Anti-Oxidant Activities, Dosage of Total Polyphenols and Flavonoids from Crossopteryx febrifuga (AFZ. Ex G. Don) Benth (Rubiaceae)
Mbaïhougadobé Séverin1,2* , Madjitoloum Bétoloum Salomon2
, Madjitoloum Bétoloum Salomon2 , Mihaela Cudalbeanu3
, Mihaela Cudalbeanu3 , Mbaïogaou Abel 2
, Mbaïogaou Abel 2 , Bianca Furdui3
, Bianca Furdui3 and Rodica Mihaela Dinică3
and Rodica Mihaela Dinică3
1Université de Moundou, BP : 206 Moundou/Tchad.
2Research Laboratory of Natural Substances, FSEA of University of N’Djaména, Chad.
3Department of Chemistry, Physics and Environment, Faculty of Sciences and Environment, Dunarea de Jos University of Galati, Romania.
Corresponding Author E-mail: ndsevess@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/390623
Article Received on : 22 Sep 2023
Article Accepted on : 03 Nov 2023
Article Published : 26 Dec 2023
Reviewed by: Dr. Ammar A Razzak Mahmood
Second Review by: Dr. Fozia Z. Haque
Final Approval by: Dr. Tawkir Sheikh
The aim of this study was to determine the total polyphenols and flavonoids in extracts of Crossopteryx febrifuga (AFZ. Ex G. Don) Benth (CF) and to evaluate their antioxidant activity. TLC also reveals the presence of free phenolic acids, flavonoids. Determination of these compound families in the extracts yielded high levels of polyphenols and flavonoids in ethyl acetate extracts, with polyphenols at 264.233 mg and flavonoids at 111.784 mg. the qualitative method for determining antioxidant activity using the DPPH TLC method, gives yellow streaks indicating activity. The ethyl acetate extract showed greater inhibition than the other two extracts with the two radicals used. The extracts inhibited the DPPH radical at 35 min and ABST at 120 min. The results obtained would justify the use of this plant species in traditional medicine in Chad.
KEYWORDS:Antioxidants; Crossopteryx febrifuga; Flavonoids; Polyphenols
Download this article as:| Copy the following to cite this article: Séverin M, Salomon M. B, Cudalbeanu M, Abel M, Furdui B, Dinică R. M. Anti-Oxidant Activities, Dosage of Total Polyphenols and Flavonoids from Crossopteryx febrifuga (AFZ. Ex G. Don) Benth (Rubiaceae). Orient J Chem 2023;39(6). |
| Copy the following to cite this URL: Séverin M, Salomon M. B, Cudalbeanu M, Abel M, Furdui B, Dinică R. M. Anti-Oxidant Activities, Dosage of Total Polyphenols and Flavonoids from Crossopteryx febrifuga (AFZ. Ex G. Don) Benth (Rubiaceae). Orient J Chem 2023;39(6). Available from: https://bit.ly/47iG9lg |
Introduction
Nowadays, modern medicine has been able to cure many illnesses. But in most cases, these treatments are accompanied by side effects. To overcome this problem of side effects, the use of compounds isolated from plants is proving more important. Traditional medicine represents a cultural and economic heritage for Africa in general, and Chad in particular. It is still very much alive in Chad, where 75% to 80% of the population lives in rural areas. In the vast majority of cases, reactive oxygen species (ROS) are the cause of many illnesses or health complications following an imbalance between them and antioxidants1. To correct this imbalance, antioxidants need to be supplied from the outside. Polyphenols and especially flavonoids extracted from plants have been shown to have powerful antioxidant properties2. They exert their antioxidant power through several mechanisms. The most common are: reactive oxygen species scavenging, metal ion chelation and enzyme inhibition3.
In this work, we propose to determine the total polyphenols and flavonoids in ethyl acetate, methanol and water-methanol extracts of Crossopteryx febrifuga (AFZ. Ex G. Don) Benth (CF), and to evaluate their antioxidant activity. CF is a 6-7 m tall tree or shrub in the Rubiaceae family. The bark is smooth, then very finely scaly, gray to light brown, with a brittle edge, brown on the surface and salmon to light orange underneath. Opposite, oval leaves. The highly fragrant white flowers give way to round, green fruits that are black when ripe. This plant species has been listed in the herbarium of Chad’s zootechnical laboratory since 1964. From an ethnobotanical point of view, the various organs of this plant are widely used in Chad and throughout Africa for their therapeutic virtues. Several sources indicate its effectiveness against coughs and fevers. Several families of secondary metabolites have been identified, notably in the plant’s various organs: terpenes/sterols, flavonoids, tannins, alkaloids, saponosides, reducing compounds and quinones. Most of the compounds isolated from this plant’s various organs are divided into four families of organic compounds. These are iridoids, terpenes, saponosides and carbohydrates4,5. Several other compounds were identified by LC-MS analysis of extracts from leaves, trunk bark and root6. One glucoside, β-quinovine, and two alkaloids, crossopterin and crossoptin, were isolated7. From a pharmacological point of view, CF has antibacterial, antitussive, analgesic, anti-inflammatory and gastroprotective properties. The compounds isolated: crossoptin A and crossoptin B have analgesic, anti-inflammatory, mucolytic and antiedematous properties5,8-13.
Materials and Methods
Plant material
The plant material for our work consists of bark from the trunk of CF harvested south of the town of Moundou in the Logone Occidentale between the villages Mankou and Faya in December 2019. The plant was identified in the national herbarium of the Institut de Recherche pour l’Elevage et le Développement (IRED) under number … A preliminary cleaning operation was carried out before cutting the bark into small pieces to facilitate drying in the dark at room temperature at the Laboratoire des Substances Naturelles. After drying, the plant material was ground and pulverized, and the pulverized powder stored in jars for later use.
Extraction
25 g sample of plant material powder was placed in a cellulose cartridge and extracted with 250 mL of Soxhlet solvent. Extractions were carried out with the solvents in ascending order of polarity, i.e. cyclohexane, dichloromethane, ethyl acetate and methanol. For the hydromethanolic extract (80/20 : V/V), the mass was used. In both cases, extraction lasted six (6) hours, then filtered. Extracts were concentrated using a rotary evaporator where the bath temperature was set to the boiling temperature of the solvent. Finally, the extracts were dried at room temperature, leaving the erlens open.
Identification of polyphenols by thin layer chromatography (TLC)
This qualitative analysis was carried out according to the protocol described by Wagner14 (1996), and polyphenols and flavonoids in particular were identified by TLC. Silica gel aluminum plates 60 F254 (MercK) and a solvent system consisting of ethyl acetate/methanol/water (8/1/1) were used for both groups. After elution, the plates are removed from the tank and dried. They are air-dried before being sprayed with chemical reagents specific to each family. For polyphenols, the Folin-Ciocalteu reagent followed by ammonia vapor fumigation then observed from the visible. For flavonoids, the Natural products-polyethylene glycol reagent (NP/PEG) known as the NEU reaction is used. For the last case, plates are observed under UV at 365 nm after reagent revelation.
Determination of phenolic compounds on microplates
After qualitative identification of phenolic compounds, this family was assayed in extracts. The assay was carried out using a multi-well UV-visible device consisting of a 96-well plate reader (Tecan Pro M200, Tecan Trading AG, Männedorf, Switzerland). The device is controlled by Tecan i-control software, and data is acquired using Ms Excel 2010. Levels are expressed in milligram equivalents of reference compounds per 100 g of plant material powder (mgECR/100g PMV), using the straight-line equation y = ax + b obtained from the calibration curve.
Total polyphenols
The content of total polyphenols in the different extracts was quantified following the Folin-Ciocalteu reagent method of the Singleton method15,16. Gallic acid was used as the standard for establishing the calibration curve. This method focuses on the reduction of the phosphotungistic and phosphomolibdic mixture constituting the Folin-Ciocalteu reagent by the groups of phenolic compounds leading to the formation of blue-colored reduction products that show an absorption maximum at 760 nm. First, 25 µL of Folin–Ciocalteu reagent was added to 10 µL of each methanolic extract. After 5 min of incubation, 25 µL of a 20% aqueous sodium carbonate solution and ultrapure water was added until the final volume reached 200 µL. Blanks were also prepared for each sample by replacing the Folin–Ciocalteu reagent with ultrapure water. after 30 min of incubation, absorbances callus of the samples were measured at 760 nm using a 96-well plate reader (Tecan Pro M200, Tecan Trading AG, Männedorf, Switzerland). The results obtained are expressed in mg gallic acid equivalent per gram of dry matter (E GA/g MS).
Total flavonoids
The aluminum trichloride method was used to quantify total flavonoids in the various CF extracts. This technique is based on the formation of an “alumino-flavonoid” complex that has an absorption maximum at 415 nm16. The calibration curve was obtained using quercetin (40–0.078 µg/mL). First, 100 µL of an aqueous 2% aluminum chloride solution was added to 100 µL of each methanolic extract sample. After 15 min of incubation, the sample absorbance values at 415 nm were read using the Tecan Pro 200 multiwell plate reader. Results are expressed expressed in mg Quercetin equivalent per gram of dry matter (EQ/g MS).
Determination of antioxidant activity using microplates
Antioxidant activity was assessed in vitro using methods based on electron transfer mechanisms. These are the DPPH (2,2-diphenyl-1-picrylhydrazyl) free radical scavenging method and the ABTS (2,2′-azinobis-(3-ethylBenzoThiazoline-6-Sulfonic Acid) reduction method.)
DPPH radical trapping test
Two methods were used: a qualitative method and a quantitative method.
Qualitative method: TLC detection
Extracts, fractions or pure products to be tested are deposited on TLC plates of aluminum GF254 silica gel and developed in the appropriate systems with a mixture of ethyl acetate/formic acid/water (8/1/1), followed by spraying with DPPH (2mg/ml in methanol) to characterize antioxidant activity.
Anti-radical activities appear as yellow-white spots on a violet background17.
Quantitative DPPH method on microplate
Method based on the absorption of the free radical (DPPH) at 517 nm.
In a 96-well plate, 100 µL of sample DPPH solution at 100 µg/mL was added to 100 µL of extract at different concentrations (0,5 – 0,0039 mg/mL). The resulting solutions were then homogenized and left in the dark at room temperature. Absorbance values were recorded at 517 nm after each period of 20 min, 35 min, 60 min and 2 h against a blank consisting of a 1 : 1 mixture of methanol and DPPH solution. Solutions of gallic acid and quercetin at concentrations of 100 µg/mL were used for comparative activity. Lower absorbance indicates greater free radical scavenging activity18.
Percentage inhibition was determined using the following formula :

Quantitative ABTS method on microplate
Anti-free radical activity was also assessed by the ABTS+- radical cation decolorization test. ABTS was dissolved in distilled water at a concentration of 7 nM. The ABTS+- cation radical solution was obtained by incubating an equal volume mixture of the ABTS stock solution with a 2.45 nM potassium persulfate solution for 12 to 16 h in the dark at room temperature. The ABTS+- solution was diluted with ethanol to an absorbance of 0.700 ± 0.02 at 734 nm before use. Then, 95 µL of ABTS+- solution was mixed with 5 μL of extract or reference (gallic acid) at different concentrations (250 – 125 – 62.5 – 31.25 – 15.62 – 7.81 μg/mL) and 100 µL of methanol was added. Absorbances were measured at 734 nm after incubation for 20 min, 35 min, 60 min and 2 hours in the dark at room temperature. Three assays were carried out for each concentration of product tested, and results expressed as Percentage Inhibition (PI) defined by :

Results
Identification of polyphenols by thin-layer chromatography (TLC)
TLC of the methanolic extract gave the following results, shown in figure 1 below.
These chromatograms clearly show the presence of free phenolic acids and flavonoids in the extracts. All the blue-black spots observed in chromatogram a) are not revealed by NEU in b).
 |
Figure 1: TLC chromatogram: Migration system: AcOEt/AF/H2O (8/1/1). |
Determination of phenolic compounds on microplate
The estimation of total polyphenol and total flavonoid content in plant extracts was carried out using separate colorimetric methods (Folin-Ciocalteux and aluminum trichloride (AlCl3)). Polyphenol and flavonoid contents were reported in milligram equivalents of each reference molecule used, and determined from the equation of a straight line of the type: y = ax + b; obtained from the calibration curve.
Total polyphenols
The total phenol content estimated by the Folin-Ciocalteu method for each extract was reported in mg gallic acid equivalent/g dry plant material. They were obtained from a calibration curve established with increasing gallic acid concentrations. The calibration curve yielded the regression line y = 0.003x + 0.1964 with correlation coefficient R2 = 0.9982.
From the regression line, we found the number of total polyphenols for each extract shown in figure 2.
 |
Figure 2: Total polyphenol content in different CF trunk bark extracts. CF ACOET : CF ethyl acetate, CF MeOH : CF methanol and CF :eau-Méthanol : CF hydromethanolic |
These results show that the quantity of polyphenols increases with solvent polarity.
Total flavonoids
Total flavonoid levels were obtained from a calibration curve based on known Quercetin concentrations (y = 0.0058x+0.0262, R2 = 0.9822). The results presented in Figure 5 show a good presence of flavonoids in plant extracts. This confirms the results obtained by thin-layer chromatography.
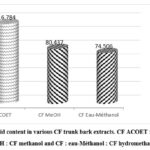 |
Figure 3 : total flavonoid content in various CF trunk bark extracts. CF ACOET : CF ethyl acetate, CF MeOH : CF methanol and CF : eau-Méthanol : CF hydromethanolic |
Due to the presence of OH in positions 3, 4, 5, 4′ and 5′, flavonoids are able to chelate Al3+ ions, forming a yellow complex proportional to the quantity of flavonoids. The results obtained show that the ethyl acetate extract contains more flavonoids than the other extracts2.
Determination of antioxidant activity using microplates
DPPH radical scavenging test
Qualitative method: Highlighting on TLC
The antioxidant capacity of extracts was assessed by measuring their free radical scavenging activity using a DPPH test. The results of thin-layer chromatography of the free radical scavenging activity of methanol (a) and methanol/water (b) extracts of plant in the following solvent systems: ethyl acetate/formic acid/water (8/1/1) are shown in figure 4.
 |
Figure 4: Antioxidant activity. Elution system: |
Anti-radical activities appear as yellow-white spots on a violet background17.
Quantitative DPPH method on microplate
Figure 5 shows the percentages of inhibition of the same extracts obtained by measuring absorbance.
The antioxidant activity assay shows that some extracts have an activity equivalent to that of controls used at high concentrations. This high activity indicates that the extracts contain substances that react with the stable DPPH radical.
 |
Figure 5: DPPH inhibition kinetics for extracts and reference compounds. |
In both reference compounds and extracts, inhibition is greatest at the thirty-fifth minute. Inhibition is around 90% for reference compounds and around 80% for extracts. The results obtained in this figure show that ethyl acetate extract has a superior antioxidant effect on the DPPH radical compared with other extracts.
Quantitative ABTS method on microplate
The results of absorbance inhibition tests on the cationic radical ABTS+-, by the various CF extracts are shown in figure 6.
 |
Figure 6: Percentage ABTS+- inhibition of CF extracts tested. |
The hydromethanolic extract showed flaible inhibition than the other two extracts. This correlates well with the assay performed. The ethyl acetate and methanol extracts showed ABTS+– absorbance inhibition in excess of 60%.
Discussion
The methanol extract revealed this chemical family. Chromatographic plate images for flavonoids shows blue spots. Blue-black spots are also observed on the plate relating to free phenolic acids. TLC also reveals the presence of free phenolic acids, flavonoids14. The two blue-black spots (Rf, 0.31 and 0.43) observed in chromatogram a) show a less intense blue coloration in b), which could explain the presence of flavonoids. This is the case for the spot, whose Rf is 0.48.
The determination of total polyphenols and total flavonoids makes it possible to assess the presence and concentration of these chemical groups in different extracts (ethyl acetate, methanol and hydromethanolic) of a sample. For polyphenols, the ethyl acetate extract has the highest concentration at 264.233 mg. The methanol extract has a concentration of 459.14 mg, while the hydromethanolic extract has a concentration of 427.211 mg. In terms of total flavonoids, the ethyl acetate extracts again showed the highest concentration at 111.784 mg, followed by the methanolic extract at 80.437 mg, and finally the hydromethanolic extract at 74.506 mg. These results indicate that ethyl acetate extracts contain the highest number of total flavonoids, while methanol extracts contain the highest number of polyphenols. It should be noted that the concentration of polyphenols and flavonoids varies according to the solvent used for extraction, which may have a variable affinity for soluble compounds. Idris M.M. and Nenge H.P19., working on the ethanolic extract of the plant’s bark, showed that flavonoid percentages were very high, as were polyphenols.
The qualitative method used to determine antioxidant power shows that the antioxidant test carried out on a TLC plate using the DPPH developer shows a trail of numerous yellow spots demonstrating the free radical scavenging activity of plant extracts.
Quantitative methods for ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid), a cationic radical, and DPPH (2,2-diphenyl-1-picrylhydrazyl), another free radical, are both used to assess the antioxidant power of compounds. Results vary slightly depending on the extracts used. In the case of ABTS, results at a concentration of 0.5 mg/ml after 120 minutes incubation give 67.77% for the ethyl acetate extract, 65.03% for the methanol extract and 58.06% for the hydromethanolic extract. The results obtained with DPPH at the same concentration after 35 minutes incubation are 81.94% for ethyl acetate extract, 79.94% for methanol extract and 77.30% for hydromethanolic extract. These values are the maximum values obtained from figures 5 and 6. Furthermore, at the same concentrations tested, the PIs obtained by the DPPH test are higher than those of the ABTS method inversely to the work of Sarr et al20. On the other hand, the work of Awika et al.21 observed high correlation between ABTS, DPPH among sorghum and its products. We note that ethyl acetate and methanol extracts showed better results in both methods, with higher percentages of antioxidant activity. This could indicate a greater capacity of the compounds extracted by these solvents to reduce free radicals and prevent oxidative damage. This leads us to say that results may vary depending on many factors such as extract concentration, incubation time and solvents used. Comparing the DPPH results of the extracts with those of the reference compounds gallic acid and quercetin, already known for their antioxidant activity, we find that the values are slightly higher.
These results are in agreement with those obtained by M. Séverin, and even more so in the work of Boungou and colleagues5,6. This antioxidant power is most likely due to the phenolic compounds present in Crossopteryx bark extracts, known as antioxidant substances with the ability to trap radical species. At concentrations above 100 mg/mL, ethyl acetate extract showed greater inhibition than the other two extracts with the two radicals used. This result for ethyl acetate extract could be explained by the presence of flavonoids shown in the assay of this family of compounds. Idriss and Neng showed that DPPH and ABTS inhibition are dose-dependent. At a concentration of 300 mg/ml, inhibition is 87.19±0.03% for ABTS and 81.65±0.00% for the DPPH radical19. The addition of an antioxidant to a solution of this cationic radical results in a reduction of the radical and a decrease in absorbance. This decrease depends on the antioxidant activity of the compounds tested, time and concentration22.
TLC analysis confirms the presence of free phenolic acids and flavonoids in the methanolic plant extract. The presence of these families was confirmed by assay. These results corroborate those obtained for antioxidant activity. Polyphenols and flavonoids are known for their antioxidant properties and their potential role in the prevention of certain diseases2. Thus, the presence of these compounds in extracts could suggest a potential health benefit.
Conclusion
Assessing the phenolic compound content of plant extracts can be an indicator for phytochemical analyses. Estimation of antioxidant power is also crucial for subsequent pharmacological analyses. It is in this context that phenolic content and antioxidant power were assessed in this study. The results showed that extracts from Crossopteryx febrifuga (AFZ. Ex G. Don) Benth trunk bark yielded satisfactory results, which could justify its widespread use in traditional medicine in Africa, and in Chad in particular. The polyphenol content of the methanol extract was higher than that of the two other extracts. The ethyl acetate extract contains more flavonoids. At concentrations in excess of 100 mg/ml, all extracts showed inhibition levels in excess of 50%. The ethyl acetate extract showed greater inhibition than the other two extracts with the two radicals used.
Acknowledgements
This work was made possible by the Eugene Ionesco grant, awarded by A.U.F., and by 3Department of Chemistry, Physics and Environment, Faculty of Sciences and Environment, Dunarea de Jos University of Galati, Romania.
Conflict of Interest
There is no conflict of interest.
Funding Sources
There are no funding sources.
References
- Talla, E., Ngenge Tamfu, A., Gade, I., Yanda, L., Tanyi Mbafor, J., Laurent, S., & Vander Elst, L. (2017). New mono-ether of glycerol and triterpenes with DPPH radical scavenging activity from Cameroonian propolis. Natural Product Research, 31(12), pp. 1379-1389.
CrossRef - Bruneton J. (2009). Pharmacognosie, Phytochimie, Plantes médicinales. 4ème Ed revue et augmentée. Editions médicales internationales, éditions Tec & Doc Lavoisier. Paris. 1269 P.
- Halliwell, B., Cross-, C. E. (1994). Oxygen-derived species: their relation to human disease and environmental stress.
- Bealem Aristide, Sakava Paul, Lidia Favier, Didier Hauchard, Jean-Paul Guegan, Nyemb Jean Noël , . . . Mbafor Tanyi Joseph. (2021). Chemical Constituents of Crossopteryx febrifuga (Afzel ex G. Don) Benth (Rubiaceae), their Phytotoxicity and Antioxidant Activities. Natural products chemistry and research, 9(1000416).
- MBAIHOUGADOBE Séverin. (2017). Phytochimie et pharmacologie des plantes utilisées dans le traitement de la goutte au Tchad. Thèse de Doctorat, Université Marien Ngouabi.173 P.
- Boungou-Tsona, G.; Gainche, M.; Decombat, C.; Ripoche, I.; Bikindou, K.; Delort, L.; Caldefie-Chézet, F.; Loumouamou, A.; Chalard, P. (2023) Chemical Profile, Antioxidant and Anti-Inflammatory Potency of Extracts of Vitex madiensis Oliv. and Crossopteryx febrifuga (Afzel ex G. Don). Plants, 12, 386. https://doi.org/10.3390/plants12020386
CrossRef - Tona, L., Kambu, K., Ngimbi, N., Mesia, K., Penge, O., Lusakibanza, M., Cimanga, K., De Bruyne, T., Apers, S., Totte, J., Pieters, L., Vlietinck, A.J. (2000) Antiamoebic and spasmolytic activity of extract of some antidiarrhoeal traditional preparations used in Kinshasa Congo. Phytomedicine 7, 31–38.
CrossRef - BALLO Mahamadou Karim. (2013). Etude phytochimique et l’évaluation de l’activité sur mycobacterium tuberculosis in vitro de 22 plantes utilisées dans le traitement traditionnel de la tuberculose au Mali. Thèse de Pharmacie de l’Université des sciences, des techniques et des technologies de Bamako/Mali.10p
- SANOGO R. (2000). Pharmacognosie et pharmacodynamie de plantes utilisées dans la médecine traditionnelle au Mali, Thèse de Doctorat de recherche, Faculté de Pharmacie, Université de Messine, Italie, 195 p.
- Adeola, SO, Yahaya, TA, Hafsatou, B, Chinwe, NA, Ezeonu, MC, Igwe, S., et Ndukuba, MA. (2011). Effet gastro-protecteur de Crossopteryx febrifuga chez le rat Wistar. Journal africain des médecines traditionnelles complémentaires et alternatives, 8(3).
CrossRef - Chouna Jean Rodolphe, Jean-de-Dieu Tamokou, Pépin Nkeng-Efouet-Alango, Bruno Ndjakou Lenta and Norbert Sewald. (2015). Antimicrobial triterpenes from the stem bark of Crossopteryx febrifuga. Z. Naturforsch. 70(7-8)c: 169–173
CrossRef - Sutovska, M., Franova, S., Priseznakova, L., Nosalova, G., Togola, A., Diallo, D., et Capek. (2009). Activité antitussive des polysaccharides isolés des plantes médicinales maliennes. International journal of biological macromolecules, 44(3), 236-239.
CrossRef - Salawu Oluwakanyinsola Adeola, Tijani Adeniyi Yahaya, Babayi Hafsatu, Nwaeze Angela Chinwe, Ezeonu Chidimma MaryJane, Igwe Sunday, Ndukuba Mary Adanna. (2011). Gastro-protective effect of crossopteryx febrifuga in wistar rats. Afr J Tradit Complement Altern Med. 8(3):300‐ 306.
CrossRef - Wagner H. et Bladt., (1996). Plant Drug Analysis, a Thin Layer Chromatography Atlas. Springer-Verlag. Ed., Berlin, second Edition.
CrossRef - Singleton VI, Lamuela-Raventos RM. (1999). Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Method in Enzymology,, 15.
CrossRef - Mihaela , C., Ioana , O., Bianca, F., Durand, D.-N., Robert, R., Teodor, C., . . . Rodica, M. (2018). Exploring New antioxidant and mineral compounds from Nymphaea alba Wild-Grown in Danub Delta Biosphere. Molecules, 23(1247). doi:doi:10.3390/molecules23061247.
CrossRef - Cavin A., (1999). Investigation phytochimique de trois plantes Indonésiennes aux propriétés antioxydante et antiradicalaire : Tinospora crispa (Ménispermacées), Merremia emarginata (Convolvulacées) et Oropea enneandra (annonacées). Thèse de Doctorat, Lausanne, 241 P.
- Milardović Stjepan , Damir Iveković, Božidar S. Grabarić. 2006. A novel amperometric method for antioxidant activity determination using DPPH free radical. Bioelectrochemistry Volume 68, Issue 2, Pages 175-180. https://doi.org/10.1016/j.bioelechem.2005.06.005
CrossRef - Idris, M., & Nenge, H. (2021). Free radical scavenging activity and histological properties of ethanol extract of crossopteryx febrifuga stem bark in alloxan-induced diabetic rats. Global Scientific Journal, 9(9), pp. 442-454.
- Sarr SO, Fall AD, Gueye R, Diop A, Diatta K, Diop N, NDiaye B, Diop YM. (2015). Etude de l’activité antioxydante des extraits des feuilles de Vitex doniana (Verbenaceae). Int. J. Biol. Chem. Sci., 9(3): 1263-1269. DOI : http://dx.doi.org/10.4314/ijbcs.v9i3.11
CrossRef - Awika, J.M., Rooney, L.W., Wu, X., Prior, R.L., Cisneros-Zevallos, L., 2003. Screening methods to measure antioxidant activity of sorghum (Sorghum bicolor) and sorghum products. Journal of Agricultural and Food Chemistry 51, 6657–6662.
CrossRef - Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. (1999). Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 26(9-10):1231-7. doi: 10.1016/s0891-5849(98)00315-3. PMID: 10381194.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









