A pH Responsive and Selective Complex Formation analysis of Catechol Based Dipodal Chelator ligand N1,N3-bis(2-(((Z)-2,3-dihydroxybenzylidene)amino)ethyl) malonamide with Trivalent Metal Ions
Amardeep1, Vijay Dangi1* , Pramod Kumar1
, Pramod Kumar1 , Meenakshi1
, Meenakshi1 , Minati Baral2
, Minati Baral2 , Brahamdutt Arya3,4* and Taruna Sheoran5
, Brahamdutt Arya3,4* and Taruna Sheoran5
1Department of Chemistry, Baba Mastnath University, Rohtak, Haryana-124021, India.
2Department of Chemistry, NIT, Kurukshetra, Haryana-136119, India.
3Department of Higher Education, Shiksha Sadan, Sec – 5, Panchkula, Haryana- 134114, India.
4Y and Y Nanotech Solutions Private Limited, Rohtak, Haryana-124001, India.
5Department of Bio and Nano Technology, GJU of S & T, Hisar, Haryana-125001, India.
Corresponding Author E-mail: 91dangi@gmail.com, brahm.chem@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/400133
Article Received on : 23 Nov 2023
Article Accepted on : 03 Jan 2024
Article Published : 18 Jan 2024
Reviewed by: Dr. Venkatesh R
Second Review by: Dr. Buddhadeb Dutta
Final Approval by: Dr. S. A. Iqbal
In the present study, we have explored the binding abilities of powerful dipodal chelator ligand N1,N3-bis(2-(((Z)-2,3-dihydroxybenzylidene)amino)ethyl)malonamide (MEC) to trivalent metal ions. We have investigated the coordination behavior of the dipodal ligand MEC with the trivalent metal ions Al3+, Fe3+, and Cr3+ in aqueous media ranging from pH 2 to 11, employing potentiometry and spectrophotometry techniques. Results shows the higher binding ability of Cr+3 metal ion among all metal ions with largest formation constant value, log β = 28.56.
KEYWORDS:Binding Affinity; Chelate; Formation Constant; Stability Constant; Spectrophotometry; Schiff’s Base
Download this article as:| Copy the following to cite this article: Amardeep A, Dangi V, Kumar P, Meenakshi M, Baral M, Arya B, Sheoran T. A pH Responsive and Selective Complex Formation analysis of Catechol Based Dipodal Chelator ligand N1,N3-bis(2-(((Z)-2,3-dihydroxybenzylidene)amino)ethyl) malonamide with Trivalent Metal Ions. Orient J Chem 2024;40(1). |
| Copy the following to cite this URL: Amardeep A, Dangi V, Kumar P, Meenakshi M, Baral M, Arya B, Sheoran T. A pH Responsive and Selective Complex Formation analysis of Catechol Based Dipodal Chelator ligand N1,N3-bis(2-(((Z)-2,3-dihydroxybenzylidene)amino)ethyl) malonamide with Trivalent Metal Ions. Orient J Chem 2024;40(1). Available from: https://bit.ly/3S6Nij0 |
Introduction
From the past few decades, synthesis of novel molecular systems for detection of metal ions from transition and post-transition series have attracted numerous scientific interest to study and analyze the various biological and environmental processes1-3. Among all elements, few trivalent metal ions like Cr3+, Fe 3+ and Al3+ are of great importance and have found numerous applications. The role of Cr3+ in the metabolism of biological active components like carbohydrates, lipids, fats, various proteins, and several essential nucleic acids makes its quite important and a vital component in a balanced diet for both humans and animals4–8. In has also been found in several studies that Cr3+ deficiencies can severely affect the lipid metabolism which can further leads to abnormal diabetic levels9,10. On the other hand, excessive Cr3+ consumption can cause toxicity to cellular level and may hugely affect the basic cellular structure11,12.
Additionally, chromium is employed in various industrial operations like electroplating, preparing chromate, tanning leather, and metal polishing. Due to excessive application and poor disposal strategies of trivalent metal ions in industrial and agricultural sector, they have emerged as a chief source of environmental pollutants. Among all trivalent metal ions, Cr3+ cause severe toxicity to environment even at very low concentrations13. Therefore, it becomes imperative to selectively detect Cr3+ ions in biological and environmental samples at lower concentration levels with high precision. Recently, a lot of efforts have been made to fabricate devices made of novel molecular systems for precise detection of trivalent metal ions in biological and environmental spheres.
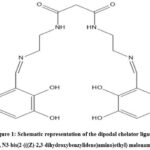 |
Figure 1: Schematic representation of the dipodal chelator ligand N1, N3-bis(2-(((Z)-2,3-dihydroxybenzylidene)amino)ethyl) malonamide. |
Schiff bases owing to their capabilities to selectively combine with metal ions to form stable complexes as well as their high reaction potential with particular metal ions have emerged as potential molecular systems for detections of metal ions in biological systems. They can be used for developing synthetic enzymes as well as for potentiometric detection of homogeneous and heterogeneous processes such as those involving polymers, colors, and chemical framework14. Edifices of metal ions with Schiff bases have been extensively studied as potent antiviral, anticancer, antibacterial, and antifungal agents in medicine and pharmaceuticals15–17. Due to their insoluble nature and hydrolysis in a water medium, very little work has been done to determine the formation constants of these frameworks in a solution. There is still plenty of room to synthesize novel Schiff bases that exhibit more promising binding capabilities, photo physical properties, and beneficial coordination conduct with metal ions of organic relevance. This can be accomplished by modelling and improving the stability of their binding domains. One of the strategies is the proton exchange between hydroxyl oxygen and imine nitrogen in excited states, which, depending on the dissolvable pH and extremity, can result in either an enol-imine form or a keto-amine form18.
In light of the aforementioned perspective, the current work examined the behavior of MEC (as shown in figure 1) in terms of coordination with the trivalent metal ions Al3+, Fe3+, and Cr3+ in a series of pH variation broadly in acidic, neutral and basic conditions. This study aims to provide the comparative experimental analysis of stability and formation constant of ligand MEC with trivalent ions and this study will provide the basis for application of ligand MEC for precise extraction of these metal ions from various environmental and industrial sources.
Materials and Methods
Metal salts were purchased from CDH and Rankem, and DMSO was bought from Loba Chemie. Solutions were prepared employing ultrapure milliQ water with a resistivity of 18.2 milli ohm cm-1. Due to DMSO’s solubility, all potentiometric titrations were conducted in a 9:1 v/v mixture of water and DMSO. Dipodal chelator ligand MEC was procured from NanoZest Incorporation, India. Spectrophotometric analysis was carried out utilizing a quartz cuvette and an Thermoscientific company made Evolution 201 UV-Vis spectrophotometer. Further, Thermo Scientific made pH meter model: Orion Star A111 and Ross made Ultra-Electrode Model: 8102BNUWP were used for the potentiometric titrations, and both instruments were calibrated according to a set procedure using buffer solutions.
Potentiometric Measurements: Determination of Protonation and Formation Constants
In order to investigate the synthesis of metal complexes and their respective formation constants, potentiometric titration analysis was carried out at 25 oC by using a double-walled titration flask. Further, the pH titration analysis was performed using a pH meter and Ross Ultra combination pH electrode. To correctly and precisely read the pH, the electrode calibration was carried out using the traditional method. Briefly, the complex formation capabilities of metal – ligand complex is studied by titrating a 1:1 metal-ligand solution of 1x 10-4 M against 0.1 M KOH, for each set of synthesized ligand with Al3+, Fe3+, and Cr3+. Further, the value of formation constants (logβ) for each of metal- ligand complexes were calculated, and distinct species that formed at various pH values were anticipated. To determine the equilibrium constants, the titration data between pH range 2 – 11 were refined using the non-linear least square refinement programme Hyperquad 200619.
Spectrophotometric Measurements
Similar procedures to those used for potentiometric titrations were used for spectrophotometric titrations. These titrations involved adding 0.1 M HCl to an H2O: DMSO (9:1, v/v) solution containing ligand and metal ions (1 x 10-4 M) to make it more acidic, and 0.1 M KCl to keep the ionic concentration/strength constant. The final product was titrated in a pH range of 2 to 11 against a freshly prepared, standardized KOH solution of 0.1 M. A thermostat glass jacketed jar was utilized to keep the temperature in the spectrophotometric titrations at 25 oC throughout the experiment. The values of binding constants were calculated employing HYPSPEC software by analyzing the titration data points20.
Table 1: Equilibrium reactions and stability constants (log β) of the ligand MEC with metal ions Al3+, Fe3+ and Cr3+ respectively, (where A stands for potentiometric titration values and B stands for spectrophotometric analysis).
|
Metal Ions |
Equilibrium |
Logβ |
|
|
|
A |
B |
|
|
Al(III) |
Al + L ⇌ AlL |
24.62 |
24.62 ± 0.01 |
|
Al + LH2 ⇌ AlLH2 |
13.93 |
13.90 ± 0.03 |
|
|
Fe(III) |
Fe + L ⇌ FeL |
24.99 |
24.99 ± 0.02 |
|
Fe + LH2 ⇌ FeLH2 |
15.34 |
15.34 ± 0.00 |
|
|
Cr(III) |
Cr + L ⇌ CrL |
28.56 |
28.55 ± 0.01 |
|
Cr + LH2 ⇌ CrLH2 |
14.65 |
14.65 ± 0.01 |
|
Results and Discussion
Complexation Behavior Analysis of Ligand MEC
By using potentiometric and spectrophotometric titrations, the complex formation capabilities of the ligand MEC with trivalent transition metal ions Al3+, Cr3+, and Fe3+ were examined in order to access their respective coordination properties. Figure 2a, demonstrates the potentiometric titrations performed in a highly aqueous media (99:1 water: DMSO mixture) at μ = 0.1 M KCl and 25 ± 1 oC in 1:1 metal-ligand molar ratios. The appearance of saturation in the potentiometric curves reveals the occurrence of hydrolysis and in response to hydrolysis, turbidity was developed in the solution after attaining pH > 10. As a result, in order to neglect the hydrolysis behavior after pH 10, all the calculations were carried out using data points up to pH 10. Further, based on the calculations for the ML, MLH2, species for M = Al3+, Cr3+, and Fe3+, the best fit model was acquired and optimized. In addition, the calculations for overall stability constants (logβ) for all the complexes were carried out using the Hyperquad 2008 program and results are shown in Table 1. The ligand and its interactions with the trivalent metal ions were also assessed spectrophotometrically in order to provide additional support for the different species that were produced in the solution.
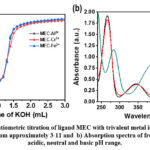 |
Figure 2: a) Potentiometric titration of ligand MEC with trivalent metal ions in varying pH media ranging from approximately 3-11 and b) Absorption spectra of free ligand MEC in acidic, neutral and basic pH range. |
In order to access the coordination behavior of the ligand MEC with trivalent metal ions viz. Al3+, Cr3+, and Fe3+ spectrophotometric titration technique was employed for confirmation of the possible complex formation in the solution. The titrations were performed in 1:1 M-L, [L] = [M] = 1 x10-4 M with increasing pH ranging from 2 to 11, at µ = 0.1 M potassium chloride (KCl) and Temperature = 25 ± 1 ºC. The electronic absorption spectra show no change in the range of pH 2-3, this pattern of electronic spectra remains same with the trivalent metal ions as shown by the ligand MEC alone. Figures 3 to 5 reveals the appearance of absorption spectra with varying λmax at different pH ranging from 3 to 11. The appearance of spectra with varying absorption wavelength and the comparative analysis of ε value for free ligand MEC and MEC-metal ion complex can be inferred for the formation of metal complexes.
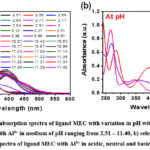 |
Figure 3: Experimental absorption spectra of ligand MEC with variation in pH with Al3+: |
Further, the appearance of turbidity after pH > 10 reveals the occurrence of hydrolysis in the metal-ligand system. As a result, the data points for metal-ligand titrations were not taken into consideration for calculation after pH >10. Furthermore, the formation of isosbestic points in the spectra can be referred to the state of equilibrium between the protonated and deprotonated structures of ligand. However, at above 3 pH, one prominent band appeared at ~266 nm with high intensity with all the metal complexes which showed, a concomitant rise in the intensity with successive increase of pH. For instance, in case of MEC-Cr3+ complex, absorption spectra of the complex shows a band at λ = 265.9 nm (Ɛ = 0.98 X 104 Lmol-1cm-1) corresponding to π→ π* transition showed a bathochromic effect to λ = 282.47 nm (Ɛ = 0.63 X 104 Lmol-1cm-1), band at λ = 344.69 nm (Ɛ = 0.21 X 104 Lmol-1cm-1) corresponding n→ π* transition experienced a red shift to λ = 391.4 nm (Ɛ = 0.418 X 104 Lmol-1cm-1) on changing pH of the solution from acidic to neutral whereas, on changing pH from neutral to basic, appearance of band for the ligand at λ = 282.47 nm (Ɛ = 0.63 X 104 Lmol-1cm-1) corresponding to π→ π* transition showed a hypsochromic effect to λ = 275.59 nm (Ɛ = 0.51 X 104 Lmol-1cm-1), band at λ = 391.4 nm (Ɛ = 0.418 X 104 Lmol-1cm-1) corresponding n→ π* transition experienced a blue shift to λ = 344 nm (Ɛ = 0.386 X 104 Lmol-1cm-1) respectively.
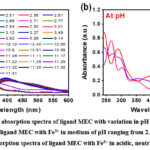 |
Figure 4: Experimental absorption spectra of ligand MEC with variation in pH with Fe3+ metal ion+ |
Further, it can be seen that the value of Ɛ for free ligand MEC at λ = 265.9 nm (Ɛ = 1.92 x 104 Lmol-1cm-1) as shown in figure 2b and that of with metal ions, a sudden drop in the value of Ɛ was observed. This impulsive fall in the Ɛ, can be inferred to the quenching of absorption being observed due to complexation of free MEC with metal ions. Similar nature of changes were also observed in the absorption spectrum of MEC ligand in the presence of other trivalent ions viz Fe3+ and Al3+ as depicted from table 2, acidic to neutral and neutral to basic, pH range signifying corresponding mode of complexation. Though, there is no additional band occurs at higher wavelength in Cr3+ complex, whereas, in Al3+ and Fe3+ complexes a charge transfer band is also observed above 400 nm.
Table 2: A comparative analysis of absorption wavelength and their respective value of molar absorptivity coefficient ε for all chemical species like free ligand MEC, MEC – Cr3+ complex, MEC – Al3+ complex and MEC – Fe3+ complexes in acidic, neutral and basic medium respectively.
|
S. No. |
Chemical Unit |
Acidic Medium |
Neutral Medium |
Basic Medium |
|
1
|
Free ligand MEC |
λ1 = 266.39 nm; ε = 1.92 x 104 λ2 = 344.77 nm; ε = 0.40 x 104 |
λ1 = 266.39 nm; ε = 1.82 x 104 λ2 = 346.14 nm; ε = 0.39 x 104 |
λ1 = 283.43 nm; ε = 1.16 x 104 λ2 = 391 nm; ε = 0.84 x 104 |
|
2 |
MEC- Cr3+ |
λ1 = 265.9 nm; ε = 0.98 x 104 λ2 = 344.69 nm; ε = 0.21 x 104 |
λ1 = 282.47 nm; ε = 0.63 x 104 λ2 = 391.4 nm; ε = 0.418 x 104 |
λ1 = 275.59 nm; ε = 0.51 x 104 λ2 = 344 nm; ε = 0.386 x 104 |
|
3 |
MEC-Fe3+ |
λ1 = 265.75 nm; ε = 0.76 x104 λ2 = 301.02 nm; ε = 0.39 x 104 λ3 = 345.50 nm; ε = 0.21 x 104 |
λ1 = 270.95 nm; ε = 0.58 x 104 λ2 = 291.90 nm; ε = 0.53 x 104 λ3 = 355.25 nm; ε = 0.23 x 104 |
λ1 = 287.99 nm; ε = 0.65 x 104 λ2 = 399 nm; ε = 0.28 x 104 |
|
4 |
MEC-Al3+ |
λ1 = 266.39 nm; ε = 0.93 x 104 λ2 = 345.49 nm; ε = 0.20 x 104 |
λ1 = 268.39 nm; ε = 0.72 x 104 λ2 = 358.53 nm; ε = 0.19 x 104 |
λ1 = 282.71 nm; ε = 0.63 x 104 λ2 = 393 nm; ε = 0.37 x 104 |
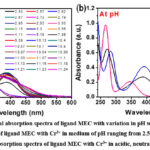 |
Figure 5: Experimental absorption spectra of ligand MEC with variation in pH with Cr3+ metal ion+: Click here to View Figure |
The HypSpec program was employed to determine the formation constants of the M3+ – MEC complexes in the spectrometric approach. When ML and MLH2 species for Cr3+, AL3+, and Fe3+ were taken into account for the calculation, the model produced the best fit curve. The values of the formation constants determined for the respective species are provided in table 1. From the investigation of coordination properties, we found that the ligand MEC shows high binding efficiency towards all the trivalent metal ions to form neutral ML complex to protonated complex (MLHn) at different pH condition, where protonated chromium complex (CrLH2) exhibits high binding constant (Log β = 28.56) compared to all other trivalent metal ions.
In a nutshell, co-ordination behavior of the ligand with the transition metal ions viz., Al3+, Cr3+, and Fe3+ in 1:1 molar ratio in a range of pH 2-11 were investigated in solution by means of potentiometric and spectrophotometric methods. The ligand exhibited a strong metal chelation efficiency with the trivalent metal ions coordinating through nitrogen of imine and oxygen of Ar-OH binding sites to form ML and MLHn kind of complexes. In the ligand, the protonated metal-ligand complex species are dominant in a highly acidic medium. Out of all the complexes of ML type, Cr3+ -MEC exhibited highest formation constant (log β = 28.56).
At pH > 10, formation of hydrolysis species occurred on account of hydrolysis of imine bonds. Considering ML, MLHn and the hydrolysis species (LH-n) in the calculations gave the best fit model to the curves obtained by using Hyperquad and HypSpec program.
Conclusion
In the present study, we have reported the pH responsive and selective complex formation capabilities of catechol based ligand MEC employing potentiometric and spectrophotometric techniques. The ligand MEC have shown excellent co-ordination capabilities with all trivalent metal ions used in this study. Further, it has shown maximum stability and formation constant (log β = 28.56) value for coordinating Cr3+ ion. It has also been found that after pH 10 i.e. in truly basic conditions, MEC gets hydrolyzed. From the above results it can be inferred that the dipodal chelator MEC have shown remarkable coordination excellence with trivalent metal ions analyzed in the study, and their potential employment for various applications as in metallurgy, chelation therapy and sequestering agents can be beneficial for mankind.
Acknowledgement
Dr Vijay Dangi and Dr Brahamdutt Arya duly acknowledge Dr B.K. Kanungo, Professor, Sant Longowal Institute of Engineering and Technology, India and Dr Surinder P. Singh, Senior Principal Scientist, CSIR- National Physical Laboratory, New Delhi for their constant support and guidance.
Conflict of Interest
There is no conflict of interest between any author regarding any financial, personal or relationship with any organization.
References
- Li, X.; Gao, X.; Shi, W.; Ma, H.; Chem. Rev., 2014, 114, 1, 590–659.
- Quang, D.T.; Kim, J.S.; Chem. Rev., 2010, 110, 10, 6280-6301.
- Kobayashi, H.; Ogawa, M.; Alford, R.; Choyke, Urano; P.L. Y., Chem. Rev., 2009, 110, 5, 2620-2640.
- Prescha, A.; Krzysik, M.; Zablocka-Slowinska, K.; Grajeta, H.; Biol. Trace Elem. Res., 2014, 159, 325-331.
- Deka, R.S.; Mani, V.; Kumar, M.; Shiwajirao, Z.S.; Tyagi, A.K.; Kaur, H.; Biol. Trace Elem. Res., 2014, 161, 57-68.
- Goswami, S.; Aich, K.; Das, S.; Das, A.K.; Sarkar, D.; Panja,, S.; Mondal, T.K.; Mukhopadhyay, S.; Chem. Commun., 2013, 49, 91, 10739 – 10741.
- Singh, A.K.; Gupta, V.K.; Gupta, B.; Anal. Chim. Acta, 2007, 585, 171-178.
- Barba-Bon, A.; Calabuig, L.; Costero, A.M.; Gil, S.; Martínez-Má˜nez, R.; Sancenón, F.; RSC Adv., 2014, 4, 8962-8965.
- Mcrae, R.; Baqchi, P.; Sumalekshmy, S.; Fahrni, C.J.; Chem. Rev., 2009, 109, 10, 4780-4827.
- Eastmond, D.A.; Macgregor, J.T.; Slesinski, R.S.; Crit. Rev. Toxicol., 2008, 38, 173-190.
- Kaur, M.; Kaur, P.; Dhuna, V.; Singh, S.; Singh, K.; Dalton Trans., 2014, 43, 5707-5712.
- Zhou, Z.; Yu, M.; Yang, H.; Huang, K.; Li, F.; Yi, T.; Hong, C.; Chem. Commun.,2008, 29, 3387-3389.
- Weerasinghe, A.J.; Schmiesing, C.; Sinn, E.H.; Tetrahedron Lett.,2009, 50, 6407-6410.
- Abu-Dief, F.A.; Ibrahim, M.A.; Mohamed, A.; Beni-Suef University J. Basic App. Sci., 2015, 4, 119 -133.
- Kumar, G.; Kumar, D.; Singh, C.P.; Kumar, A.; Rana, V.B.; J. Serb. Chem. Soc., 2010, 75, 5, 629–637.
- Chang, E.L.; Simmers, C.; Knight, D.A.; Pharmaceuticals, 2010, 3, 1711-1728.
- Creaven, B.S.; Duff, B.; Egan, D.A.; Kavanagh, K.; Rosair, G.; Thangella, V.R.; Walsh, M.; Inorg. Chim. Acta, 2010, 363, 4048–4058.
- Abdullah, M.; Asiri, A.M.; Badahdah, K.O.; Khan, S.A.; g. Al-sehem, A.; s. Al-Amoudi; M., Bukhari A.A.; Org. Chem. Insights., 2010, 3, 1–8.
- Gans, P.; Sabatini, A.; Vacca, A.; Talanta., 1996, 43, 1739-1753.
- Gans, P.; Sabatini, A.; Vacca, A.; Ann. Chim. (Rome), 1999, 89, 45-49.

This work is licensed under a Creative Commons Attribution 4.0 International License.









