of cancer development involves three stages 13 (Figure 1):
Initiation
In this phase, a somatic cell’s genetic makeup undergoes a permanent alteration due to factors like free radical-induced DNA damage. This initial event may be caused by exposure to various factors, including environmental toxins, genetic mutations, or viral infections.
Promotion
Following the initiation stage, the mutated cell clone undergoes expansion and multiplication, resulting in the growth of a precancerous lesion or tumour. This stage is characterized by the accumulation of additional mutations and alterations that provide the mutated cell with a growth advantage over normal cells.
Progression
In the final stage of cancer development, the precancerous lesion or tumour becomes malignant, invading surrounding tissues and spreading to other parts of the body. This stage is characterized by a further accumulation of genetic alterations that promote tumour growth, invasion, and metastasis.
Free radicals’ involvement in the initiation and progression of cancer highlights the significance of oxidative stress in the disease’s development as well as the promise of antioxidant therapies for both cancer prevention and treatment 14.
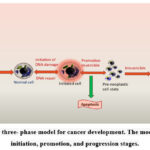 |
Figure 1: The three- phase model for cancer development. The model represents initiation, promotion, and progression stages. |
Tumor promotion
Oxidative stress plays a significant role in promoting carcinogenesis, with several tumour promoters believed to stimulate the production of endogenous oxygen radicals by altering cellular metabolic processes 15. In addition to reactive nitrogen species (RNS), reactive oxygen species (ROS) can temporarily modify genes related to cell proliferation or death, leading to the expression of mutated cell clones. Low levels of oxidative stress can stimulate cell division and promote tumour growth, while high levels can halt proliferation through cytotoxic effects. Thus, encouraging intracellular reactive species production is thought to be the main way that free radical-mediated tumors are caused 16.
Toyokuni et al. conducted in vitro experiments which demonstrated that specific transcription factors, including p53, nuclear factor (NF)-κB, and activator protein (AP)-1, were repressed in an oxidative environment and activated in a reductive one. On the other hand, oxidation had the opposite effect on other transcription factors, activating some while repressing others. 17.
Tumor progression
The process of carcinogenesis entails tumor cells acquiring malignant characteristics, which are typified by accelerated cell proliferation, immune surveillance evasion, tissue invasion, and metastasis 17. Certain tumors have the potential to transform, impede anti-protease activity, and cause harm to nearby tissues due to the production of copious amounts of free radicals and an increase in oxidatively modified DNA bases 18. On the other hand, elevated amounts of altered DNA bases might also be linked to genetic instability and the capacity of fully grown cancer cells to spread 19. Studies indicate that while damage within a certain range may be active and that excessive damage may have an anti-cancer effect by inducing apoptosis, oxidative DNA base damage alone may not be sufficient to cause the development of cancer 20.
Healthy people normally produce ROS through aerobic metabolism; however, in order to prevent and neutralize ROS activity and repair cell damage, cells have evolved a variety of antioxidant mechanisms. Oxidative stress, however, can result from an imbalance between ROS and antioxidants. Overexposure to reactive oxygen species (ROS) can damage the intracellular environment, resulting in diseased cells and altering gene expression that contributes to the pathogenesis of cancer21.
ROS like O2•-have the ability to cross cell membranes and modify lipids, proteins, and nucleic acids, which can have deleterious effects on embryonic cells, mitochondria, ATP levels, and apoptosis. Furthermore, ROS causes lipid peroxidation, which impacts mitochondrial dysfunction, metabolic transport, and cell division22. Table 1 represents ROS and RNS with the associated cancer development.
Table 1: The roles of ROS and RNS in the development of cancer with involved interactions
|
Reactive Species |
Production |
Interactions |
Associated cancer |
Half-life (seconds) |
Reference |
|
ROS Hydroxyl radical (OH•) |
Excess iron levels in the body can lead to the production of free radicals through the Fenton reaction. |
carbohydrates, nucleic acids, lipids and proteins |
Bronchogenic and colorectal carcinoma |
10-9 |
23 |
|
Superoxide (O2•-) |
Produced in mitochondria and cardiovascular system |
reactive oxygen species (ROS), can react with and inactivate enzymes that contain iron-sulfur clusters |
Colorectal carcinoma |
10-6 |
23 |
|
Hydrogen peroxide (H2O2) |
Generated during metabolic reactions |
Interacts with lipids, proteins and nucleic acids |
Hepatocellular carcinoma |
stable |
23 |
|
RNS Nitric oxide (NO•) |
Neurotransmitter |
nucleic acids deamination and breakdown |
Breast cancer |
5 |
24,25 |
|
Peroxynitrite (ONOO–) |
Produced from NO• and O2• – |
Activation of COX gene |
Breast cancer, Cervical cancer |
10-3 |
26 |
Free radicals, inflammation and cancer
Inflammation is a contributing factor to the development of cancer as it triggers oxidative stress, which in turn promotes further inflammation. The processes of oxidative stress and inflammation are essential to the development of cancer. Oxidative stress produces reactive oxygen species (ROS), which have the potential to damage DNA and trigger signaling pathways that disturb the cell cycle, thereby elevating the risk of cancer development. Inflammation and ROS interact, intensifying their effects and promoting the development of cancer. Through its impact on immune surveillance, the inflammatory response also affects the development of tumors. Tumor-invading immune cells collaborate with cancerous cells to orchestrate various pathways that jointly foster the development of tumors 23. Figure 2
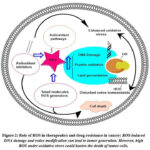 |
Figure 2: Role of ROS in therapeutics and drug resistance in cancer: ROS-induced DNA damage and redox modification can lead to tumor generation. |
Role of mediators Involved in the Inflammation and Carcinogenesis
Chemokines are important in cancer-related inflammation, and normal cells can become cancerous due to downstream genetic events involving their receptors and ligands. These components are often expressed in chronic inflammation, which increases the risk of cancer. The chemokine system affects various pathways involved in tumor progression, such as recruitment of immune cells, formation of new blood vessels, cell growth and division, survival, invasion, and spread of cancer cells to other parts of the body.
Both preclinical and clinical studies have shown that targeting the chemokine system could be a promising approach for developing new cancer therapies. By intervening in this system, it may be possible to slow or stop the growth and spread of cancer cells24.
Cytokines and their role in development of cancer
Interleukins
Nitric oxide (NO) and reactive oxygen species (ROS) produced by epithelial cells can damage DNA. IL-1β also stimulates the production of IL-6, IL-11, and IL-22 from myeloid and epithelial cells, as well as IL-22 from type 3 innate lymphoid cells (ILC3s). Hence, in situations of unchecked chronic activation, the inflammatory responses start cellular programs and may be directly linked to the development of tumors.26,27.
TNF-α (Tumor Necrosis Factor- α)
Through its ability to stimulate the production of genotoxic molecules like nitric oxide (NO) and reactive oxygen species (ROS), TNF-α plays a role in the initiation of tumors. These substances have the ability to damage and mutate DNA, which can activate oncogenes and deactivate tumor suppressor genes. Furthermore, TNF-α promotes the recruitment of inflammatory cells, such as macrophages, to the tumor microenvironment. Growth factors and cytokines released by these cells encourage the survival and growth of tumor cells while inhibiting the function of cytotoxic T lymphocytes, which are essential for the immune system’s defense against cancer.
Overall, the induction of NF-кB-dependent anti apoptotic molecules and the recruitment of inflammatory cells in the tumor microenvironment by TNF-α contribute to the growth, survival, and progression of tumors, and represent attractive targets for the development of novel cancer. 28,29,30,31,32.
Angiogenesis in cancer
The growth and spread of tumors rely on the formation of new blood and lymphatic vessels, which are triggered by chemical signals from cancer cells during periods of rapid growth. Studies have shown that cancer cells grew to a certain size without vascular support, but required angiogenesis to continue growing beyond that point. Without proper blood supply, tumors may become necrotic or apoptotic.33.
Antioxidants against free radicals
Biological cells have their own protective mechanisms against harmful free radicals, such as ROS and RNS, which can damage cellular structures. Antioxidants are substances that can neutralize these free radicals and prevent them from causing harm. These substances can scavenge free radicals and disrupt oxidative chain reactions. Vitamin E, reacts with soluble free radicals in lipid membranes to prevent lipid peroxidation.34,35,36,37,38,39.
Antioxidant enzyme level in cancer
Antioxidants come in two varieties: enzymatic and non-enzymatic. Catalase, glutathione reductase, GSH-Px, SOD, and glutathione peroxidase are examples of enzymatic antioxidants that function by oxidizing glutathione and reducing hydrogen peroxide. Synthetic or dietary supplements like taurine, hypotaurine, zinc, selenium, glutathione, beta-carotene, and vitamins C and E are examples of non-enzymatic antioxidants 40.
The effect of antioxidants on cancer cells is complex and depends on various factors. According to recent research, radiation and chemotherapy may slightly damage DNA, causing apoptosis—programmed cell death—instead of necrosis. Antioxidant therapies have the ability to trigger apoptotic pathways and may work in concert with chemotherapy or radiation therapy. It is well known that many cancer cells have compromised defense mechanisms, which prevents them from utilizing additional antioxidants for repair41,42.
Studies have shown that antioxidant enzymes and detoxifiers can inhibit tumor initiation and promotion. However, the antioxidant status of cancer patients can vary depending on the stage of the cancer and the type of cancer. Studies have found that the antioxidant content of cervical cancer in its late stages is lower than that of the disease in its early stages, but other studies have found that certain antioxidant levels are higher in certain tumor types 43,44,45,46,47.
Polymorphisms in MnSOD (a type of SOD enzyme), has been linked to a number of cancer types and may predispose people to developing cancer. Selenium’s chemopreventive defense mechanism is thought to work by scavenging reactive oxygen species (ROS) and enhancing the synthesis of the enzymatic antioxidant GSH-Px. Selenium has been demonstrated to have a preventive role against cancer in various organs and species. Patients with breast and colon cancers have lower levels of the GSH/GSSG ratio in their blood, particularly when the cancer has progressed to an advanced stage. This could be because of increased peroxide generation and altered GSH-related enzymes. When tumors’ levels of protein synthesis and cell proliferation decline, their antioxidant content also does 48,49,50,51,52,53,54,55.
Antioxidants in cancer therapy
Antioxidant enzymes protect cells by neutralizing harmful free radicals. The potential of antioxidants to prevent cancer has been well studied, however there are concerns with using them during chemotherapy [56]. Combining antioxidants with chemotherapy may lessen its efficacy because certain chemotherapeutic agents produce free radicals that harm and kill tumor cells. Chemotherapy’s main objective is to kill tumor cells by causing irreversible DNA damage. Supplementing with antioxidants may help lower toxicity and enhance long-term results, but the results vary depending on the patient’s metabolic state, the stage and location of the disease, and the type of treatment used57.
Chemotherapy with antioxidants
Chemotherapeutic or anticancer medications only effect dividing cells; they specifically target DNA synthesis. The percentage of cells that are actively dividing determines how effective these medications can be. Free radicals are not necessary for the majority of anticancer medications; however, some, like bleomycin, doxorubicin, and cisplatin, create them as part of their therapeutic process58. Doxorubicin, for example, is more toxic to hypoxic cells. Antioxidants can protect normal cells during all treatments, including those that do not involve free radicals59. They support the preservation of normal tissue integrity and shield it from the damaging effects of circulating free radical-producing cytokines, which are more prevalent in cancer patients as the disease progresses.
Enhancing the availability of antioxidants can also be achieved by genetically modifying plants to produce vegetables with increased concentrations of these compounds. For instance, researchers have developed tomatoes with a longer shelf life and up to three times the concentration of lycopene. Additionally, “orange cauliflower” is a rich source of carotene. To get the most antioxidant benefits, it is advised to eat fruits and vegetables with ORAC values of 3000–5000 per day60.
Bioactive nanoparticles, which can function as efficient and focused drug delivery platforms by overcoming biological, biophysical, and biomedical barriers, are the result of the application of nanotechnology in biomedicine. Nanotechnology-based targeted cancer chemotherapy has been suggested as a way to boost drug absorption at particular target sites while avoiding issues related to traditional cancer chemotherapy. For this reason, a variety of nanotechnologically based systems have been developed, including stealth nanoparticles, liposomes, stealth liposomes, pH and temperature-sensitive liposomes, nanoparticles, nanofibers, nanocapsules, nanorods, nanocrystals, and nanotubes61. These delivery materials minimize toxicity, maximize therapeutic index, and enhance drug biodistribution by allowing the drug to be selectively and effectively localized at pre-identified target sites (e.g., overexpressed receptors in cancer) at therapeutic concentrations while preventing access to non-target sites. This is essential to the effectiveness of cancer chemotherapy62.
Novel advancement to minimize free radical damage and their future aspects
In an effort to improve human health, new methods for researching antioxidants and free radicals have been developed. Free radicals and antioxidants may decrease in cancer patients due to physiological and genetic changes. Consuming foods high in dietary antioxidants may help lower the risk of cancer and other diseases caused by free radicals, according to studies SOD mimetics derived naturally, which have a lower molecular weight, greater stability, and do not elicit an immune response in the body, may serve as effective drugs. They can also increase the antitumor effects of interleukins and act as radio protectors. Antioxidant enzymes (such SOD, GPx and reductase, CAT, etc.) that are highly selective and detoxify particular free radicals make up enzymatic antioxidants. The initial line of defense against free radicals/ROS is comprised of non-enzymatic antioxidants or antioxidants derived from nutrients, such as ascorbic acid, Vitamin E, carotenoids, and glutathione, etc.63 Table 2.
 |
Table 2: Medicinal Importance of Non-enzymatic Antioxidants |
Conclusion
Several studies have shown that changes in cell metabolism are essential for the emergence of different forms of cancer. Oxidative stress’s effects on the development, spread, and response to treatment of cancer are still being extensively studied. Reactive oxygen species have both positive and negative effects, and researchers are exploring their potential therapeutic benefits. There is ongoing debate about whether antioxidant supplementation or inhibition of ROS modulation is helpful or harmful in cancer treatment. Cancer cells depend on antioxidants for survival and growth, and they need a higher antioxidant capacity to combat elevated ROS levels. Antioxidant inhibitors show promise as a therapeutic strategy for the treatment of cancer.
Acknowledgments
The management’s assistance and facilities are greatly appreciated by the writers.
Author contribution
The first author is thanksful to the management for providing the assistance and facilities to accomplish this work.
Conflict of Interest
There is no conflict of interest.
Funding Sources
The authors reported no funding received for this study.
References.
- Kim J.; Kim J.; Bae J.S. Experimental & molecular medicine, 2016, 48, e269.
CrossRaf - Traverso N.; Ricciarelli R.; Nitti M.; Marengo B.; Furfaro A.L.; Pronzato M.A.; Marinari U.M.; Domenicotti C. Oxid Med Cell Longev., 2013, 2013:972913.
CrossRaf - Liou G.Y.; Storz P. Free radical research, 2010, 44, 479-96.
CrossRaf - Phaniendra, A.; Jestadi D.B.; Periyasamy L. Ind J Clin Biochem., 2015, 30, 11–26.
CrossRaf - Halliwell; Barry; John M. C.; Gutteridge. Free Radicals in Biology and Medicine, 5th edn (Oxford, 2015; online edn, Oxford Academic, 22 Oct. 2015), accessed 6 June 2023.
- Raha S.; Robinson B.H. Trends in biochemical sciences. 2000, 125, 502-8.
CrossRaf - Turrens J.F.; Freeman B.A.; Crapo J.D. Archives of biochemistry and biophysics, 1982, 217, 411-21.
CrossRaf - Nishino T.; Okamoto K.; Eger B.T.; Pai E.F.; Nishino T. The FEBS Journal, 2008, 275, 3278-89.
CrossRaf - Jomova K.; Valko M. Toxicology, 2011, 283, 65-87.
CrossRaf - Danse T.B.; Wirtz K.W. IUBMB Life, 2001, 51, 223–230.
CrossRaf - Wright D.T.; Cohn L.A.; Li H.; Fischer B.; Li C.M.; Adler K.B. Environmental health perspectives, 1994, 102, 85-90.
CrossRaf - Arvind P.; Malaya K.S.; Diana O.; Sanjay B. Cell Molec. Immunol., 2015, 12, 5–23.
- Ahmed M.I.; Fayed S.T.; Hossein H.; Tash F.M. Disease markers, 1999, 15, 283-91.
CrossRaf - Agarwal A.; Gupta S.; Sikka S. Current opinion in obstetrics and gynaecology. 2006, 18, 325-32.
CrossRaf - Trueba G.P.; Sánchez G.M.; Giuliani A. Frontiers in Bioscience-Landmark, 2004, 9, 2029-44.
CrossRaf - Burdon R.H. Free Radical Biology and Medicine, 1995, 18, 775-94.
CrossRaf - Cooke M.S.; Evans M.D.; Dizdaroglu M.; Lunec J. The FASEB Journal, 2003, 17, 1195-214.
CrossRaf - Malins D.C.; Polissar N.L.; Gunselman S.J. Proceedings of the National Academy of Sciences. 1996, 93, 2557-63.
CrossRaf - Schmielau J.; Finn O.J.; Cancer research, 2001, 61, 4756-60.
- Thomas E.; Brewster D.H.; Black R.J.; Macfarlane G.J. Int J Cancer, 2000, 88, 497-502.
CrossRaf - Agarwal A.; Said T.M. BJU international, 2005, 95, 503-7.
CrossRaf - Kaushal N.; Kudva A.K. Journal of Postdoctoral Research, 2013, 1, 89-101.
- Yeung M.L.; Jeang K.T. Pharmaceutical research, 2011,28, 3043-9.
CrossRaf - Zhang H.J.; Zhao W.; Venkataraman S.; Robbins M.E.; Buettner G.R.; Kregel K.C.; Oberley L.W. J Biol Chem., 2002, 277, 20919-20926.
CrossRaf - Allavena P.; Germano G.; Marchesi F.; Mantovani A. Experimental cell research, 2011, 317, 664-73.
CrossRaf - Raman D.; Baugher P.J.; Thu Y.M.; Richmond A. Cancer letters, 2007, 28, 256137-65.
- Hoesel B.; Schmid J.A. Mol Cancer, 2013, 12, 1-15.
CrossRaf - Liang Y.; Zhou Y.; Shen P. Cell Mol Immunol., 2004, 1, 343–350.
- Fan Y.; Mao R.; Yang J. Protein & cell. 2013 Mar, 4, 176-85.
CrossRaf - Fernandes J.V.; Cobucci R.N.; Jatobá C.A.; de Medeiros Fernandes T.A.; de Azevedo J.W.; de Araújo J.M. Pathology & Oncology Research. 2015 Jul, 21, 527-34.
CrossRaf - Parangi S.; O’Reilly M.; Christofori G.; Holmgren L.; Grosfeld J.; Folkman.; Hanahan D. Proceedings of the National Academy of Sciences. 1996 Mar 5, 93(5), 2002-7.
CrossRaf - Silva F.; Marques A.; Chaveiro A. The Open Veterinary Science Journal. 2010, 4(1), 127-133.
CrossRaf - Agarwal A.; Said T.M. BJU international. 2005 Mar, 95(4), 503-7.
CrossRaf - Pierce J.D.; Cackler A.B.; Arnett M.G. Rn. 2004 Jan 1;67(1), 38-42.
- Packer L.; Colman C. John Wiley & Sons, New York, 1999,
- Wolf G. The Journal of nutrition. 2005 Mar 1;135(3):363-6.
CrossRaf - Kirsch M.; De Groot H. The FASEB Journal. 2001 Jul, 15(9):1569-74
CrossRaf - Vivekananthan D.P.; Penn M.S.; Sapp S.K.; Hsu A.; Topol E.J. The Lancet. 2003, 9374, 2017-23
CrossRaf - Sies H. Academic Press. New York, NY, USA. 1996,9.
- Noguchi T.; Shibata T.; Fumoto S.; Uchida Y.; Mueller W.; Takeno S. Annals of surgical oncology, 2002, 9, 1017-22.
CrossRaf - Mediavilla M.D.; Cos S.; Sanchez-Barcelo E.J. Life sciences, 1999, 4, 415-20.
CrossRaf - Ahmed M.I.; Fayed S.T.; Hossein H.; Tash F.M. Disease markers, 1999, 4, 283-91.
CrossRaf - Oberley T.D.; Oberley L.W. Histology and histopathology, 1997,12, 525-535.
- Trueba G.P.; Sánchez G.M.; Giuliani A. Frontiers in Bioscience-Landmark, 2004, 3, 2029-44.
CrossRaf - Dasari S.; Wudayagiri R.; Valluru L. Free Radicals and Antioxidants, 2013, 2, 87-92.
CrossRaf - Kong Q.; Lillehei K.O. Medical hypotheses, 1998, 5, 405-9.
CrossRaf - Czarnecka A.M.; Campanella C.; Zummo G.; Cappello F. Cancer biology & therapy. 2006, 7, 714-20.
CrossRaf - Izutani R.; Asano S.; Imano M.; Kuroda D.; Kato M.; Ohyanagi H. Journal of gastroenterology, 1998, 33, 816-22.
CrossRaf - Kinnula V.L.; Crapo J.D. Free Radical Biology and Medicine, 2004,6, 718-44.
CrossRaf - El-Bayoumy K.; Narayanan B.A.; Desai D.H.; Narayanan N.K.; Pittman B.; Amin S.G.; Schwartz J.; Nixon D.W. Carcinogenesis, 2003, 24, 1505-14.
CrossRaf - Subramanyam D.; Subbaiah K.C.; Rajendra W.; Lokanatha V. Experimental oncology, 2013, 35, 97-100.
- Patrick L. Alternative medicine review, 2004, 9, 239-258.
- Carretero J.; Obrador E.; Anasagasti M.J.; Martin J.J.; Vidal-Vanaclocha F.; Estrela J.M. Clinical & experimental metastasis, 1999, 17, 567-74.
CrossRaf - Navarro J.; Obrador E.; Carretero J.; Petschen I.; Avino J.; Perez P.; Estrela J.M. Free Radical Biology and Medicine, 1999, 26, 410-8.
CrossRaf - Singh D.K.; Lippman S.M.; Oncology, 1998, 12, 1643-53.
- Vande Creek L.; Rogers E.; Lester J. Alternative Therapies in Health and Medicine, 1999, 5, 71.
- Borek C. Integrative cancer therapies, 2004, 3, 333-41.
CrossRaf - Sözen S.; Coskun U.; Sancak B.; Bukan N.E.; Günel N.; Tunc L.; Bozkirli I. Neoplasma, 2004, 51, 25-9.
- Lachnicht D.; Brevard P.B.; Wagner T.L.; DeMars C.E. Nutrition research, 2002, 12, 1389-99.
CrossRaf - Grodzinski P.; Silver M.; Molnar L.K. Expert review of molecular diagnostics, 2006, 6, 307-18.
CrossRaf - Kakde D.; Jain D.; Shrivastava V.; Kakde R.; Patil A.T. Journal of Applied Pharmaceutical Science, 2011, 01, 01-10.
- Haider K.; Haider M.R.; Neha K.; Yar M.S. European Journal of Medicinal Chemistry, 2020, 204, 112607.
CrossRaf - Gülçin, İ.; Innovative Food Science & Emerging Technologies, 2010, 1, 210-218.
CrossRaf - Espíndola K.M.M; Ferreira R.G.; Narvaez L.E.M.; Silva Rosario A.C.R.; da Silva A.H.M.; Silva A.G.B.; Vieira A.P.O.; Monteiro M.C. Frontiers in oncology, 2019, 9, 541.
CrossRaf - Song Y.; Tang L.; Han J.; Gao Y.; Tang B.; Shao M.; Yuan W.; Ge W.; Huang X.; Yao T.; Bian X.; Li S.; Cao W.; Zhang H. Oxidative Medicine and Cellular Longevity, 2019, 2019:3435174.
CrossRaf - Cutler R.G.; Camandola S.; Feldman N.H.; Yoon J.S.; Haran J.B.; Arguelles S.; Mattson M.P.; Neurobiology of Aging, 2019, 75, 159-168.
CrossRaf - Olszowy M.; A.L. Dawidowicz, and M. Jóźwik-Dolęba. European Food Research and Technology, 2019, 245, 1473-1485.
CrossRaf - Gülçin İ. Toxicology, 2006, 2-3, 213-220.
CrossRaf - Jadhao K.; Poonam R.G. Journal of Global Biosciences, 2016, 5, 4638-4642.
- Varvara M.; Bozzo G.; Celano G.; Disanto C.; Pagliarone C.N.; Celano G.V. Ital J Food Saf., 2016, 5, 4313.
CrossRaf - Ullah A.; Munir S.; Badshah S.L.; Khan N.; Ghani L.; Poulson B.G.; Emwas A.-H.; Jaremko M. Molecules 2020, 25, 5243.
CrossRaf - Darband S.G; Kaviani M.; Yousefi B.; Sadighparvar S.; Pakde, F.G.; Attari J.A.; Mohebbi I.; Naderi S.; Majidinia M. J. Cell. Physiol. 2018, 233, 6544–6560.
CrossRaf

This work is licensed under a Creative Commons Attribution 4.0 International License.










