The Emergence of CaO-MgO based Binary Oxides of Alkaline Earth Metals as Cost-effective Solid Base Heterogeneous Catalysts and Sorbents: A Mini Review
Siddaramagoud Bandalla1,2 , Satyanarayana Mavurapu1
, Satyanarayana Mavurapu1 , Sreekantha B jonnalagadda3
, Sreekantha B jonnalagadda3 and Chandra Sekhar Vasam1*
and Chandra Sekhar Vasam1*
1Department of Pharmaceutical Chemistry, Telangana University, Nizamabad-503322, India.
2Department of Integrated Chemistry, Palamuru University, Mahbubnagar-509001, India.
3School of Chemistry and Physics, University of KwaZulu-Natal, Durban, South Africa.
Corresponding Author E-mail: csvasamsa@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/390602
Article Received on : 16 Nov 2023
Article Accepted on : 20 Dec 2023
Article Published : 27 Dec 2023
Reviewed by: Dr. Aditya Bhattacharyya
Second Review by: Dr. Abdul Aziz Abdul Raman
Final Approval by: Dr. Masami Okamoto
The mono or single oxides of alkaline earth metals such as CaO and MgO are a type of non-toxic and non-corrosive solid-base heterogeneous catalysts. Nevertheless, these mono oxide particles can agglomerate and form larger and less active particles at certain temperatures and reduces their catalytic activity. Therefore, the use of thermally stable CaO-MgO binary oxides is recommended. Further, the possible synergistic interactions between two metal centers provides cooperative catalytic behavior to improve catalytic activity compared to their single oxide counterparts. Therefore, the main theme of this review article is to highlight the ability of reported CaO-MgO based alkaline earth binary oxides as cost-effective and efficient solid-base catalysts in variety of organic transformations and to expand their scope in many other unexplored non-asymmetric organic transformations. Literature survey reveals that CMBOs are highly considerable in optimizing recognized organic transformations such as Transesterification, Knoevenagel/Aldol condensations, Isomerization, Oligomerization, Acetylation, Henry reaction, Alcoholysis, Aza-Michael addition, Cracking of Alkanes, H2-production via steam reforming, Photodegradation of organic pollutants and so forth. The literature survey further visualizes that the surface properties of CMBOs such as Brønsted/ Lewis’s basicity, surface area, particle size, structural diversity, Ca: Mg ratios and synergism between Ca and Mg in CMBOs are very useful to promote them as efficient catalysts compared to their single oxide counterparts (pure CaO and pure MgO). The rightness of proposed mechanisms of abovementioned organic reactions by CMBO catalysts is elicited by this review. Moreover, the precursors for CMBOs are inexpensive, highly abundant and eco-compatible. Apart from the catalytic applications, the suitability of the CMBOs in sorption studies including CO2 uptake, ethanol steam reforming, and heavy metal ion removal is also covered.
KEYWORDS:Alkaline earth metal oxides; CaO-MgO (CMBOs); Solid-base heterogeneous catalysts
Download this article as:| Copy the following to cite this article: Bandalla S, Mavurapu S, Jonnalagadda S. B, Vasam C. S. The Emergence of CaO-MgO based Binary Oxides of Alkaline Earth Metals as Cost-effective Solid Base Heterogeneous Catalysts and Sorbents: A Mini Review. Orient J Chem 2023;39(6). |
| Copy the following to cite this URL: Bandalla S, Mavurapu S, Jonnalagadda S. B, Vasam C. S. The Emergence of CaO-MgO based Binary Oxides of Alkaline Earth Metals as Cost-effective Solid Base Heterogeneous Catalysts and Sorbents: A Mini Review. Orient J Chem 2023;39(6). Available from: https://bit.ly/47dzMjd |
Introduction
When considered heterogeneous catalysis, non-noble metal based solid base heterogeneous catalysts (SBHCs) including oxides of alkali metal (AMOs) and alkaline earth metals (AEMOs), zeolites incorporated with AMs and AEMs, MOFs formed with AMs and AEMs, organic base functionalized polymers, hydroxyapatites, layered-double-hydroxides (LDHs), and hydrotalcites (HTs), are known as special type of heterogeneous catalysts for certain transformations. Figures 1 and 2 represent the types of common non-noble metal based SBHCs and their application in a wide variety of organic syntheses.1,2
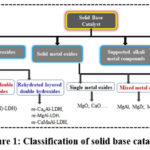 |
Figure 1: Classification of solid base catalysts. |
 |
Figure 2: Some applications of solid base metal oxides. |
Thecatalysts of alkaline earth metal oxides (AEMOs) are a type of solid base heterogeneous catalysts (SBHCs) that are particularly effective in base-catalyzed reactions.3 The important nature of these catalysts relies on the interplay between the Lewis acidic metal cation and an oxide ion exhibiting Brønsted basic characteristics.4 These catalysts and their precursors are cost-effective, highly abundant, reusable, and corrosion-resistant. When compared to the non-noble transition metal oxide precursors, the precursors for CMBOs (CaO-MgO) such Ca(NO3)2, Mg(NO3)2 and dolomite mineral are relatively cheaper as observed in many chemical business catalogues. Simple MgO and CaO are well known single oxides among AEMOs and used as catalyst supports as well as main catalysts in heterogeneous catalytic process. However, these signle oxide particles can agglomerate and form larger and less active particles at certain temperatures and reduces their catalytic activity. Therefore, the use of binary oxides of alkaline earth metals is recommended. Interestingly, the synergistic interactions between two metal centers provides cooperative catalytic behavior,5 thereby improved catalytic activity and selectivity compared to their single oxide counterparts. Hence, from the above-mentioned statements we are interested in reviewing literature reports on the emergence of binary alkaline earth oxide mixture, especially the CaO-MgO (abbreviated as CMBOs) in various heterogeneous reactions. This review gives a summary of the advantages of CMBOs as efficient SBHCs for a variety of reactions in synthetic organic/ inorganic chemistry as well as useful materials in sorption studies.
The main contents of this micro review are divided into the following three (3) sections:
CMBO catalyzed transesterification reactions
CMBO catalyzed C-C, C-heteroatom bond forming organic synthesis other than transesterification
CMBO assisted Sorption studies
CMBO catalyzed transesterification reactions
Transesterification (TE) reaction is one of the key industrial chemical processes that exchanges the alkyl substituents between an alcohol (usually methyl or ethyl alcohol) and an ester functionality of triglycerides of certain vegetable oils in the presence of a base to form the mixture of new mono fatty acid esters (FAEs) and glycerol (Scheme 1). The mixture of FAEs is known as biodiesel and the TE reaction is also termed as alcoholysis. Biodiesel is emerging as a renewable and environmentally friendly alternative to fossil fuels. Generally, NaOH and KOH are traditional and cost-effective homogeneous catalysts employed extensively in industrial transesterification processes. Nevertheless, these catalysts are highly hygroscopic, absorbs moisture from the environment during storage, generates water when mixed with alcohol reactants and influences the overall yields. Moreover, the removal of homogeneous base catalysts is not easy from the system. Hence the use of noble and non-noble metal based heterogeneous catalysts especially CaO-MgO (CMBOs) in TE reaction is increasing. Definitely, these CMBOs are non-corrosive, eco-friendly, cost-effective, recyclable and suitable to employ in fixed-bed reactors. In this context, various researchers emphasized the efficacy of CMBOs in TE reaction to produce biodiesel.
 |
Scheme 1: Transesterification reaction and products. |
CMBO catalysts were extensively used in the TE process by different compositions, precursors, and synthetic methods (Figure 3).
 |
Figure 3: CMBO (CaO-MgO) catalyst for TE reaction. |
Ibrahim et al, developed a novel CMBO catalyst by mixing hydrated lime and magnesium oxide (50:50) and subsequent calcination.6 The presence of CMBO catalysts in the TER process of jatropha curcas seed oil with methyl alcohol gave 100% yield of biodiesel within in 90 minutes. Maimoonah and colleagues reported a sunflower oil-derived biodiesel synthesis via transesterification conducted at moderate temperatures.7 The CMBO catalyst was synthesized via hydration-dehydration process and designed a series of nanostructured CMBO catalysts with varied molar ratios of Ca: Mg. The authors optimized the catalytic conditions to produce 97% biodiesel, using atmospheric pressure, 5% catalyst weight, alcohol and oil molar ratio of 9:1. Notably, the CMBO catalyst used in this report also showed significant promise for large-scale biodiesel production from sunflower oil.
Yan et al, investigated the effectiveness of CMBO catalysts in the TE reaction between rapeseed oil and methyl alcohol. According to the results, the catalytic performance of CMBO was found to be superior to pure CaO.8 The performance of the catalysts was optimized by Ca loading, and calcination temperatures. The CMBO catalyzed transesterification of rapeseed oil provided 92% at ~65 °C. Teo and colleagues investigated the CMBO catalyzed transesterification of Elaeis guineensis oil with methyl alcohol. The CMBO catalyst was synthesized using co-precipitation.9 Various mole ratios of Ca to Mg (1:1, 1:2, 2:1) were used to synthesize CMBOs. The presence of calcium was aimed to enhance the basic nature of the CMBO system, thereby to enhance its performance in the abovementioned transesterification. The reaction conditions were optimized mainly by using 4 wt% of catalyst loading, a 15:1 methyl alcohol-to-oil ratio. A maximum of 99% yield of the product was obtained under the optimized reaction conditions.
A report by Hu and coworkers investigated the impact of Ca: Mg ratios in the CMBO catalyzed TE reaction of soybean oil and methyl alcohol.10 A series of CMBO catalysts were synthesized by coprecipitation by varying the Mg:Ca molar ratios and found that the incorporation of Mg into the CMBO structure enhanced basic sites by reducing CaO lattice spacing for improving the catalytic activity. However, it was also noticed that an excessive Mg portion in CMBO influenced the pore structure and specific surface area. The 1Mg3Ca catalyst with optimal surface area (50.72 m2/g) performed well in the transesterification.
Buasria and the team examined the natural dolomitic rock derived CMBO as an eco-friendly catalyst in microwave-assisted TE reaction between Jatropha Curcas oil and methyl alcohol.11 The mineral rock dolomite produced the CMBO catalyst upon calcination, which has a 14.8 m2/g surface area. The influence reaction parameters like alcohol/oil molar ratios, reaction time, and catalyst loading was established. A conversion of 95% oil was achieved using 4 wt% of CMBO catalyst, 18:1 molar ratio between alcohol and oil. Taufiq et al also reported the efficacy of CMBO in the transesterification of Jatropha curcas oil (JCO) with methyl alcohol and compared the activity with the single oxide counterparts.12 The authors reported the synthesis of CMBO using a co-precipitation technique. Under optimized transesterification conditions, the CMBO catalyst achieved JCO conversion rates exceeding 80%. The catalytic efficiency of CMBO was ascribed to the presence of robust basic sites on its surface, primarily associated with Ca2-O2 pairs. Abdulloh and colleagues employed calcined dolomite catalyst in the TE reaction process of tamanu oil to yield biodiesel.13 The catalyst’s basic strength was assessed using Hammett indicators. This report mentioned ~98% transesterification conversion of Tamanu oil using 65 °C reaction temperature, alcohol to oil molar ratio of 1:30, 1g of CMBO.
Olivia and colleagues investigated the CMBO catalyzed TE process between palm oil and methyl alcohol to obtain biodiesel.14 They prepared four samples of CMBO by calcining dolomite at four distinct temperatures: 700 °C, 800 °C, 900 °C, and 1000 °C to employ them abovementioned transesterification and observed that the CMBO catalyst obtained at 900 °C, exhibited notable efficacy in facilitating the transesterification reaction.
A report by Abdelrahman et al described the results of TE process of waste cooking oil with methyl alcohol in producing biodiesel.15 A calcined dolomite i.e., CMBO was used as a catalyst in this TER process to provide approximately ~97% of biodiesel. The optimized reaction conditions include 6 wt% of CMBO catalyst, 1:15 molar ratios of oil and methyl alcohol, and 90 °C of reaction temperature. Tahvildari et al, worked on the usefulness of a nano CMBO catalyst in the TER process of recycled cooking oil.16 A sol-gel procedure was followed to obtain CMBO catalyst. Higher proportions of CaO to MgO were found to enhance the yield of biodiesel production from recycled cooking oil. Under the optimized conditions, the nano CMBO gave ~99% yield of biodiesel.
A report by Lee and colleagues provided a comparison of catalytic activity between CMBO and other non-noble metal oxide catalysts in producing biodiesel from non-edible jatropha oils.17 The solid base binary metal oxides combinations such as CaO-MO (M = Mg, Zn, La and MgO-ZnO were prepared by coprecipitation. When these binary oxides were explored as catalyst in TE process of jatropha oils, the CMBO catalysts showed highest catalytic activity in producing >90% yield of biodiesel with minimal metal leaching. Korbag and Korbag studied the transesterification of oils such as sunflower, olive, and corn in the production of biodiesel using CMBOs.18 The CMBO catalysts were prepared from commercial hydroxide samples that decomposed at 600 °C. The study mentioned the optimal reaction conditions such as a methyl alcohol to oil ratio of 6:1 and temperature range from 30 to 60 °C. A maximum biodiesel yield (99%) was obtained at a reaction temperature of 60 °C.
Abukhadra et al, investigated the catalytic efficiency of CMBO in the TE reaction of non-edible castor oil into biodiesel.19 The authors prepared the CMBOs with nanorod morphology via microwave irradiation. The CMBOs catalyst has a distinct rod-like shape and a large BET surface area of 112.8 m2/g. The optimized reaction conditions facilitated a biodiesel yield of ~97% using a 6 wt% catalyst, and a 15:1 molar ratio of ethanol to oil at moderate temperatures.
Vahid and colleagues reported the results of a CMBO catalyzed TE process of n-butyl acetate.20 The CMBO catalyst was prepared by co-precipitation with varied mass ratios of Ca:Mg. The characterization results of CMBO showed a combination of a cubic and hexagonal phases. It was noticed that an adjustment in the Ca: Mg ratio in the CMBO resulted a variation in surface area and pore diameter. The transesterification experiment performed at a temperature 95 °C, an atmospheric pressure, with the 8:2 Ca:Mg mass ratio in CMBO provided ≥83% conversion. This best performance of the catalyst was attributed to the robust basic sites associated with Ca2+–O2− pairs.
Albuquerque et al, disclosed the accomplishment of TE process between ethyl butyrate and methyl alcohol using the CMBO catalysts.21 A series of CMBO catalysts were synthesized using coprecipitation by changing the Ca to Mg ratios. A CMBO catalyst with Ca:Mg being 1:3 is found to be highly efficient in TE reaction than the single oxide counterparts. This phenomenon was ascribed to the presence of effective basic sites on the CMBO surface, primarily linked to Ca2+–O2− pairs, as well as a significantly greater surface area compared to that of pure CaO. In contrast, MgO displayed inactivity in this process.
Finally, we have made a comparative statement of the results and optimized conditions reported with various transesterification reactions studied in the presence of CMBO catalysts as shown in Table 1.
Table 1: Comparative statement of transesterification over CMBO catalysts
|
S.No |
Oil |
Alcohol:Oil |
Cat. Wt% |
Temp(°C) |
Time |
Yield% |
Ref |
|
1 |
Jatropa curcus |
5.5:1 |
1.5 |
60 |
90min |
100 |
6 |
|
2 |
Cooking oil |
15:1 |
1.5 |
90 |
2h |
97 |
15 |
|
3 |
n-butyl acetate |
5:1 |
0.1 |
95 |
2h |
95 |
20 |
|
4 |
Sunflower |
9:1 |
5 |
65 |
3h |
97 |
7 |
|
5 |
Jatropa |
25:1 |
3 |
120 |
3h |
94 |
17 |
|
6 |
Rapeseed |
18:1 |
16.5 |
65 |
2h |
92 |
8 |
|
7 |
Elacis guineensis |
15:1 |
4 |
60 |
6h |
99 |
9 |
|
8 |
Waste cooking |
7:1 |
3 |
6 |
6h |
99 |
16 |
|
9 |
Ethyl butyrate |
4:1 |
3 |
60 |
1h |
100 |
21 |
|
10 |
Soybean |
7:1 |
4 |
65 |
2h |
100 |
10 |
|
11 |
Jatropa curcus |
18:1 |
4 |
80 |
1h |
95 |
11 |
|
12 |
Castor |
15:1 |
6 |
70 |
1.1h |
96.2 |
19 |
|
13 |
Jatropa |
15:1 |
4 |
65 |
6h |
85 |
12 |
|
14 |
Olive, sunflower, Corn |
6:1 |
2 |
60 |
4h |
99 |
18 |
|
15 |
Lamann |
30:1 |
5 |
65 |
5h |
97.96 |
13 |
|
16 |
Glycerol |
– |
2 |
220 |
24h |
51 |
22 |
|
17 |
Palm |
11:1 |
15 |
60 |
1h |
78 |
14 |
Plausible reaction mechanism of TE process
This reaction can follow either an Eley-Rideal or a Langmuir-Hinshelwood mechanism in the presence of SBHCs (Scheme 2). Firstly, the alcohol activates at a surface basic site. This surface basic site, O2−, removes H+ of the alcohol. The alkoxide anion is formed by the adsorption of R–O– group (with R as a hydrocarbon chain) on the active site of CMBO. In the process, the carbonyl group adsorbs on the adjacent active site of CMBO to form a cationic complex. Next, there will be a nucleophilic interaction between activated alkoxide and the carbonyl group (Step-II), followed by the addition process of alcohol to generate a tetrahedral intermediate. Next, the intermediate obtains H+ from the CMBO surface basic site and undergoes rearrangement to produce the ester. Finally in step III, desorption of organic molecule through the alignment of charges on the oxygen attached to the carbonyl group forms an ester and a diglyceride. This phenomenon appears to be important in the case of CMBOs that have strong basic sites. Furthermore, the TE phenomenon repeats twice, involving the diglyceride and the monoglyceride to produce three ester molecules along with one glycerol molecule.
 |
Scheme 2: CMBO catalyzed reaction mechanism of transesterification. |
Literature survey reveals that the CMBOs efficiency is not limited to TE reaction but is also suitable to perform other valuable organic synthesis because of their base nature. Suttibut et al, reported the application of CMBO catalysts in the isomerization of 1-butene (Scheme 3). The CMBO catalyst was synthesized using co-precipitation method.23 When the CaO concentrations were varied in CMBO, there was an increase in basic sites, but a decrease in BET surface area due to reduced pore volume. The modified structure of CMBO improved OH− adsorption and enhanced catalytic activity. The CMBO catalyst with CaO wt% of 1.77, displayed the highest performance to give 99% selectivity for 2-butene, which indeed is superior to single oxide counterpart.
 |
Scheme 3: CMBO catalyzed isomerization of 1-butene. |
Omata and colleagues explored the performance of CMBO catalyst in methane oxidative coupling.24 A CMBO catalyst synthesized via co-precipitation was employed in this investigation. According to the catalyst characterization results, the incorporation of calcium ions (Ca2+) as dopants has increased the catalyst’s basicity compared to pure MgO. When the CMBO catalyzed oxidative coupling of methane was performed at 750 °C, the catalyst achieved a 9.2% conversion of methane and 67.1% selectivity for C2 products (Scheme 4).
 |
Scheme 4: Dimethyl carbamate synthesis via oxidative coupling of methane. |
A report by Vin’s group disclosed the usefulness of CMBO catalyst in an alcoholysis reaction to synthesize methyl formate from CO2-derived formamides and green methyl alcohol (Scheme 5).25 A series of CMBO catalysts with varied CaO mass percentages (3.8%, 7.2%, 10.5%, 13.5%, and 16.3%) were obtained via the impregnation method. The catalyst with 13.5% CaO performed well to get ~95% conversion of N-formylmorpholine and 100% selectivity for methyl formate. The study also established a direct correlation between strong basic site density and alcoholysis reaction efficiency, with higher base density resulting in superior reaction rates.
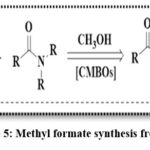 |
Scheme 5: Methyl formate synthesis from CO2 |
Alarcon et al, investigated the efficiency of CMBOs in naphthalene steam gasification to produce H2 gas and compared the activity with the single oxide counterparts i.e. CaO and MgO (Scheme 6).26a,b The competence of CMBO catalyst with Ca:Mg being 1:9 in said process was attributed to the rise of cooperative interactions between the two metals centers. The role of MgO in preventing the formation of bidentate carbonate and deposition of carbonaceous material on CaO surface, endorsing the creation of unidentate carbonates.
 |
Scheme 6: Naphthalene steam gasification reaction for H2 production. |
Tang et al, demonstrated the CMBO efficacy in accelerating streptomycin hydrolysis degradation from waste water samples.27 When compared with homogeneous catalytic conditions, the performance of heterogeneous CMBO solid-base was found to be superior. The strong base sites present CMBO solid base surface interacts with the ether C-O bonds of streptomycin at two positions to produce four hydrolysis products and indicates the viability of improved hydrolysis. The metal-mediated hydrolysis pathway of streptomycin is depicted in the following Scheme 7.
 |
Scheme 7: Hydrolysis of streptomycin mediated by CMBO. |
Taralas and the team evaluated the role of CaO, MgO, and CMBO as catalysts during cracking of n-heptane in the presence of steam.28 The results of CMBO catalyzed thermal cracking of n-heptane were compared with results obtained with the pure MgO, and CaO. Deactivation of catalysts was noticed when the cracking process was conducted in the absence of steam. The results highlight the effectiveness of calcined dolomite (CMBO) as compared to single oxide counterpart (Scheme 8) in said process of steam cracking of n-heptane.
 |
Scheme 8: Catalytic cracking of n-heptane. |
Philipp and colleagues synthesized the CMBO catalysts with varied Mg: Ca ratios, and employed them in methane oxidative coupling.29 A maximum selectivity (67%) and activity were achieved with CMBO catalyst containing higher percentages of MgO. The incorporation of CaO into MgO resulted in enhanced basicity of the CMBO catalyst, which was associated with changes in surface morphology. Additionally, the surfaces of the MgO-rich oxides were mostly covered by MgO (Scheme 9a).
 |
Scheme 9a: Oxidative coupling of methane reaction. |
Dang’s group found the application of CMBO catalysts in the reaction of urea with propylene glycol to produce propylene carbonate (PC).30 A series of CMBO catalysts were prepared by adjusting the Mg:Ca ratios using different precipitating agents such as NaOH, Na2CO3. The most effective catalyst had a 1:1 Mg/Ca ratio, which gave 96% PC, 99% selectivity, and 96% urea conversion. FE-SEM analysis evidenced the presence of small MgO particles and large CaO aggregates on the surface of CMBO (Scheme 9b).
 |
Scheme 9b: PC synthesis reaction over CMBO. |
Tamaddon and colleagues reported the application of natural Dolomite (CaMg(CO3)2) mineral, a precursor for CaO-MgO, as a recyclable natural catalyst in (i) Henry reaction, (ii) Knoevenagel condensation, and (iii) Michael reactions in pure aqueous medium to provide nitroalkanols, trisubstituted alkenes, b-amino and b-thio substituted carbonyl compounds respectively.31 (Scheme 10 a & b) The characteristics of dolomite catalyst are extensively analyzed by various physico-chemical techniques. The basic strength of the catalyst was evaluated by Alam following the Hammett indicators procedure. Structure-activity relation between the Dolomite catalyst and the substrates of the abovementioned three reactions was deduced.
 |
Scheme 10a: Dolomite catalyzed C-C and C-hetero atom bond forming reactions. |
 |
Scheme 10b: Dolomite catalyzed reaction mechanism of Knoevenagel condensation reactions. |
Alam et al, investigated a valorization pathway for 6-amyl-α-pyrone (6PP) during the aldol condensation of nonanones with furfural and HMF.32 The reaction was performed under neat conditions using CMBO catalyst to get excellent yields of the aldol products. The liquid aldol products then participated in hydrodeoxygenation (HDO) to yield branched C14 or C15 alkanes that could be used as diesel and jet range fuels (Scheme 11).
 |
Scheme 11: CMBO catalyzed hydrodeoxygenation |
Jose et al studied the production of glycerol oligomers using natural dolomite and calcinated dolomite (CMBO) as catalysts.33 The CMBO exhibited a decrease in the particle size (297 to 153 nm), an increase in the surface area (1.57 to 37.7 m2/g) and basic strength. The calcined dolomite (CMBO) was superior to natural dolomite during the oligomerization in terms of glycerol conversion and selectivity for diglycerol and triglycerol. The interaction between the catalyst and the substrates in catalyzing the oligomerization is depicted in Scheme 12.
 |
Scheme 12: Glycerol oligomerization. |
Yang et al, reported the use of calcined dolomite based CMBO as a competent recyclable catalyst to accomplish Knoevenagel condensation (KC) between aldehydes and active methylene reagents for the synthesis of α,β-unsaturated carbonyls.34 The authors mentioned that calcination temperature is an important factor to modify the surface properties of the catalysts. The dolomite calcined at 700 °C was served as the best catalyst in the KC reaction. Reaction parameters such as the effect of solvent, catalyst amount, and catalyst basicity were assessed. The model reaction was also checked in the presence of natural dolomite and recorded longer reaction times as compared to the calcined dolomite. Further, it was also recognized that the calcined catalyst is recyclable four times without a significant decrease in reactivity (Scheme 13 a & b).
 |
Scheme 13a: CMBO catalyzed Knoevenagel condensation reaction. |
 |
Scheme 13b: Knoevenagel condensation mechanism catalyzed by CMBO. |
Bandalla et al, used CMBOs as efficient and reusable heterogeneous catalysts for the selective oxidation of cyclohexanol to cyclohexanone under solventless condition.4 The CMBOs were synthesized by co-precipitation method with different mass ratios of Ca: Mg. The CMBO with 1CaO–1.5MgO catalyst composition displayed a higher conversion of cyclohexanol (~85%) with superior selectivity toward cyclohexanone product (~92%) at 140 °C. According to the results, data analysis, the basic sites on CMBO have played a key role in the alcohol deprotonation and activation of the C–H bond on the associated α–carbon of the alcohol (Scheme 14).
 |
Scheme 14: Selective oxidation of cyclohexanol to cyclohexanone. |
Yan and colleagues used a CMBO catalyst in the degradation of lignin (Scheme 15).35 The catalyst was prepared by impregnating calcium acetateon a MgO carrier followed by calcination at 700 °C. The catalyst exhibited high surface basicity (30.2 mmol/g) when the CaO/MgO ratio in CMBO was 0.08. During the catalytic degradation of lignin, there was a substantial reduction in the lignin’s average molecular weight (from 3,000 to 800) and a notable increase in hydroxyl content (from 200 to over 500). FT-IR analysis revealed a reduction in ether bonds and a significant increase in hydroxyl bonds.
 |
Scheme 15: Lignin degradation using CMBO. |
He and colleagues discovered the efficacy of CMBOs as solid base catalyst during the conversion of ethylene glycol dimethyl ether (EGDE) to vinyl methyl ether (VME) (Scheme 16).36 It is known that VME is an important precursor for functional polymers and fine chemicals. Generally, Reppe vinylation is conducted in industry to obtain VME. However, this additionreaction between methyl alcohol to acetylene needs high pressures and strong bases as catalysts. Hence, biomass-derived EGDE via the CMBO catalyzed elimination of methyl alcohol was optimized to produce VME in a sustainable way. Among the CMBOs investigated, the one with a Ca:Mg molar ratio of 1: 2 displayed a 100% EGDE conversion and a ~94% selectivity to VME. The authors explained that the CMBO catalyst surface possesses strong basic sites and eases the production of VME.
 |
Scheme 16: Synthesis of vinyl methyl ether (VME). |
Elumalai’s research group described about the catalytic application of CMBO nanocomposite for the profitable generation of D-fructose and D-allulose from glucose in aqueous media (Scheme 17).37 The CMBO with equal proportions of MgO and CaO helped to manipulate the surface properties and basicity. Further, the location/ dispersion of CaO and MgO in CMBO influenced the basicity and consequently the path of entire transformation. Overall, the CMBO catalyst augmented the interconversion of glucose selectively to get fructose in good yields in shorter reaction times.
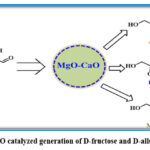 |
Scheme 17: CMBO catalyzed generation of D-fructose and D-allulose from glucose. |
Acetylation of glycerol using CMBO catalyst was reported by Ramirez and colleagues.38 Simple co-precipitation method, with variable CaO compositions of by weight was used to prepare the CMBO catalyst. A CMBO with an equal mixture of MgO and CaO displayed the highest glycerol conversion rate and lowest production of diacetin and monoacetin (Scheme 18).
 |
Scheme 18: Glycerol acetylation. |
Delgado and Aznar investigated the effectiveness of CaO, MgO, and a CMBO (calcined dolomite) in hot gas from biomass gasification with steam.39 Due to larger surface area, the calcined dolomite performed well than pure CaO and MgO in gasification. Shahid et al, studied methylene blue degradation using CMBO and CaO catalysts. Various preparation methods have been explored, including conventional heating and hydrothermal processes.40 Higher temperatures (up to 160 °C) led to increased catalytic efficiency, but excessive heat caused particle aggregation, reducing surface area. CaO performed better in low dielectric constant solvents, while CaO/MgO based CMBO showed the opposite trend.
CMBO assisted Sorption studies
Fossil fuel combustion produces unsafe CO2 emissions, which have negative effects on the environment, health and the economy. The process of capturing of carbon, its utilization and storage is a crucial concern in order to suppress this phenomenon. Therefore, it is essential to create solid absorbent with high absorption capacities, fast reactivity, stability, low decomposition temperatures, and quick rates at high temperatures. Due to their affordability, high CO2 absorption capability, and quick chemical reactivity, CMBOs are interesting choices (Figure 4).
 |
Figure 4: CO2 absorption studies over CMBO catalyst. |
Park and Yi investigated the impact of preparation methods of CMBOs on CO2 absorption and hydrogen production.41 A co-precipitation method followed by hydration step is reported to obtain the CMBOs that contain different Ca: Mg ratios. Nitrate precursors of the metals were used in co-precipitation. The CMBOs prepared just by co-precipitation were effective in CO2 absorption. Therefore, the authors added an additional hydration step during the preparation of CMBOs to improve its CO2 absorption efficacy. In particular, Ca75Mg25 material was found to be highly efficient as CO2 absorbent in a multi-cycle test.
Nethravathi and colleagues also studied the use of porous Nano composite CMBOs for CO2 capture.42 A series of Nano porous CMBO with varied mole percentages of MgO (10–40) worked well in the absorption of CO2. A porous CMBO with Ca: Mg in 8:2 molar ratios absorbed ~62 mass % of CO2. Further, the CMBO exhibited maximum efficacy even after 100 carbonation–decarbonation cycles. The high CO2 absorption efficiency and cycling stability by CMBOs are ascribed to their large surface area, a nanoporous structure and the given MgO content.
Lan´s research group observed the influence of quantity of MgO content in the porous Nano structured CMBO based adsorbents for the purpose of CO2 adsorption uptake.43 The CMBO with higher mole ratios of MgO had a positive impact on the reaction rate and the overall durability of the nano CMBO adsorbent. This could be due to the increased surface area of the resulting CMBO. Notably, these adsorbents displayed good durability and high conversion rates even at elevated temperatures to absorb the higher concentrations of CO2.
Yan and colleagues developed a dolomite derived CMBO as CO2 sorbent.44 The sorption study explained the influence of several reaction parameters such mass ratio between dolomite and carbide slag, calcined temperatures, presence of steam to determine the CO2 absorption by CMBO in multiple calcium looping cycles. Notably, the CMBO designed with achieved highest CO2 absorption when the mass ratio between carbide slag and CMBO was set at 74:26. The CMBO sorbent material with a mass ratio of Ca to Mg of 90:10 was recommended for this process.
A report by Al-Awaji and the team described the application a porous CMBO as sorbent in removing cobalt ions (Co (II)) from aqueous solutions.45 A sol-regime was applied in designing the nanoporous structured CMBO by incorporating gum arabic extract. This CMBO has a relatively large surface area and large pores to display better performance in removing the cobalt ions. The Co (II) adsorption process of CMBO material followed Langmuir adsorption isotherm and proved its potential in removing maximum quantity. This report further highlighted the scope for the removal capacity of other heavy metal ions and organic pollutants by CMBO.
Olivas et al, studied the hydrogen production by ethanol steam reforming combined with CO2 absorption using CaO and CMBO based solid adsorbents in the presence of Ni/Al2O3 catalyst.46 The authors highlighted the necessity for the development of clean and green energy sources like hydrogen energy. The reported system comprises ethanol as a feedstock that combined with the in-situ absorption of CO2 by CMBO is useful to produce H2. The use of H2 in the fabrication of fuel cells is a well-known phenomenon.
Zhu and the team reported the synthesis of a CMBO composite by ball-milling method and its application in CO2 uptake.47 The results indicates that the presence of MgO prevented the agglomeration of CaO particles in the CMBO and improved the CO2 uptake/ absorption. An effort was made to relate CO2 uptake and the surface/structural properties of CMBO material. Inter-particle distance was found to be a key factor for adsorption capacity. The CaO derived from D-gluconic acid calcium salt monohydrate achieved 80% conversion with minimal loss over cycles.
Diana and the team synthesized the CMBO based sorbents by co-precipitating CaO with varied MgO levels.48 The addition of MgO reduced the sintering effects in CaO sorbents. Sorbents with 5% Ca and 10% MgO maintained CO2 adsorption efficiency through multiple cycles. Notably, when the CaO doped with 10% MgO in CMBO consistently adsorbed CO2 in thirty carbonation cycles and indicated enhanced performance, structural stability, and surface area.
Yan et al, reported a CMBO material as CO2 sorbent that was created from a carbide slag (composed of Ca(OH)2) and dolomite by combustion method.49 The carbide slag served as a source for CaO and the dolomite for both MgO and CaO. Optimal results were achieved with a 74:26 mass ratio between carbide slag and dolomite. This hybrid sorbent exhibited excellent CO2 uptake, even after 20 cycles. The CO2 sorption capacity of CMBO material was superior to the sorbents made from other analytical reagents during the calcium looping method.
AbuKhadra et al, reported the synthesis of new CMBO based nanorods on diatomite frustules by combining hydrothermal synthesis with microwave irradiation.50 Pharmaceutical residues from levofloxacin were successfully eliminated by this CMBO substance. The CMBOs featured with rod-shaped nanomaterials with a size of ~52 nm and a surface area of ~113 m2/g BET. The ideal circumstances led to theoretical levofloxacin absorption of 106.7 mg/g, with an equilibrium time of 720 minutes at pH 7. CMBO Nano rod catalysts prepared via hydrothermal synthesis showed better adsorption performance than CMBO catalysts made via precipitation.
Ahmed et al, reported a template-free method to create hierarchically porous CMBOs and their adsorption performance for phosphate and methyl orange (MO).51 As the Mg2+/NH3 feeding ratio increased, the average pore size and porosity decreased. A sample designated MgO-50 displayed relatively highest BET surface area (121 m²/g). All the CMBO samples followed pseudo second-order kinetics for phosphate adsorption and pseudo second-order and Freundlich isotherms for MO adsorption. MgO-25 demonstrated the most significant phosphate removal capacity at ~479 mg/g and the highest MO removal capacity at ~4484 mg/g among all investigated samples (Fig 5).
 |
Figure 5: Adsorption studies on CaO-MgO based CMBOs. |
Conclusions
The review of literature reveals that CMBOs are versatile solid-base catalysts, capable of exhibiting adjustable structure-activity relationship for catalytic organic synthesis. The method of preparation of CMBO and the ratio between Ca: Mg are found to influence their particle size, BET surface area, surface basicity, crystallinity and thermal stability. The CMBOs are usually prepared via coprecipitation and sol-gel method using appropriate Ca and Mg precursors. However, some of the reports mentioned the use of dolomite as precursor to obtain CMBO catalyst.
Concerning the application part, all the reported CMBOs mentioned in this review have shown efficiency as main catalyst in many C-C and C-heteroatom bond forming reactions. Majority of the reports disclosed the use of CMBO’s mainly in transesterification process. However, there is a gradual progress observed in literature in using the CMBOs as metal catalysts in many other important organic reactions like Knoevenagel Condensation, Aldol Condensation, Oligomerization, Hydrolysis, Alcohol Oxidation, Steam Reforming, Oxidative Coupling, Henry Reaction, Aza-Michael Reaction, Acetylation and So Forth. The CMBO catalysts have shown superior catalytic activity than their single oxide counterparts. It is now understood that these CMBOs are no longer considered just as catalyst supports but also as efficient main catalysts of solid base category. Further, The CMBOs can be used as effective alternative catalysts to hydrotalcite and layered double hydroxide (LDH) catalysts in many base-catalysed organic reactions. The reaction mechanisms proposed for reported CMBO catalytic cycles of various organic transformations provide the information about their redox properties, tunable crystal and electronic structure, suitable active sites and the scope to promote them in many other relevant reactions. Besides, these CMBOs are also recognized as good sorbents in industrial and environmental processes. It is always not necessary to combine other non-noble or noble metal catalyst materials with CMBOs.
Conflict of Interest
There is no conflict of interest
Funding Sources
There are no funding sources.
References
- Bing, W.; Wei, M. J. Solid State Chem. 2019, 269, 184–194.
CrossRef - Dresp, S.; Strasser, P. ChemCatChem. 2018, 10, 4162–4171.
CrossRef - Tsiotsias, A. I.; Charisiou, N. D.; Yentekakis, I. V.; Goula, M. A. Catalysts, 2020, 10, 36.
CrossRef - Tavizon-Pozos, J. A.; Chavez-Esquivel, G.; Suarez-Toriello, V. A.; Santolalla-Vargas, C. E.; Luevano-Rivas, O. A.; Valdes-Martinez, O. U.; Talavera-Lopez, A.; Rodriguez, J. A. Energies, 2021, 14, 1031.
CrossRef - Siddaramagoud, B.; Vijay, D.; Ganesh, B.; Madhu, R.; Jyothi, Y.; Parikshith, G.; Mallesham, B.; Srikantha, B, J.; Chamdrasekar, V. J. Mol. Catal. 2022, 533, 112759.
- Ibrahim, H.; Nwakuba, D. C. U.; Abubakar, G.; Nwobi, B. E. Asian J. Eng. Tech. 2013, 01, 2321–2462.
- Qasim, M. K. Egypt. J. Chem. 2019, 62, 475–485.
CrossRef - Yan, S.; Lu, H.; Liang, B. Energy Fuels. 2008, 22, 646–651.
CrossRef - Teo, S. H.; Rashid, U.; Thomas Choong, S. Y.; Taufiq-Yap, Y. H. Energy Convers. Manag. 2017, 141, 20–27.
CrossRef - Hu, M.; Pu, J.; Qian, E. W.; Wang, H. Bioenergy Res. 2013, 1, 13.
- Buasri, A.; Rochanakit, K.; Wongvitvichot, W.; Masa-Ard, U.; Loryuenyong, V. Energy procedia, 2015, 79, 562-566.
CrossRef - Taufiq-Yap, Y. H.; Lee, H.; Hussein, V. M. Z.; Yunus, R. Biomass Bioenergy, 2011, 35, 827–834.
CrossRef - Abdulloh, A.; Widati, A. A.; Arizal, O. J. Chem. Technol. Metall, 2017, 52, 1150–1156.
- Olivia, R.; Jamarun, N.; Arif, S.; Sirin, Y. A. Rasayan J. Chem. 2017, 10, 160–164.
- Rabie, A. M.; Shaban, M. M.; Abukhadra, R.; Hosny, R.; Ahmed, S. A.; Negm, N. A. J. Mole. Liq. 2019, 279, 224–231.
CrossRef - Tahvildari, K.; Anarak, Y. N.; Fazaeli, R.; Mirpanji, S.; Delrish, E. J. Environ. Health Sci. Eng. 2015, 13, 1–9.
CrossRef - Lee, H. V.; Juan, J. C.; Yun Hin, T. Y.; Ong, H. C. Energies, 2016, 9, 611.
CrossRef - Korbag, S.; Korbag, I. Eur. Chem. Commun. 2023, 5, 382–391.
- Abukhadra, M. R.; Mohamed, A. S.; El-Sherbeeny, A. M.; Soliman, A. T. A.; Abd Elgawad, A. E. E. Chem. Eng. Process.: Process Intensif. 2020, 154, 108024.
CrossRef - Mahdavi ,V.; Abedini, F. Chem. Eng. Commun. 2016, 203, 114–122.
CrossRef - Albuquerque, M. C.; Azevedo, G. D.; Cavalcante, C. L.; Santamaria-Gonzalez, J.; Merida-Robles, J. M.; Moreno-Tost, R.; Rodríguez-Castellon.; Jimenez-Lopez, E. A.; Maireles-Torres, P. J. Mol. Catal. A: Chem. 2009, 300, 19–24.
- Barros, F. J. S.; Cecilia, J. A.; Moreno-Tost, R. M.; Oliveira, F.; Rodríguez-Castellon, E.; Luna, F. M. T.; Vieira, R. S. Waste Biomass Valor.2020, 11, 1499–1512.
CrossRef - Suttibut, P.; Suriye, K.; Praserthdam, P.; Panpranot, J. J. Nanosci. Nanotechnol.2017, 18, 439–444.
CrossRef - Omata, K.; Aoki, A.; Fulimoto, Cat. Lett. 1990, 4, 241–244.
CrossRef - He, X.; Guo, X.; Xia, G.; Xu, R.; Wu, Y.; Luan, X. Catalysts, 2023, 13, 487.
CrossRef - a) Alarcón, N.; García, X.; Centeno, M.; Ruiz, P.; Gordon, A. Surf. Interface Anal. 2001, 31, 1031–1041; b) Alarcon, N.; Garcıia, X.; Centeno, M. A.; Ruiz, P. A.; Gordon, Appl. Catal. A: Gen. 2004, 267, 251-265.
CrossRef - Tang, M.; Dou, X.; Tian, Z.; Yang, M.; Zhang,Y. Chem. Eng. J. 2019, 355, 586-593.
CrossRef - Taralas, G.; Vassilatos, V.; Sjostrom, K.; Delgado, J. Can. J. Chem. Eng. 1991, 69, 1413–1419.
CrossRef - Philipp, R.; Omata, K.; Aoki, A.; Fujimoto, K. J. Catal. 1992, 134, 422–433.
CrossRef - Dang, K.; Kumar, N.; Srivastava, V. C.; Park, J.; Naushad, M.; Materials, 2023, 16, 735.
CrossRef - Tamaddon, F.; Tayefi, M.; Hosseini, E.; Zare, E. J. Mol. Cat. A: Chem. 2013, 366, 36–42.
CrossRef - Alam, M. I.; Gupta, S.; Bohre, A.; Ahmad, E.; Khan, T. S.; Saha, B.; Haider, M. A. Green Chem. 2016, 18, 6431–6435.
CrossRef - Barros, F. J. S; Cecilia, J. A.; Moreno-Tost, R.; de Oliveira, M. F.; Rodríguez-Castellón, E.; Luna, F. M. T.; Vieira, R. S. Waste. Biomass Valor. 2020, 11, 1499–1512.
CrossRef - Yang, H.; Dong, H.; Zhang, T.; Zhang, Q.; Zhang, G.; Wang, P.; Liu, Q. Cat. Lett. 2019, 149, 778–787.
CrossRef - Yan, W.; Yong-mei, C.; Ping-yu, W.; Yan-ming, H.; Te-fu, Q. Chem. Ind. For. Prod. 2012, 32, 81-86.J
- He, X.; Guo, X.; Xia, G.; Xu, R.; Wu, Y.; Luan, X. Catalysts, 2023, 13, 487.
CrossRef - Arumugam, S. M.; Singh, D.; Mahala, S.; Devi, B.; Kumar, S.; Jakhu, S.; Elumalai, S. Ind. Eng. Chem. Res. 2022, 61, 2524-2537.
CrossRef - Ramirez, M. E. M.; Elizalde, I.; Lopez, R.; Valdez, M. T. Rea. Kine. Mech. Cat. 2022, 130, 417-431.
- Delgado, M;. Aznar, P.; Corella, J. Ind. Eng. Chem. Res. 1997, 36, 1535–1543..
CrossRef - Shahid, M.; Farrukh, M. A.; Umar, A. A.; Khaleeq-Ur-Rahman, M. Rus. J. Phy.Chem. A, 2014, 88, 836–844.
CrossRef - Park, J.; Yi, K. B. Int. J. Hydrogen Energy. 2012, 37, 95–102.
CrossRef - Rajamathi, R.; Bojraj.; Nethravathi, C. ACS Appl. Nano Mater. 2021, 4, 10969-10975
CrossRef - Lan, P.; Wu, S. Chem.Eng.Tech.2014, 37, 580–586.
CrossRef - Yan, X.; Li, Y.; Ma, X.; Zhao, J.; Wang, Z.; Liu, H. New J. Chem. 2019, 43, 5116–5125.
CrossRef - Al-Awaji, N.; Boukriba, M.; Idriss, H.; Ben Aissa, M. A.; Bououdina, M.; Modwi, A. Adv. Mat. Sci. Eng. 2022, 12.
CrossRef - Olivas, D.; Y. A.; Guerrero, M. R. B.; Bretado, M. A. E.; Paula, M. M. D.; Gutiérrez, J. S.; Velderrain, V. G.; Ortiz, A. L.; Martínez,V. C. Int. J. Hydrogen Ene. 2014, 39, 16595–16607.
CrossRef - Zhu, Q.; Zeng, S.; Yu, Y. Env. Sci. Technol. 2017, 51, 552–559.
CrossRef - Diana, F.; Vignesh, K.; Sreekantan, S.; Rahman Bin, A. New J. Chem. 2015, 40, 1–11.
- Yan, X.; Li, Y.; Ma, X.; Zhao, J.; Wang, Z.; Liu, H. New J. Chem. 2019, 43, 5116–5125.
CrossRef - AbuKhadra, M.; Basyouny, R. M. G.; AlHammadi, A. A.; El-Sherbeeny, A. M.; Salam, M. A. Sci. Rep.2020, 10, 1–11.
CrossRef - Ahmed, S.; Guo, Y.; Li, D.; Tang, P.; Feng, Y. Env. Sci. Pollut. Res. 2018, 25, 24907–24916.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









