Study on Solution Behavior of Green Gemini-Conventional Mixed Systems and their Solubilization Capability Towards Phenanthrene
Department of Petroleum Studies, Aligarh Muslim University, Aligarh, U. P. India.
Corresponding Author E-mail: nsardar@rediffmail.com
DOI : http://dx.doi.org/10.13005/ojc/390514
Article Received on : 08 Aug 2023
Article Accepted on : 25 Sep 2023
Article Published : 09 Oct 2023
Reviewed by: Dr. Ayssar Nahle
Second Review by: Dr. Ioana Stanciu
Final Approval by: Dr.. Malinee Sriariyanun
In the present study green ester bonded cationic gemini surfactants, Ethane-1,2-diyl bis (N,N-dimethyl-N-hexadecylammonium acetoxy)dichloride (16E216) and 2,2’-{(oxybis(ethane1,2-diyl)bis(oxy)bis(N-alkyl-N,N-dimethyl-2-hexadecyl oxoethanaminium)dichloride (16E316) were synthesized and evaluated for their various physicochemical and other interaction parameters with their mixtures with conventional surfactants namely (cetyltrimethylammonium bromide (CTAB) and cetyltrimethylammonium chloride (CTAC) by tensiometry. Much lower cmc values of the order of 250 times were observed for 16E216 and 16E316 than conventional surfactants. Further, synergistic interaction occurs between the gemini and conventional surfactants as a result of negative interaction parameters. The mixed systems were also investigated for the solubilization of polyaromatic hydrocarbon (PAH), Phenanthrene. Among the pure surfactants, 16E216 has the highest and CTAB has the lowest efficiency for phenanthrene. Solubility enhancement was observed for almost all gemini-conventional mixed systems and shows better solubilization properties than pure surfactant systems due to synergism. The morphologies of pure and mixed systems were examined by TEM imaging. Findings of the present study could be exploited to use such mixed surfactant systems as the successful remediation technology for PAH contaminated soils.
KEYWORDS:Gemini Surfactants; Mixed Micellization; Solubilization; Surfactant Washing
Download this article as:| Copy the following to cite this article: Ahmad M, Sardar N. Study on Solution Behavior of Green Gemini-Conventional Mixed Systems and their Solubilization Capability Towards Phenanthrene. Orient J Chem 2023;39(5). |
| Copy the following to cite this URL: Ahmad M, Sardar N. Study on Solution Behavior of Green Gemini-Conventional Mixed Systems and their Solubilization Capability Towards Phenanthrene. Orient J Chem 2023;39(5). Available from: https://bit.ly/3RRbGH8 |
Introduction
Surfactants are surface active agents; they contain both hydrophilic as well as hydrophobic parts, therefore they are amphiphilic in nature. Depending upon the charge present on the polar head, they are categorized as Ionic (anionic, cationic and zwitterionic) and Non-ionic surfactants 1˗4. When a surfactant is added to a system, after a certain amount of concentration in a solution, a concentration is reached, known as critical micelle concentration (cmc), surfactants arrange themselves into small aggregates and micelles are formed. This value of cmc is different for different types of surfactants depending on their molecular structure, chemical compounds etc. As the concentration of surfactants increases, the solubilization of insoluble compounds (PAHs) increases, but the physical properties do not change above cmc 5.
Polycyclic aromatic hydrocarbons, PAHs, are organic pollutants having very poor solubility and high interfacial tension with water and due to this fact, it can stay for an extended amount of time in the atmosphere. Further, their volatility and biodegradability are low 6. The environment is endangered by the accumulation of these toxic hydrocarbon compounds in the food chain. Therefore, removal of these toxic hydrocarbons is the prior need to save the ecosystem 7. To treat PAHs contaminated sites, the physical characteristics which are important are the solubility of PAHs in water and the interfacial tension of PAHs with water. Generally, PAHs have low solubility. Therefore, it is quite difficult to solubilize the PAHs simply in water. Therefore, simple water flushing will be less efficient 8. The solubility of PAHs can be enhanced due to the partitioning of PAHs into hydrophobic cores of surfactant micelles at concentrations greater than cmc 9. Therefore, added surfactant flushing is a promising approach to treat a PAHs contaminated site 10. However, synthetic surfactants are toxic to the environment and when their concentration increases, for example in industrial and commercial applications, the toxicity of these chemicals has a significant impact on the ecosystem 11. To overcome these problems, biodegradable surfactants can be used 12.
Gemini surfactants are a type of surfactant that has a spacer linked between more than one hydrophilic head group and hydrophobic tail groups at or near the head group. It has unique properties such as low cmc, very high water solubility, being very efficient in reducing oil/water interfacial tension, unusual rheology, viscosity, and so on 13. Additionally, gemini surfactants are biodegradable, less toxic than conventional surfactants, and have their lower cmc values. Although gemini surfactants alone or when combined with conventional surfactants find various applications, among them solubilization is a recent development and gaining importance due to environmental applications 14. A large number of studies have been conducted on nonionic surfactants and their mixtures with gemini surfactants. But very few systematic studies to get insight into the micellization and solubilization phenomena of cationic-cationic mixed surfactant systems and a detailed systematic study of the properties and structure of these mixed systems are required to make wise and optimum utilization of gemini-based systems for such applications and hence increased bioavailability of hydrophobic compounds. Therefore, the present study is aimed to systematically Study the interaction of green ester-bonded cationic gemini surfactants and conventional cationic surfactants and to investigate enhancement in the solubilization of phenanthrene using a single/binary mixture of cationic surfactants.
Experimental
Materials
The PAH phenanthrene (99.5%, Koch-Light laboratories, Haverhil, England (U.K)) were used as received. The cationic surfactants CTAB (≥99%, Sigma-Aldrich, St. Louis, Missouri, USA) & CTAC (≥99%, Sigma-Aldrich, USA) were used as received. Chloroacetyl chloride (98%, Spectrochem, Mumbai, Maharashtra, India), ethylene glycol (99%, Fisher Scientific, Waltham, Massachusetts, USA), diethylene glycol (99%, Fisher Scientific, Waltham, Massachusetts, USA) and N,N-dimethylhexadecylamine (≥90.0%, TCI, Hyderabad, Telangana, India) were used for gemini synthesis.
Table 1: Chemicals used
|
Surfactant |
Molecular formula |
Molecular weight (g/mol) |
cmc (mM) |
Literature cmc value (mM) |
|
16E216 [Ethane-1,2-diyl bis (N,N-dimethyl-N-hexadecylammonium acetoxy)dichloride] |
C42H86N2O4Cl2 |
753 |
0.0036 |
0.00126 [27] |
|
16E316 [2,2’-[(oxybis(ethane1,2-diyl)bis(oxy)bis(N-alkyl-N,N-dimethyl-2-hexadecyl oxoethanaminium)dichloride]
|
C44H90N2O5Cl2 |
797 |
0.0042 |
0.0062[34] |
|
CTAB [Cetyl trimethyl ammonium bromide] |
C19H42BrN |
363 |
0.90 |
0.776 [24] |
|
CTAC [Cetyl trimethyl ammonium chloride] |
C19H42ClN |
320 |
1.20 |
1.24 [27] |
|
PHE [Phenanthrene] |
C14H10 |
178 |
– |
– |
Synthesis of Gemini Surfactants (16E216 & 16E316)
Synthesis of desired gemini surfactants was carried out in two steps depicted in Figure 1 and described elsewhere 15. First, chloroacetyl chloride and diethylene glycol (for the synthesis of 16E316) or ethylene glycol (for the synthesis of 16E216) (2:1 molar ratio) were heated for 8 h at 50°C to produce oxybis(ethane-1,2-diyl)bis(2-chloroacetate). Then, in the second step (same for 16E316 & 16E216), N,N-dimethylhexadecylamine and oxybis(ethane-1,2-diyl)bis(2-chloroacetate) (spacer) (2.1:1) were refluxed for a period of 10 h in ethyl acetate. The solvent was then evaporated under vacuum, and cationic gemini surfactant (16E316 & 16E216) was produced. After recrystallization with ethyl acetate-ethanol combination, FT-IR and 1H NMR proved the purity of the products 16.
 |
Figure 1: Scheme for synthesis of Ester bonded cationic gemini surfactan |
Methods
Surface tension method
Stock solutions of CTAB, CTAC, 16E316, 16E216, and equimolar binary mixture of CTAB and CTAC with 16E216 and 16E316 were made by adding the known weight of surfactant in double distilled water (specific conductance 1 to 2 x10-6 Scm-1).
To determine the cmc values, surface tension measurements were carried out with a digital tensiometer (Kromtek, SEO DST 60 (Surface Tension, Subjong-dong, Ansleng City, Gyeongi-do, Korea) by platinum ring detachment method at 25°C using circulating water bath. The platinum ring went through two acetone washes and correction a burn in a red-hot ethanol flame to get rid of any remaining impurities. The tensiometer had been previously calibrated using double-distilled water. cmc values were obtained from the plots of surface tension versus the ln[surfactant]. The experiments were repeated three times and average values were taken and the surface tension values were accurate up to five decimal places.
UV-spectrophotometry method
Solubilization is defined as the formation of a thermodynamically stable isotropic solution of a solute which is insoluble or very slightly soluble in a given solvent by the addition of component(s) or by a surfactant 17. Micellar solubilization increased polycyclic aromatic hydrocarbons’ water solubility (PAHs). The PAHs’ solubility was evaluated in both pure conventional/gemini micellar solution and in their mixtures.
Surfactant solutions of varying concentrations were made in deionized water that had been subjected to two distillations at concentrations exceeding cmc. An extra amount of PAH was mixed with 10 mL of surfactant solution in a tightly sealed screw-capped vial followed by stirring for 24 hours in a magnetic stirrer to ensure maximum solubilization.
The surfactant and PAHs mixture were centrifuged at 12000 rpm for 15 minutes in order to remove excess undissolved solutes. The centrifuged mixture was analyzed at wavelength 470nm using a spectrophotometer (Make: Labman, Chennai, Tamil Nadu, India) to determine the absorbance of a surfactant system containing phenanthrene. To decrease the UV Absorbance error, both reference and measurement cells had the same surfactant concentration. The amount of solubilized phenanthrene was obtained using the Lambert-Beer law18
The molar extinction coefficient of Phenanthrene in ethanol at the specified wavelength was calculated using the Lambert Beer law and was found to be 22609.5 Lmol-1cm-1.
TEM Measurement
The morphologies of the aggregates of pure 16E316 and Phenanthrene solubilized in 16E316 were examined by the JSM-2100F TEM (JEOL, Japan) system at 200kV. The samples were placed on a grid and then dried at room temperature.
Results and discussions
Characterization of synthesized cationic gemini surfactant
FT-IR Spectroscopy
The synthesized gemini surfactants were preliminarily characterized by FT-IR spectroscopy with KBr as medium and the FT-IR spectra of 16E216 and 16E316 are presented in Figure 2. The purity and the structure of the synthesized gemini surfactants were in accordance with the literature [19]. Different peaks of FT-IR of 16E216 are as follows-(-CH3)-2922, (-CH2)-2848), (-C-O)-1194, 3425 is the absorption peak of hydroxyl in water. Peaks of 16E316 are as follows- (-CH3)-2918, (-CH2)-2843, (-C-O)-1135 and 3126 is the absorption peak of hydroxyl in water.
 |
Figure 2: FT-IR spectra of synthesized gemini surfactant (16E216 and 16E316) |
1H-NMR
Brucker Advance NMR spectrometer (Central Drug Research Institute, Lucknow) was used to record 1H-NMR spectra of synthesized gemini surfactant at 3000MHz in CDCl3 with 1H chemical shifts relative to internal standard tetramethylsilane. NMR spectra of 16E316 and 16E216 are presented in Figure 3 and results of the NMR are in agreement with the literature values 15.
 |
Figure 3: 1H-NMR spectra of gemini surfactant (a) 16E316 and (b) 16E216 in CDCl3 at 300 MHZ |
Surface Tension Results
Determination of cmc
The surface tension method was used to calculate the cmc and physiochemical parameters of pure and mixed surfactants systems. The surface tension versus ln [surfactant] plots for pure as well as mixed gemini-conventional surfactant systems are shown in Figure 4 and Figure 5.
 |
Figure 4: Plots of surface tension versus ln [surfactant] for pure Gemini & Conventional surfactants. (Error bars were calculated using triplicate data sets). |
 |
Figure 5: Plots of surface tension versus surfactants concentration for mixed systems at different concentrations (a) 16E216 + CTAC (b) 16E216 + CTAB (c) 16E316 + CTAC & (d) 16E316 + CTAB. |
The cmc for each system was obtained from the cut points as reported in Table 2. The order of decrease of cmc values for pure surfactant systems is: CTAC>CTAB>16E316>16E216.
Table 2: Various Physicochemical parameters for pure and mixed surfactant systems.
|
Surfactant System |
p1 |
cmce |
cmci |
X1m |
Xmideal |
βm |
f1m | f2m |
ΔGex |
|
16E216 + CTAB |
0.0 |
0.9000 |
0.9000 |
– |
– |
– |
– |
– |
|
|
0.2 |
0.0076 |
0.0179 |
0.5155 |
0.9840 |
-0.8816 |
0.8130 |
0.7911 |
-554.66 |
|
|
0.4 |
0.0057 |
0.0090 |
0.6669 |
0.9939 |
-0.5035 |
0.9456 |
0.7993 |
-281.74 |
|
|
0.6 |
0.0038 |
0.0060 |
0.6589 |
0.9973 |
-0.4339 |
0.9507 |
0.8283 |
-245.63 |
|
|
0.8 |
0.0031 |
0.0045 |
0.7113 |
0.9989 |
-0.3283 |
0.9730 |
0.8469 |
-169.86 |
|
|
1.0 |
0.0036 |
0.0036 |
– |
– |
– |
– |
– |
||
|
16E216 + CTAC |
0.0 |
1.2068 |
1.2068 |
– |
– |
– |
– |
– |
|
|
0.2 |
0.0091 |
0.0179 |
0.5728 |
0.9880 |
-0.7163 |
0.8774 |
0.7905 |
-441.53 |
|
|
0.4 |
0.0067 |
0.0090 |
0.7555 |
0.9954 |
-0.4079 |
0.9759 |
0.7922 |
-189.79 |
|
|
0.6 |
0.0047 |
0.0060 |
0.7895 |
0.9979 |
-0.3272 |
0.9856 |
0.8154 |
-136.98 |
|
|
0.8 |
0.0036 |
0.0045 |
0.7955 |
0.9992 |
-0.2704 |
0.9887 |
0.8427 |
-110.81 |
|
|
1.0 |
0.0036 |
0.0036 |
– |
– |
– |
– |
– |
||
|
16E316 + CTAB |
0.0 |
0.9000 |
0.9000 |
– |
– |
– |
– |
– |
|
|
0.2 |
0.0103 |
0.0209 |
0.5676 |
0.9814 |
-0.8077 |
0.8591 |
0.7709 |
-499.36 |
|
|
0.4 |
0.0055 |
0.0105 |
0.5750 |
0.9929 |
-0.6353 |
0.8916 |
0.8105 |
-390.05 |
|
|
0.6 |
0.0043 |
0.0070 |
0.6392 |
0.9968 |
-0.4664 |
0.9410 |
0.8265 |
-270.99 |
|
|
0.8 |
0.0031 |
0.0053 |
0.6259 |
0.9988 |
-0.4064 |
0.9447 |
0.8528 |
-239.70 |
|
|
1.0 |
0.0042 |
0.0042 |
– |
– |
– |
– |
– |
||
|
16E316 + CTAC |
0.0 |
1.2068 |
1.2068 |
– |
– |
– |
– |
– |
|
|
0.2 |
0.0098 |
0.0210 |
0.5445 |
0.9860 |
-0.7933 |
0.8485 |
0.7901 |
-495.58 |
|
|
0.4 |
0.0075 |
0.0105 |
0.7316 |
0.9947 |
-0.4374 |
0.9689 |
0.7912 |
-216.37 |
|
|
0.6 |
0.0053 |
0.0070 |
0.7690 |
0.9976 |
-0.3475 |
0.9816 |
0.8142 |
-155.47 |
|
|
0.8 |
0.0046 |
0.0053 |
0.8784 |
0.9991 |
-0.2564 |
0.9962 |
0.8205 |
-68.96 |
|
|
1.0 |
0.0042 |
0.0042 |
– |
– |
– |
– |
– |
The cmc values for the pure surfactants (gemini as well as conventional) determined from the plots of surface tension versus ln[surfactant] (Figure 6) show agreement with the literature values 23,24,25,26. CTAB and CTAC have much higher cmc values (approx. 250 times) than the corresponding cationic gemini surfactants. It is because of the fact that gemini surfactant contains two polar head groups and two hydrophobic chains27. Further, the presence of oxy-diester moity (E2O) via hydrogen bonding enhances the aggregation of surfactant monomers resulting in this noticeable decrease in cmc and hence brings them under the category of green surfactants as an extremely small quantity of gemini will be sufficient comparing to conventional surfactants when comparing the performance.
Table 2 reports the cmc values for all the systems. We can see that the cmc values drop as the concentration of gemini surfactant increases, as reported earlier also25 and a synergistic effect was observed concentrations of gemini surfactants. Due to the synergistic effect, especially at higher concentrations of gemini surfactant, the cmc values drop below the pure gemini surfactant cmc values.
The cmc values for the pure surfactants (gemini as well as conventional) determined from the plots of surface tension versus ln[surfactant] (Figure 6) show agreement with the literature values 23,24,25,26. CTAB and CTAC have much higher cmc values (approx. 250 times) than the corresponding cationic gemini surfactants, it is because of the fact that gemini surfactant contains two polar head groups and two hydrophobic chains[27], further, the presence of oxy-diester moity (E2O) via hydrogen bonding enhances the aggregation of surfactant monomers resulting in this noticeable decrease in cmc and hence brings them under the category of green surfactants as an extremely small quantity of gemini will be sufficient comparing to conventional surfactants when comparing the performance.
Table 2 reports the cmc values for all the systems, we can see that the cmc values drops as the concentration of gemini surfactant increases as reported earlier also25 and synergistic effect was observed at all concentration of gemini surfactants. Due to the synergistic effect, especially at higher concentrations of gemini surfactant, the cmc values drops below the pure gemini surfactant cmc values.
Interaction Studies of Mixed surfactant systems
The interactions between the surfactants in the mixed micelles are described by the following physicochemical parameters and equations. Using Clint’s equation (1) the optimal cmc values for ideal mixing of gemini and conventional surfactants are determined.

Where cmc1 is the ideal cmc, p1 is mole fraction of gemini surfactant and p2 is the mole fraction of conventional surfactants , cmc1 is cmc of gemini surfactant and cmc2 is cmc of conventional surfactant. Lower experimental cmc (cmcexp) than ideal cmc (cmci) values in all the conventional-gemini mixed systems indicates non-ideal solution behaviour. Many factors, like as structural variations, types of charge, types of hydrophobic chains etc effects the interactions between the surfactant interaction in mixed surfactant systems.
The experimental cmce of mixed surfactant systems are lower than those obtained for ideal mixed solution, which is calculated from Clint’s equation, explaining the non-ideal behavior of the mixed systems as well as synergism between the surfactants in micelles. The difference in cmc values of experimental and ideal solution confirms mixed micelle formation between gemini and conventional surfactants24. Also, the mixed system shows synergistic behavior between the surfactant micelles.
The gemini micellar mole fraction (X1m) in the mixed micelle was calculated using Rubingh’s equation [23].

cmce gives the experimentally determined values of cmc for pure and mixed systems. The micellar mole fraction is a term that describes the nature and degree of the interaction between gemini and conventional surfactant molecules in a mixed micelle. The ideal micellar mole fraction was obtained using motomura’s approximation25.

Interaction parameters (βm) can be evaluated using the following equations (4), (5) and (6) and are presented in Table 3:

The βm values for all mixed systems as reported in Table 2 are negative, which again indicates that the interaction between the components of the mixed surfactant is found to be synergistic in nature. When the value of βm is negative, a decrease in the free energy of micellization is observed, resulting in a more thermodynamically stable system28. , for any binary mixture shows the ideal behavior during mixed micelle formation which was not found in any of the binary systems. Within the mixed micelle, the interaction parameter and activity coefficient f1m of an individual surfactant are related through the following equations.

f1m & f2m values less than unity indicate the formation of mixed micelle. Also, f1m values are greater than f2m values, indicating more participation of gemini surfactant in comparison to conventional surfactant in the mixed micelle.
The mole fractions of gemini surfactants in the mixed micelles are different and are found to be greater than p1 with more tendency to form micelles due to lower cmc of gemini surfactant and synergistic interaction between the surfactants was observed as a result of lower experimental cmc(cmce) values than ideal cmc (cmci) and negative βm values for all mixed system. The ΔGex values calculated from activity coefficient are presented in table 2 and found to be negative, hence showing greater stability of the mixed micelles systems as compared to single surfactant micelles.
Determination of Interfacial Parameters of Mixed Surfactant Systems
When surfactant molecules are present at the air/water interface they alter the surface properties of water as a result of adsorption per unit surface area. At the interface, the maximum surface excess concentration (τmax)can be obtained using Gibbs adsorption equation28 and higher τmax values were obtained for CTAC than 16E216.

where γ represents the surface tension (mNm-1) of the solution, C represents the surfactant concentration of the solution, T (K) represents the absolute temperature of the solution and R = 8.314 Jmol-1K-1, n represents the constant whose values depends on the number of species that make up the surfactant and the value of n for conventional surfactants is 2, for the gemini surfactants value of n is 3 and for the mixture of conventional surfactant and gemini surfactant it is taken as 5 22. For pure surfactants, the order of τmax value is as follows CTAC>CTAB>16E216>16E316. When 16E216 is mixed with the conventional surfactant, the order of decrease is 16E216+CTAB > 16E216+CTAC, similarly when 16E316 is combined with CTAC and CTAB, the order of decrease is 16E316+CTAB > 16E316+CTAC due to the inter-molecular head group distance.
The minimum surface area of surfactant molecules Amin can be obtained from equation (8) 21 and summarized in Table 3.
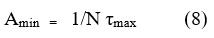
Amin is the area of exclusion per surfactant monomer having units Å2 molecule-1. N represents the Avagadro’s number in equation (8). For Pure surfactants, the Amin values of conventional surfactants are found to be less than gemini surfactants and have the order 16E316>16E216>CTAB>CTAC. Higher Amin values of mixed surfactants result from the bulky hydrophobic part which finds difficulty in adjusting at the air/water interface. In gemini + conventional mixed micelles, both are cationic in nature, having equal flexible hydrophobic chains, hence their mixed interface is densely populated in them being easily assembled in a small space. At cmc, the surface pressure value is calculated by equation (9).

Where γ0 is the surface tension of pure water and γcmc is the surface tension at critical micelle concentration. The surface pressure parameter measures the greatest reduction in water’s surface tension induced by surfactant dissolution23.
PC20 values for adsorption efficiency are determined using equation (10)

Where C is the surfactant concentration, and C20 is the surfactant concentration needed to drop the surface tension value by 20 units. As a result, it is a measure of surfactant molecule adsorption efficiency at the air/solution interface. The PC20 values of gemini surfactant is higher than conventional surfactants in their respective groups, which means gemini lowers the surface tension by 20 units at low concentration than conventional surfactants. In the mixed surfactant system, as the concentration of gemini surfactant increases, the value of PC20 increases, showing the effect of gemini surfactant in the mixed surfactant systems. The order of the surfactant molecule adsorption efficiency is 16 E2 16>16 E3 16>CTAB>CTAC.
Table 3: Values of τmax, Amin, πcmc and PC20 at different concentrations of different surfactant systems.
|
Surfactant System |
P1 |
τmax *10-7 |
Amin (A°)2 |
πcmc |
PC20 |
|
16E216 + CTAB |
0.0 |
4.06 |
36.6 |
40.86 |
3.50 |
|
0.2 |
2.38 |
103 |
24.62 |
5.61 |
|
|
0.4 |
3.03 |
83.0 |
24.31 |
5.67 |
|
|
0.6 |
4.22 |
76.4 |
24.25 |
6.21 |
|
|
0.8 |
2.47 |
167 |
24.71 |
6.31 |
|
|
1.0 |
3.77 |
50.5 |
34.42 |
7.82 |
|
|
16E216 + CTAC
|
0.0 |
4.37 |
21.4 |
36.70 |
3.22 |
|
0.2 |
2.15 |
104 |
23.97 |
5.52 |
|
|
0.4 |
2.33 |
98.6 |
23.63 |
5.64 |
|
|
0.6 |
3.89 |
72.9 |
23.77 |
6.01 |
|
|
0.8 |
2.24 |
162 |
23.50 |
6.21 |
|
|
1.0 |
3.77 |
50.5 |
34.42 |
7.82 |
|
|
16E316 + CTAB
|
0.0 |
4.06 |
36.6 |
40.86 |
3.50 |
|
0.2 |
1.81 |
124 |
22.70 |
4.93 |
|
|
0.4 |
2.92 |
92.7 |
22.63 |
5.71 |
|
|
0.6 |
2.25 |
126 |
23.25 |
6.03 |
|
|
0.8 |
2.41 |
166 |
23.00 |
6.12 |
|
|
1.0 |
3.12 |
133 |
33.14 |
7.41 |
|
|
16E316 + CTAC
|
0.0 |
4.37 |
21.4 |
36.70 |
3.22 |
|
0.2 |
1.93 |
101 |
23.94 |
5.54 |
|
|
0.4 |
2.72 |
112 |
22.59 |
5.84 |
|
|
0.6 |
2.18 |
125 |
23.42 |
6.01 |
|
|
0.8 |
2.04 |
151 |
22.80 |
6.14 |
|
|
1.0 |
3.12 |
133 |
33.14 |
7.41 |
Solubilization of Phenanthrene in Pure/ Mixed Surfactants
Solubility was traditionally measured in terms of solubilization capacity, called the MSR (molar solubilization ratio). MSR is the measure of the surfactant effectiveness to solubilize the contaminant 28. MSR value can be determined by empirical equation (11) as well from the slope of solubilization curve. Higher values from the slope gives higher MSR values. Therefore, higher will be the solubilization of PAHs. Another metric term the micelle-water partition coefficient (Km) can be used to assess a surfactant’s solubilization capacity given by equation (12) 29.

Where Vm = 0.0189 L/mole i.e., volume of water at 30°C and Km can be obtained by equation (15)

Where,
Scmc the apparent solubility of PAH at cmc
St the total PAH solubility in the single/mixed surfactant solution at a given total surfactant concentration
Xm the mole fraction of PAH in the micellar phase and
Xa the mole fraction in the aqueous phase.
The slope of the plot between the concentrations of solubilizate versus the concentration of the surfactant can be used to calculate MSR.
The solubility of phenanthrene in pure water is very low (0.0089×10-3 mol/L). Enhancement in the water solubility of phenanthrene was investigated using mixtures of pure gemini and pure conventional surfactants and their binary mixtures.To calculate MSR values for binary systems; the total concentration of the equimolar surfactant mixture was used. In the case of pure gemini systems, the aqueous solubility of the phenanthrene is very high as compared to pure conventional surfactant systems and increased in a linear manner with the surfactant concentration above cmc as depicted in Figure 6.
While in case of pure conventional surfactant systems, the aqueous solubility of phenanthrene increases almost linearly up to concentration of 10 M and then increases gradually upto 20 M. Figure 7 shows the variation of Phenanthrene solubility as a function of the total concentration of the binary equimolar mixtures of gemini and conventional mixed surfactants. MSR values were used to compare the solubility (Table 4 and 5). Mixed systems have much higher capacity to solubilize phenanthrene as compared to pure surfactants as depicted by MSR values. Further, in mixed systems, solubility increases linearly as the concentration of gemini surfactant increases due to the more and greater number of micelles of gemini surfactants as a result of very low cmc values of gemini surfactants.
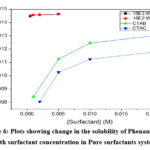 |
Figure 6: Plots showing change in the solubility of Phenanthrene with surfactant concentration in Pure surfactants system. |
Molecular structure of the Phenanthrene along with spaces within the head groups of the surfactants in the micelles determine the extent of solubilization30,31. The solubility increases as the micellar core size increases, further swollen micelles also affect solubilization 32.
In gemini and conventional mixed surfactant systems, the order of solubility for phenanthrene is
16E216+CTAC > 16E216+CTAB > 16E316+CTAC > 16E316+CTAB
In cationic gemini- cationic conventional systems, electrostatic repulsive force within similar charged head groups of surfactants leads to the development of loosely packed mixed micelles, whereas the greater MSR and Km values in binary systems relative to single surfactant systems may be associated with creation of large mixed micelles. For 16E216 +CTAC/CTAB the formation of more compact micelles as compared to 16E316+CTAC/CTAB system was expected. Hence, solubilization capacity was assumed to decrease, owing to very small cmc, extra mixed micelles are formed, providing more and more space for the Phenanthrene to get into the spaces and hence solubility increases33.
 |
Figure 7: Plots showing change in the solubility of Phenanthrene in different concentrations of gemini surfactant in their equimolar binary mixtures |
In order to find the best result of gemini-conventional mixed surfactant system, the deviation ratio (R) was determined31.

MSRideal can be determined by following equation

Where X1, X2, MSR1, MSR2 are mole fractions and molar solubilization ratio.
From Table 5, we can see that the deviation ratio R depends on the molecular microstructures of surfactants in mixed systems and also on the interaction between the solute and the mixed micelles, besides the structure of surfactants and solute. R is greater than 1 for all the surfactant systems. It is observed that Km value of 16E216 + CTAC is higher than all other surfactant systems. It clearly reveals that binary systems have higher MSR values compared to single surfactant systems, implying that mixed surfactant systems show enhancement in PAH solubility.
The mixed surfactant systems having the chloride group in conventional surfactants show better solubilizing strength. 16E216 + CTAC surfactant has the maximum solubilizing strength among all mixed surfactant systems33.
Thermodynamics of solubilization
The Gibb’s free energy of solubilization is represented by:
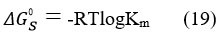
The universal gas constant is R, while the absolute temperature is T. The calculated values of ΔG0s are mentioned in table 4 and table 5 and are found to be negative in all cases, which shows the process of solubilization is spontaneous in single as well as in mixed surfactant systems.
Table 4: Molar Solubilization ratio (MSR), ln (Km) values of Phenanthrene (PAH) in Single Surfactants.
|
Surfactant system |
MSR |
ln (Km) |
ΔG0s(kJmol-1) |
|
16E216 |
0.0002 |
7.08 |
-17.542 |
|
16E316 |
0.0002 |
7.08 |
-17.542 |
|
CTAB |
0.0021 |
9.43 |
-23.363 |
|
CTAC |
0.0018 |
9.27 |
-22.982 |
Table 5: Molar Solubilization ratio (MSR), ln (Km), and R values of Phenanthrene in mixed surfactants system.
|
Surfactant system |
MSRexp |
ln (Km) |
R |
MSRideal |
ΔG0s (kJmol-1) |
|
16 E2 16 +CTAB |
0.0041 |
10.14 |
3.5 |
0.0011 |
-25.12 |
|
16 E2 16 +CTAC |
0.0081 |
10.82 |
8.1 |
0.0010 |
-26.80 |
|
16 E3 16 +CTAC |
0.0069 |
10.66 |
6.9 |
0.0010 |
-26.41 |
|
16 E3 16 +CTAB |
0.0057 |
10.47 |
4.9 |
0.0011 |
-25.94 |
Morphological Study by TEM
To obtain the morphologies present in pure and mixed systems, TEM micrographs were acquired. Figure 8 shows the TEM images of pure 16E316, mixed 16E316+CTAC and solubilized Phenanthrene in 16E316+CTAC mixed system (equimolar) at the maximum solubility of Phenanthrene. From Figure 8 we can clearly see that pure surfactant system shows spherical and rod-shaped micelles (Figure 8(a)).Also, micelles size is small in comparison to micelle size in mixed system (Figure 8(b)) for the same magnification. Further, for solubilized PHE in 16E316+CTAC mixed system (Figure 8(c)), it can be observed that some of the micelles are larger in size for the same magnification, hence showing phenanthrene molecules are entrapped in the micelles resulting in expansion of micelles due to solute addition (swollen micelles).
 |
Figure 8: TEM image at 25000-X zoom. (a) pure 16E316, (b) 16E316 + CTAC Mixed system (c) 16E316 + CTAC Mixed system (Equimolar) with solubilized phenanthrene. |
Conclusions
The IR and NMR spectra for the synthesized ester bond cationic gemini surfactants (16E216 and 16E316) are in accordance with the literature, hence confirming their structures. A systematic and comprehensive study has been carried out to get insight into the interfacial properties and solubilization capabilities of these gemini surfactants along with their mixtures with conventional surfactants. The results show that due to unusual properties the cmc of gemini surfactants are very low (250 times approx.) as compared to cationic conventional surfactants. For mixed systems, as the concentration of gemini surfactants increases, cmc values decrease, hence a synergistic effect is observed in mixed surfactant systems. Further, in mixed surfactant systems, cmcexp are lower than cmcideal, again showing synergism, and mixed micelle formation exhibits negative deviation from ideal behavior, again indicating a strong synergistic interaction. Interestingly, the mixed surfactant systems show much better solubilization power for phenanthrene as compared to single/pure surfactant systems due to the packing pattern or surfactant micellar microstructure of mixed micelles. TEM images also confirm the results. The findings can provide valuable information for analyzing, predicting, and enhancing the solubilization of phenanthrene in a mixture of cationic-cationic mixed surfactant systems which can be used as a successful remediation technology for PAH removal from soil.
Acknowledgment
The author would like to thank, (Department of Petroleum Studies, Aligarh Muslim University, Aligarh) for their guidance and support to complete this article.
Conflict of Interest
The authors declare that there is no conflict of interest.
Funding Sources
This research received no external funding.
References
- Porter, M. R. Handbook of Surfactants, First Edition, 1991.
CrossRef - Schmitt, T. M. Analysis of Surfactants, Second Edition, 2001.
- Tadros, T. F. Applied Surfactants: Principles and Applications, 2005.
CrossRef - Ying, G. G.Fate, behavior and effects of surfactants and their degradation products in the environment, Environmental International. 2006,32(3) 417-431.
CrossRef - Zhu, L.; Feng, S, Synergistic solubilization of polycyclic aromatic hydrocarbon by mixed anionic-nonionic surfactants, Chemosphere. 2003,53(5), 459-467.
CrossRef - Nakama, Y. Cosmetic Science & Technology: Theoretical principles and applications, 2017.
- Lee, K. S.; Lee, J. H. Hybrid Enhanced Oil Recovery Using Smart Water-flooding, 2019.
- Zhentian, S.; Jiajun C.; Jianfei L.; Ning, W.; Zheng, S. Anionic-nonionic mixed surfactant-enhanced remediation of PAH-contaminated soil. Environ. Sci. and Poll. Res. Int. 2015, 22(16).
CrossRef - Makkar, R. S.; Rockne, K.J. Comparison of synthetic surfactants and biosurfactants in enhancing biodegradation of polycyclic aromatic hydrocarbon. Environ. Toxicol. Chem. 22(10), 2003, 2280-2292.
- Bamforth, S. M.; Singleton, I. Bio-remediation of polycyclic aromatic hydrocarbons: current knowledge and future directions. J. Chem. Tech. Biotechnol. 2005, 80, 723-736.
CrossRef - Badmus, S. O.; Amusa, H. K.; Oyehan, T. A.; Saleh, T. A. Environmental risks and toxicity of surfactants: overview of analysis, assessment and remediation techniques. Env. Sci. and Poll. Res. 2021, 28, 62085-62104.
CrossRef - Paria, S. Surfactant-enhanced remediation of organic contaminated soil and water. Advances in Colloid and Interface Sci. 2008, 138, 24-58.
CrossRef - Rosen, M.J.; Tracy, D.J. Gemini Surfactants. J. Surfactants Deterg. 1998, 1, 547-554.
CrossRef - Zhou, W.; Zhu, L. Efficiency of surfactant-enhanced desorption for contaminated soils depending on the component characterstics of soil-surfactant-PAHs system. Environment Pollution. 2007, 147, 66-73.
CrossRef - Zhinong, G.; Shuxin, T.; Qi, Z.; Yu, Z.; Bo, L.; Yushu, G.; Li, H.; Xiaoyan, T. Synthesis and surface activity of biquaternary ammonium salt gemini surfactant with ester bond. Wuhan Univ J. of Nat. Sci. 2008, 13, 227-31.
CrossRef - Bhadani, A.; Endo, T.; Sakai, K.; Abe, M. Physicochemical evaluation of micellar solution and lyotropic phases formed by self-assembled aggregates of morpholinium geminis, Colloid Polym. Sci. 2014, 2(9), 5324-5334.
CrossRef - Tadros. T. F. Encyl. Of Phy. Sci. and Tech., Third Edition, 2003.
- Swinehart, D.F. The beer-lambart law, Jornal of Chemical Education. 1962, 39(7), 333.
CrossRef - Abadallah, M.; Hegazy, M.; Alfakeer, M.; Ahmed, H. Adsorption and inhibition performance of the novel cationic gemini surfactant as a safe corrosion inhibitor carbon steel in hydrochloric acid. Green Chemistry Letter and Reviews .2008, 11(4), 457-468.
CrossRef - Zhinong, G.; Shuxin, T.; Qi, Z.; Yu, Z.; Bo, L.; Yushu, G. Li, H ; Xiaoyan, T.Synthesis and surface activity of biquarternary ammonium salt gemini surfactant with ester bond. Wuhan University J. Natural Sci. 2008, Vol.13 No.2, 227-231.
CrossRef - Zhou, Q.; Rosen, M. J. Molecular interaction of surfactants in mixed monolayers at the air/aqueous solution interface and in mixed micelles in aqueous media: the regular solution approach. Langmuir. 2003, 19, 4555-4562.
CrossRef - Berriche, L.; Badache, L.; Benhariz, S. H.; Gharbi, A.; Talhi, W. Mixed micellization and surface properties of non-ionic/cationic surfactants. J. Dispersion Sci. Technol. 2018.
CrossRef - Yu, T. Z.; Ding, W.; Luo, Q.S.; Luan, X.H. Enthalpy-entropy compensation of micellization of alkyl aryl sulfonates in aqueous solutions. Acta. Phys. Chem. Sin. 2010, 3, 638-642.
CrossRef - Chattoraj, D.K.; Birdi, S. Adsorption and the gibbs surface excess. Plenum Press, New York, 1984, 21-38.
CrossRef - Sugihara, G; Miyazono, A.; Nagadome, S; Oida,T; Hayashi, Y.; Ko, J.S. Adsorption and Micelle Formation of Mixed Surfactant Systems in Water. II: A Combination of Cationic Gemini-type Surfactant with MEGA-10. J. Oleo Sci. 2003, 55(5), 449-461.
CrossRef - Rubingh, D.N.; Mittal, K.L.; Solution Chemistry of surfactants. Plenum Press New York 1, 1979, 337.
CrossRef - Motomura,K.; Yamanaka, M.; Aratono, M.Thermodynamic consideration of the mixed micelles of surfactants. Colloid Polym. Sci.1984, 51(11), 948-955.
CrossRef - Fatma, N.; Ansari, W. H.; Panda, M.; Kabir-ud-Din. Solubilization of polycyclic aromatic hydrocarbons by novel biodegradable cationic gemini surfactant ethane-1,2-diyl bis(N,N-dimethyl-N-hexadecylammoniumacetoxy) dichloride and its binary mixture with conventional surfactants. Z. Phys. Chem.2013, 9, 1478-1487.
CrossRef - Ghosh, S.; Chakraborty, T. Mixed micelle formation among anionic gemini surfactant (212) and its monomer (SDMA) with conventional surfactants (C12E5 and C12E8) in brine solution. J. Phys. Chem2007, 28, 8080-8088.
CrossRef - Panda, M.; Kabir-ud-Din. Study of surface and solution properties of gemini-conventional surfactant mixtures and their effects on solubilization of polycyclic aromatic hydrocarbons. J. Mol. Liq. 2011, 163, 93-98.
CrossRef - Panda, M.; Shafi, M.; Kabir-ud-Din. Solubility enhancement of polycyclic aromatic hydrocarbons (PAHs) using synergistically interacting gemini-conventional surfactant systems. Z. Phys. Chem.,2011,225(4), 427-439.
CrossRef - Koneva, A. S.; Ritter, E.; Anufrikov, Y.A.; Lezov, A. A.; Klestova, A. A.; Smirnova, N. A.; Sofonova, E. A.; Smirnova, I. Mixed aqueous solution of non-ionic surfactants Brij 35/Triton-X 100: Micellar properties, solutes partitioning from micellar liquid chromatography and modelling with COSMOmic. Colloids and surface A: Physicochemical and engineering aspects. 2018 Vol. 538, 45-55.
CrossRef - Cang, H.; Brace, D. D.; Fayer, M.D. Dynamic partitioning of an aromatic probe between the head group and core regions of the cationic micelles, The Journal of Physical Chemistry B. 2001, 105, 10007-10010.
CrossRef - Akram, M.; Anwar, S.; Ansari, F.; Bhatt, I. A.; Kabir-ud-Din. Bio-Physicochemical analysis of ethylene oxide linked diester-functionalized green cationic gemini surfactants, RSC Adv. 2016, 6, 21697-21705.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.










