Kinetic Study and Hammett Correlations in the Chemistry of M-Nitro and M-Amino Benzoic Acid Hydrazides by Using Thallium (Iii) in 1,4-Dioxane Medium
1Department of Chemistry, A.C.S. College, Lanja, Dist-Ratnagiri Maharashtra State, India.
2Department of Chemistry, A.S.P. College, Devrukh, Dist-Ratnagiri Maharashtra State, India.
Corresponding Author E-mail: amitvarale@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/390626
Article Received on : 19 Sep 2023
Article Accepted on :
Article Published : 10 Nov 2023
Reviewed by: Dr. Asif Khan
Second Review by: Dr. Mohd. Tofiq
Final Approval by: Dr. Gavat Cristian
Iodometric analysis is used to determine the kinetics and mechanism of oxidation of benzoic, m-amino, and m-nitro benzoic acid hydrazides by Thallium(III) in acidic medium at constant ionic strength. The complex decomposes to produce the product. The rate of the reaction is decreased by the rise in [H+] and [Cl-]. Additionally, Activation parameters were established, and a mechanism is proposed. The analysis of reaction constants (ρ) and substituent constants (σ) for m-nitro BAH and m-amino BAH indicates good agreement with the values from the literature.
KEYWORDS:Hammett parameter; Kinetics, Thallium (III); m-nitro benz acid hydrazide (m-NO2 BAH); m-amino benz acid hydrazide (m-NH2BAH); Oxidation; Thermodynamic parameter
Download this article as:| Copy the following to cite this article: Nikalaje S. H, Varale A. S. Kinetic Study and Hammett Correlations in the Chemistry of M-Nitro and M-Amino Benzoic Acid Hydrazides by Using Thallium (Iii) in 1,4-Dioxane Medium. Orient J Chem 2023;39(6). |
| Copy the following to cite this URL: Nikalaje S. H, Varale A. S. Kinetic Study and Hammett Correlations in the Chemistry of M-Nitro and M-Amino Benzoic Acid Hydrazides by Using Thallium (Iii) in 1,4-Dioxane Medium. Orient J Chem 2023;39(6). Available from: https://bit.ly/49so5Yh |
Introduction
One of the most used tools for the analysis of chemical reactions and their processes is the Hammett equation. It is remarkable that sigma constants, calculated simply from the ionization of organic acids in solution, have an empirical basis, usually able to accurately estimate the velocity and equilibrium of a number of types of reactions in solutions 1.
Physical-Organic chemists used the Hammett equation to explain the reaction mechanisms of various organic chemical reactions in the second half of the 1930s.2 As recorded, the equation only is applicable to reactions involving aromatic compounds and their derivatives.3 QSAR has been a very helpful tool for the study of substituent effect on the rate of reaction. Hydrazides are products prepared from hydrazine hydrate, ester and carboxylic acid4. In aqueous alkali media, a recent initial review based on the oxidation of aromatic acid hydrazides using hexacyanoferrate-(III) has been reported5,6. There is no change in ionic strength, according to a recent report on the kinetics study and mechanism of fluorenone hydrazine’s permanganate oxidation in alkaline media7. Anderson type hexamolybdochromate (III) catalyses the rate at which bromate oxidizes benzoic acid hydrazide in an acidic aqueous medium 8. Interest in the use of Thallium(III) in the oxidation of organic compounds has increased only recently and research in this regard is not been extensive. The potential of this oxidant is realized more and more as is evident from the considerable amount of work that is lately being done. Thus, the selectivity of Thallium (III) is higher than its neighbours in the periodic table, Mercury(II) and lead (IV) and also Thallium (III) is a better oxidant than the other two. The kinetics of oxidation of simple olefins was studied in detail by Henry9. Hence, the present work deals with the kinetics and mechanism of oxidation of benzoic, m-nitro, m-amino benzoic acid hydrazides by Thallium(III) in acidic medium10.
Experimental
The AR/AG quality of all the chemicals used during the experiment. The reagents’ impurities normally impact the rate of the chemical reaction; Therefore, every safety measure practicable was taken to keep substances out of the reaction system. In the laboratory, m-nitro and m- amino benzoic acid hydrazides were synthesized11. After recrystallization, their purity was examined by measuring their physical constants and by TLC.
A stock solution of m-nitro and m- amino benzoic acid hydrazides was prepared in 1,4-dioxan – water. medium12. The solutions were kept in a dark space. Pyrex glass was used for the preparation and storage of the solution. An amber-colored bottle containing the hydrazide stock solution was kept in a darkened room.
Using a graduated pipette, the calculated amounts of standard 0.1 M Thallium (III), 1 M HCl, and 1 M perchloric acid solutions were obtained in one flask. By using a graduated pipette to add the required quantity of distilled water, the volume of 50 ml was adjusted.
Using a graduated pipette, the calculated amount of standard 1 M hydrazide solution was added to another conical flask. Using a graduated pipette, add a solution of 1M perchloric acid. By using a graduated pipette to add the necessary amount of distilled water, the volume of 50 ml was adjusted. Both flasks were thermostated at least for 20 minutes.
The thermostated solution of hydrazide was added to the thermostated solution of thallium (III), which contained a combination of HCl and HClO4, to start the reaction. The two solutions’ time of mixing was recorded as zero time.
5 ml of the reaction mixture are pipetted out at zero time and added to a fresh conical flask. Then 5ml of 2M H2SO4 solution and 5 ml of 5% KI solution is added to the reaction mixture, starch solution is then added to the reaction mixture and it is titrated with standard Na2S2O3 solution till color changes from violet to colorless. Similarly, at various time intervals, the reaction mixture was pipetted out, and the burette reading was recorded.
Results and Discussion
The reaction has been observed extensively was successfully proceeded by using combination of HCl and HClO4. At constant concentrations of [HCl] and [HClO4] and at µ = 0.6 mol /dm3, The influence of reaction rates on the kinetics of reaction was investigated. Oxidant concentration was diversified since 6.5×10-4 near 6.5×10-3 mol/ dm3 andmaintaining the hydrazide concentration at 10×10-2 mol/dm3. The pseudoUni-molecular velocity constant is constant for m-amino BAH and m-nitro BAH and are 5.04 ± 0.2×10-4 s-1, 8.32 ± 0.1×10-4 per second the order is equal to one when compared to the order of the [oxidant].
In the further study the concentration of hydrazide was determined by changing concentration from 0.01 to 0.1 mol /dm3 by putting the concentration of oxidant constant at 3.0×10-3 mol/ dm3. It is observed that the big rise in the pseudo first order rate constants from 0.85 x 10-4 s-1 to 5.20 x 10-4 s-1 for m-NH2 BAH and 1.53 x 10-4 per second to 12.96 x 10-4 s-1 for m-NO2 BAH with hydrazide. The order of this reaction is found in the fractional.
To understand more about how [Cl–] and [H+] affect reaction rate m-amino BAH and m-nitro BAH, the [oxidant], [hydrazide] and ionic strength were remains constant and these are 0.003, 10 x 10-2, µ = 0.6 mol/ dm3 respectively. For the determination of [H+] and [Cl–], the chemical reagents used are HClO4 and NaCl. It is observed that rise in [H+] from 13 x 10-2 to 60 x 10-2 mol /dm3 reduces k x 10-4 (s-1) from 1.62 to 0.42 for m-amino BAH and 3.06 to 0.99 x10-4 k (per second) m-nitro BAH at 25oC. Further the rise in [Cl–] from 13 x 10-2 to 60 x 10-2 mol dm–3 decreases k x 10-4 (s-1) from 3.02 to 1.52 for m-NH2 BAH and 1.06 to 0.22 x10-4 k (s-1) m-NO2 BAH at 25oC.
By adjusting the solvent concentration from 5 to 40% v/v, the relative dielectric phenomena were changed. It is detected that, rate of reactions for m-amino BAH and m-nitro BAH was reduced by reducing the percentage of 1, 4-dioxan. The values for Michaelis-Menten plot for m-amino BAH and m-nitro BAH is revealed in Table 1. Figure 1 displays the Michaelis-Menten plot for temperatures between 15°C and 30°C.
Table 1: Michaelis-Menten plot for m-amino BAH and m-nitro BAH.
|
Temperature |
m-nitroBAH and m-aminoBAH |
1/ m-nitroBAH and 1/ m-amino BAH |
Kobs x 10-4 per second |
1/ Kobs x 10-4 per second |
||
|
Meta-nitro BAH |
Meta -aminoBAH |
Meta-nitro BAH |
Meta -aminoBAH |
|||
|
15oC |
0.01 0.03 0.05 0.064 0.1 |
100.33 33.33 20.00 15.625 10.00 |
0.75 1.25 2.51 4.79 6.12 |
0.42 1.71 1.14 1.78 2.24 |
1.3333 0.8000 0.3920 0.2087 0.1633 |
2.3809 1.4084 0.8771 0.5617 0.4464 |
|
20oC |
0.01 0.03 0.05 0.064 0.1 |
100.33 33.33 20.00 15.625 10.00 |
1.14 1.72 3.14 7.28 9.32 |
0.63 1.06 1.70 2.67 3.35 |
0.8771 0.5813 0.2673 0.1373 0.1072 |
1.5873 0.9433 0.5882 0.3745 0.2985 |
|
25oC |
0.01 0.03 0.05 0.064 0.1 |
100.33 33.33 20.00 15.625 10.00 |
1.53 2.52 5.01 9.77 12.96 |
0.85 1.41 2.27 3.56 4.46 |
0.6535 0.3968 0.1996 0.1023 0.0771 |
1.1764 0.7092 0.4405 0.2808 0.2242 |
|
30oC |
0.01 0.03 0.05 0.064 0.1 |
100.33 33.33 20.00 15.625 10.00 |
2.26 3.40 7.42 14.20 18.20 |
1.25 2.13 3.39 5.32 6.51 |
0.4424 0.2941 0.1347 0.0704 0.0552 |
0.8000 0.4694 0.2949 0.1879 0.1522 |
 |
Figure 1: Effect of Temperature and Michaelis-Menten plots for-m-NitroBAH. |
 |
Figure 2: Effect of Temperature and Michaelis-Menten plots for m-AminoBAH. |
Table 2: Determination of Kc and k1 [HCl] = 10 x 10-2 mol /dm3, [HClO4] = 10 x 10-2 mol /dm3, [TlIII] = 0.3 x 10-2 mol /dm3, µ = 0.6 mol /dm3
|
Hydrazide for kinetic sudy |
Kc.in (moldm-3 ) |
|||
|
15oC |
20oC |
25oC |
30oC |
|
|
BAHydrazide |
9.32 |
9.61 |
9.31 |
9.01 |
|
m-nitro BAH |
14.50 |
14.47 |
14.94 |
14.53 |
|
m-amino BAH |
11.42 |
11.07 |
11.11 |
11.13 |
From the kinetic study of the reaction, it was found that the order of hydrazide is fractional and it is unity with respect to thallium (III) oxidizing reagent. The complex formed in the reaction have an equilibrium with the substrates. This shows an effect on the fractional order of substrate concentration11.
Due to the complex’s production throughout the reaction, against 1/[Hydrazide] the 1/kobs Michaelis-Menten graphs had an intercept and were linear. As a result, findings, Scheme 1 can be used to identify the reaction’s probable mechanism.
 |
Scheme 1 |
By plotting the graph of per kobs versus per [Hydrazide] at varying temperatures, rate law in is validated. Kc and k1 values were calculated. using the value of slopes and intercept obtain through graphs shown in Table 2. These depict marginally positive intercept values.
It has been noted that the reaction is influenced by the protonation-induced concentrations of hydrogen and chloride ions of hydrazides12 and the presence of various chloro-complexes13 of thallium (III). Ionic strength and the finding that one of the reactants employed in the reaction is neutral support the idea that free hydrazide plays a significant role in this reaction.
“Oxidant forms solid complexes with chloride ions that have the formula TlCln3-n, where n is the number of complexes with chlorides. The values of specific stability constants”14 is as following:
K1 = 13.8 X 107, K2 = 39.8 X 1012, K3 = 60.2 X 1014 and K4 = 10 X 1017 per mole dm3.
Thallium (III) will be present in this reaction as TlCl2+ and A.15 illustrates its concentration. [TlCl2] +free where, b1=K3/K2=151 and b2=K4/K3=166.
 |
Scheme 2 |
The rate laws were confirmed by when plotting per kobs against both per [Hydrazide] and per [H+], both of which linearity was found with positive intercepts. as shown in Figure 1. The values were determined using the slopes and intercepts of graphs of KH and Kc. The Kc values can be found in Table 1. For BAH and m-nitro BAH, they were found to be 13 and 16 per mol dm3 respectively. The thallium (III) chloro complex with the highest electrophilicity, TlCl2+, is the reactive species.
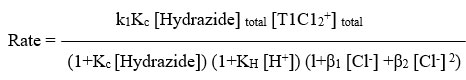
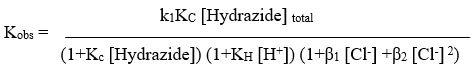
Below is an illustration of the plausible mechanism for the entire reaction.
Mechanism
 |
Scheme 3 |
An N-Tl bond is created by the electrophilic substitution of the hydrazide’s N2 in the mechanism. The intermediate is produced via a two-electron shift from substrate to oxidant. This N-Tl bond formation is thought to result from the oxidation of nitrogen-containing molecules by thallium (III)15.
The computed values for thermodynamic variables are (ΔH )KJ mol-1), ΔG (KJ mol-1) and ΔS (JK-1mol-1) remained 12.11, 21.53 and -31.89 for m-amino BAH and 16.64, 21.37, -15.92 for m-nitro BAH. According to Scheme 3, The entropy of activation significantly decreases as the transition state becomes more organized. The reaction can be tolerated by the shift in ionic strength since the method utilizes hydrazides that are neutral as the active substrate. The rate reduces as the concentration of 1, 4-dioxan increases; this effect of the solvent is brought about by the complex equilibrium between the reactants16 in a medium with a low relative permittivity17,18,19,20.
Calculated Value of Hammett Parameters
The pKa value of BAH at (V1/2) is 5.7 and the pKa value of the m-nitro BAH (V2/2) is 5.2 (δ = 1.69 for 50% ethanol). The substituent constant (𝛔) value of the m-nitro BAH is 0.30 and reaction constant (𝛒) value of m-nitro BAH is 1.67.
pKa values for BAH are 5.7 at (V1/2) and 8.5 at (V2/2) (= 1.69 for 50% ethanol). The substituent constant (σ) value for the m-amino BAH is (-1.69), while the reaction constant (ρ) value is 1.65.
pH metry method is used to determine the substituents constant (𝛔) and the reaction constant (𝛒) value of m-nitro BAH and m-amino BAH. Moreover, the purpose of this research work was to use the Hammett equation to determine how the BAH and m-substituted BAH’s substituents affect their equilibrium constants or up of reactions.21
Pko – pKa = 𝛒𝛔
Where, Pko and pKa represents the acid dissociation constant of BAH and substituted BAH, 𝛔 is a constant that is characterized by substituents m-NO2 and m-NH2 is independent of the substituents. The ionic strength (0.6) kept constant during the experimental condition.
pH measurements were performed using a serial number 259731 for an Adel model Equipped with a combined pH electrode E-201-C, the pH ionometer from Bio Era life sciences. In aqueous 50% ethanol (v/v), pH metry measurements were prepared at room temperature. The value was recorded at the beginning of the pH metry titration (in the acidic area). with the value of pH at the end of titration where the titration solution was sufficiently basic. At regular intervals throughout the titration, pH was monitored. (2-3 min) for equilibrium after every 0.2 N NaOH solution addition. Pko, pKa value of BAH and substituted BAH were calculated from this method and pH measurement data.
 |
Figure 3: pH measurement data with m-aminoBAH |
Table 3: Hammett Parameters
|
Hammett Parameters |
||
|
|
(σ)-Value |
(𝛒)-Value |
|
m-nitro BAH |
0.30 |
1.67 |
|
m-aminoBAH |
-1.66 |
1.65 |
Conclusion
The examined hydrazides are reactive in the following order:
m-nitro BAH < BAH < m-amino BAH.
The calculation of the Hammett parameters yields the reaction constant (ρ) value of 1.67 for the m-nitro BAH and the substituent constant (σ) value of 0.30.
The increased electron-donating inductive action of the alkyl group in the case of m-amino benz hydrazide has no effect on efficiency. According to Hammett parameters, the m-amino BAH the values of the reaction constant (ρ) and substituent constant (σ) are 1.65 and -1.66, respectively. Because they raise the ionization constant in comparison to parent benzoic acid, Sigma values for electron withdrawing groups are positive, whereas those for electron donating groups are negative. These constants provide us with a qualitative and quantitative sequence effect caused by electron donating and withdrawing groups on the rate of reaction in addition to providing us with an understanding of whether a specific substituent is relative to hydrogen electron-withdrawing or electron-donating. Reaction rates are slowed or equilibrium is suppressed by electron withdrawing groups.
References
- Hanch, C.; Leo, A.; Taft, R. W.; Chem.Rev, 1991, chapter 91, 165-195.
CrossRef - Vandanapu, J.; Rachuru, S.; Advances in Physical Chemistry. 2012, 2012, 1-4.
CrossRef - Gallup,G.R .; Gilkerson, W.R.; and Jones, M.M.; A Theoretical Formulation of the Hammett Equation . 1952, 32, 233-236.
- Koeevar, M.; Mihorko, P.; Polanc, S.; J. Org. Chem. 1995, 60, 1466–1469
CrossRef - Shimpi, R.; Fadat, R.; Janrao, D. M.; Farooqui, M.; Arab J. Phys. Chem. 2014, 2, 52-59
- Pore, S. V.; Int. J. Curr. Res. 2016, 8, 28330–28338
- Fawzy, A.; Ahmed, S. A.; Althagafi, I. I.; Morad, M. H.; Khairou, K. S.; Adv. Phys. Chem. 2016, 20, doi:10.1155/2016/4526578.
CrossRef - Kadam, S. D.; Supale, A. R.; Gokavi, G. S.; J. Phys. Chem.2008, 222, 635-646.
CrossRef - Henry, P.M. ; J. Am. Chem. Soc., 1965, 87, 990; Henry, P.M. Ibid .,1965, 87, 4423; Henry, P.M. Ibid ., 1966, 88,1597.
CrossRef - Kocevar, M.; Mihorko, P.; Polanc, S.; J. Org. Chem.1995, 60, 1466–1469
CrossRef - Vogel, A. I.; A Text Book of Quantitative Inorganic analysis IV Edn. (E.L.B.S. and Longman’s Press) 385.
- Kazo K.; Hirakazoo, T.; Hisashi, K.; Zenzo, T.; Chem. Pharm. Bull. 1963,11, 797
- Lee, A. G.; The Chemistry of Thallium, Elsevier, 1971, London, 48
- Amis, E. S.; Academic Press, New York, 1966.
- Gokavi, G. S.; Raju J. R.; Polyhedron, 1987, 6, 1721
CrossRef - Vogel, A. I.; ELBS and Longman Group, 3rd Edition, 1975
- Varale, A. S.; Hilage N. P.; Oxidation Communications. 2015, 38, 1204–1212.
- Deokar, H. P.; Varale,A. S.; International Journal of Grid and Distributed Computing.,2020, 13, 119 –125
- Deokar, H. P.; Tendolkar, N. P.; Varale, A. S.; High Technology Letters. 2023, 29, 245-256
- Nikalaje,S.H.; Varale, A.S.; Shinde,B.T.;Tendolkar N.P.; Manjare,S.B.; Rasayan Journal of Chemistry.2023, 16 ( 4 ),2042-2048.
- Nikalaje,S.H.; Varale, A.S.; Eur. Chem. Bull. 2023, 12 (s3), 6541–6550.

This work is licensed under a Creative Commons Attribution 4.0 International License.










