Preparation of Gbsa (Guargum 1-Butane Sulphonic Acid) Resin and Study of their Physicochemical Characterization and Use for Removal of Toxic Metal from Effluents
Sunita Gaur*, Renu Joshi and Aresh Vikram Singh
Department of Chemistry, Jai Narain Vyas University, Jodhpur, Rajasthan (INDIA).
Corresponding Author E-mail: areshvikram04@rediffmail.com
DOI : http://dx.doi.org/10.13005/ojc/390515
Article Received on : 10 Aug 2023
Article Accepted on : 15 Sep 2023
Article Published : 25 Sep 2023
Reviewed by: Dr. anuradha Jape
Second Review by: Dr. Devendra Gupta
Final Approval by: Dr. Naeem Uddin
The adsorption behavior of a functional group 1-butane sulphonic acid, is used for synthesis of the natural resin based on guar gum is GBSA resin. Many hazardous metal ions have been studied utilizing batch method and column separation experiment. The Gaur gum-based resin (GBSA) was evaluated based on its bulk density, FTIR spectra, and ion exchange capability. The hazardous metal ions have been removed from industrial effluents using GBSA resin. The resin utilized to remove iron and cobalt metal from contaminated water using GBSA resin. Iron metal ions show maximum adsorption on GBSA resin at pH 5 while cobalt metal ion show maximum adsorption at pH 6.This paper described about removal of iron and cobalt metal ion on GBSA resin.
KEYWORDS:Adsorption Behavior; Hazardous Metal Ions; Industrial Waste; Natural Resin
Download this article as:| Copy the following to cite this article: Gaur S, Joshi R, Singh A. V. Preparation of Gbsa (Guargum 1-Butane Sulphonic Acid) Resin and Study of their Physicochemical Characterization and Use for Removal of Toxic Metal from Effluents. Orient J Chem 2023;39(5). |
| Copy the following to cite this URL: Gaur S, Joshi R, Singh A. V. Preparation of Gbsa (Guargum 1-Butane Sulphonic Acid) Resin and Study of their Physicochemical Characterization and Use for Removal of Toxic Metal from Effluents. Orient J Chem 2023;39(5). Available from: https://bit.ly/46lS4yL |
Introduction
Heavy metal ions, including ferrous, copper, zinc, lead, and others, have been found in the effluent from the mining, tannery, electroplating, battery, and steel sectors. When they exceed tolerance thresholds, heavy metal ions have detrimental effects on both human and environmental life.
Heavy metals, similarly to posing huge dangers to people, additionally directly harm soil ecosystems because of their toxicity. Plants developing in polluted soils may additionally enjoy slowed increase, photosynthetic inhibition, decreased chlorophyll manufacturing, and reduced enzyme hobby. Heavy metals in excessive concentrations within the soil impair plant vitamins absorption, metabolic sports, chlorosys, and other procedures. Additionally, the great of receiving water our bodies, consisting of rivers and seas; will be impacted through heavy metal-polluted commercial effluent. This impact varies and is largely based on the chemical composition of the effluent launched via industries 1.
Cobalt
Cobalt is totally risky steel whose extensive use has wreaked havoc on the surroundings and harmed human fitness in lots of areas of the globe. In a dry environment, cobalt is a vivid silvery metal this is rather bluish. When it comes into contact with air, it starts off evolved to tarnish, generating a complex mixture of compounds that varies relying on the scenario.
Iron
Iron is the second one maximum ample metallic on the earth’s crust 2 Iron occupies the 26th elemental position inside the periodic table. Iron is a most crucial detail for boom and survival of virtually all dwelling organisms. It is one of the crucial components of organisms like algae and of enzymes which includes cytochromes and catalase, in addition to of oxygen transporting proteins, together with hemoglobin and myoglobine.
Ion exchange is a chemical exchange wherein loose cell ions from a solid, the ion exchange, are exchanged for different ions in answer with equal prices. The exchangers have to have an open network shape, both organic and inorganic, that transports ions and allows them to pass through.
An ion exchange is a water-insoluble substance that can alternate a number of its ions for similarly charged ions within the medium with which it comes into touch; this is a huge definition. Many exchanges are referred to as “materials” in place of “compounds,” some of which can be herbal merchandise without a well-described makeup. Ion exchange can occur in aqueous and non-aqueous solutions, molten salts, or maybe in interaction with vapors, because the name “medium” implies. Because a few chemical solvents which are immiscible with water can remove ions from aqueous section via an ion alternate mechanism 3, the term isn’t always constrained to solids.
Modified Natural Ion Exchangers
Some evidently taking place natural ion exchangers were modified to improve trade potential and selectivity; for instance, cellulose-primarily based cation exchangers may additionally have phosphate, carbonic, or other acidic functional groups introduced to improve change ability and selectivity.
A chemical and warmth treatment can change the sorption properties of natural materials; for instance, by treating clinoptilolite with a suitable solvent of acids or sure salts, a greater selective sort of sorbent for a particular radionuclide 4 may be created.
Therefore, technology for the acceptable and practicable removal of heavy metal ions from industrial wastewater must be developed. Diverse technologies, such as precipitation 5, solvent extraction 6,7, chemical and electrochemical technology 8, and complex oxidation procedures 9,10 can be used to remove heavy metal ions from wastewater. These techniques cost too much, don’t work, or pollute the environment 11.
Di-hydroxybenzene was used to investigate the adsorption of metal and chromium VI in aqueous solution using the modified polymer PAA 12-14. In recent years, one of the most widely used techniques for removing heavy metals from industrial effluent has gained a lot of attention: the ion exchange method 15,16.
This study set out to synthesized and characterized a unique GBSA resin that selectively filters out heavy metal ions from industrial wastewater 17.Because this type of natural modified resin have capacity to consume sodium metal ion on surface. During the ion exchange process heavy metal ions can be exchanged by these sodium metal ions. Metal ions transferred from solution to surface of natural polymer and sodium ion transferred surface to solution. GBSA resin had good ion exchange capacity thus GBSA behave as promising natural resin.
Instruments
pH meter
A Digital pH meter model 5651 from Electronics Corporation of India (ECI) was used to calculate the pH readings.
Varian model 175 (AAS)
The procedure of quantitatively determining the concentration of metal ions in traces by spectrophotometer.
Methodology
Preparation of epoxy propyl ether of 1-butane sulphonic acid
16.01 gm of 1-butane sulphonic acid (0.1 mole) was dissolved in minimum amount of methanol in a round bottom flask. 9.25ml (0.1 mole) of epichlorohydrine was added in the above solution and reaction mixture was stirred for 4 hrs on magnetic stirrer. 4 gm of sodium hydroxide (0.1mole) was added in reaction mixture and reaction mixture was further stirred for 5hrs on magnetic stirrer and left it overnight.
Preparation of guar gum 1-butane sulphonic acid resin
In a round bottom flask, 81 g of guar gum (0.5 mole) was combined with dioxane and 4 gm (0.1mole) NaOH solution added and stirred on magnetic stirred at 65OC. After this, epoxy propane ether of 1-butane sulphonic acid was added in it and mixture was stirred continuously for 4.5hrs at 65OC. After being washed with methanol and HCl to remove any remaining inorganic impurities and to neutralize any excess alkali. Guar gum 1-butane sulphonic Acid resin yield was found 92.6 gram.
 |
Figure 1: Depicts the reaction scheme for the preparation of GBSA resin. |
For synthesis of GBSA resin can be explain by this above reaction mechanism. In which functional group 1- butane sulphonic acid was reacted with epichlihydrine and formed epoxy propyl ether of 1-butane sulphonic acid, and it again reacted with NaOH and formed sodium salt of epoxy propyl ether of 1-butane sulphonic acid.
At 65OC powder of guar gum mixed with epoxy propyl ether of 1- butane sulphonic acid and final product formed was guar gum 1- butane sulphonic acid (GBSA) resin.
Stock Solution of Iron Metal Ion
The stock solution for the Fe (II) metal ion was ferrous ammonium sulphate (NH4)2Fe (SO4)2.6H2O. To generate a 1000 ppm Fe (II) solution, 7.02 gm ferrous ammonium sulphate was combined in 2 ml con. H2SO4 and the capacity were increased to 1000 ml.
Distribution Coeficient (Kd)
The molar distribution coefficient (Kd) of metals with strong adsorption on chelating resins, such as Co (II), Ni (II), and Cr (II), were measured using the batch method. Weighed amounts of many resins were used.
The concentration of metal ions in the filtrates were measured using a calibration curve. Finally, using the formula, the distribution coefficient was computed.
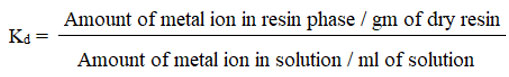
Iron metal ion chelating on GBSA resin
0.2 M sodium acetate and 0.2 M acetic acid were added to many glass stopper conical flasks to provide buffers with the requisite pH range of 3 to 8. A pH of 8.0 was attained by adding enough 0.2 M NH4OH and 0.1 M NH4Cl to other glass stopper conical flasks. Each container has 1 ml of 1000 ppm Fe (II) solution and 0.070 g of dried GBSA resin.
The contents were filtered after 1 hour of magnetic stirrer equilibration. The iron concentration of the filtrates was determined by AAS. Table 1 summarized the findings.
Table 1: Kd Value Of Iron Metal Ion On Gbsa Resin
|
pH |
Absorbance |
concentration (ppm) |
metal ion in filtrate(mg) |
metal ion in resin(mg) |
Kd |
% absorbance of iron metal ion on resin |
Metal exchange capacity (mg/g) |
|
3 |
0.987 |
8.88 |
0.3222 |
0.6778 |
2897.89 |
67.78 |
9.87 |
|
4 |
0.876 |
7.67 |
0.3111 |
0.6890 |
2987.56 |
68.90 |
10.56 |
|
5 |
0.786 |
5.56 |
0.2999 |
0.7001 |
3245.67 |
70.01 |
11.12 |
|
6 |
0.567 |
6.54 |
0.3010 |
0.6990 |
2567.89 |
69.90 |
10.78 |
|
7 |
0.332 |
7.89 |
0.3124 |
0.6876 |
2456.98 |
70.00 |
7.67 |
|
8 |
0.331 |
7.99 |
0.3622 |
0.6378 |
2103.90 |
63.78 |
6.43 |
*Maximum adsorption of iron metal ion was found on GBSA resin at pH 5.
Chelation of cobalt metal ion on GBSA resin
To generate buffers with the required pH range of 3 to 8, sufficient amounts of 0.2 M sodium acetate and 0.2 M acetic acid were introduced to numerous glass stopper conical flasks. Other glass stopper conical flasks received a suitable amount of 0.2 M NH4OH and 0.1 M NH4Cl to achieve a pH of 8.0.
0.070g dry GBSA resin and 1 ml 1000 ppm Co (II) solution were added to each flask. After 1 hour of magnetic stirrer equilibration, the contents were filtered. The filtrates were tested for cobalt using AAS. The findings are summarized in Table 2
Table 2: Kd Value Of Cobalt Metal Ion On Gbsa Resin
|
pH |
absorbance |
concentration (ppm) |
metal ion in filtrate(mg) |
metal ion in resin(mg) |
Kd |
% absorbance of cobalt metal ion on resin |
Metal exchange capacity (mg/g) |
|
3 |
1.112 |
15.67 |
0.5010 |
0.4990 |
967.78 |
49.90 |
7.65 |
|
4 |
1.879 |
14.65 |
0.4477 |
0.5523 |
1098.78 |
55.23 |
6.45 |
|
5 |
0.675 |
11.34 |
0.4322 |
0.5678 |
1324.67 |
56.78 |
5.55 |
|
6 |
0.0112 |
8.67 |
0.4223 |
0.5777 |
1987.67 |
57.77 |
5.76 |
|
7 |
0.665 |
9.56 |
0.4655 |
0.5345 |
1546.78 |
53.45 |
4.34 |
|
8 |
0.435 |
9.88 |
0.4455 |
0.5545 |
1785.89 |
55.45 |
3.23 |
Maximum adsorption of cobalt metal ion was found on GBSA resin at pH 6.
Table 3: Removal of Iron and Cobalt Metal Ion
|
pH |
Fe(II)% removal |
Co(II) % removal |
|
3 |
49.9 |
67.78 |
|
4 |
55.23 |
68.9 |
|
5 |
56.78 |
70.01 |
|
6 |
57.77 |
69.9 |
|
7 |
53.45 |
70.01 |
|
8 |
55.45 |
63.78 |
 |
Figure 2: % Removal of iron and cobalt metal ion |
The maximum value of Fe (II) metal adsorption on GBSA resin was found 70.01% and at pH 6 for Co (II) metal ion was found 57.77%.
Discusions
Ion exchange capacity of GBSA resin
The ion exchange capacity of a resin is determined by the total amount of charge or active ion groups per unit mass of material. The exchanger’s ion exchange capacity grows as the number of active ions grows. Milli moles per gram of exchanger 18 are the unit used to express the resin’s total ion exchange capacity (IEC).
In the lab, the amount of milli moles of sodium ion consumed by 1 gram of dry polymer 19 in hydrogen form can be used to calculate the ion exchange capacity of a cation exchanger. The anion exchange capability of the anion exchanger is determined by the amount of Cl– ion overtaken by 1 gm of powdered polymer in the OH– form.
Moderate cross-linking may not be able to digest large ions.
Process
A back titration procedure was used to determine the resin capacity. 1.0g of resin was placed in an Erlenmeyer flask, followed by 200 ml of standardized NaOH (0.05N) containing 5ml of 5% NaCl solution and allowed to stand overnight. A 25ml volume of supernatant liquid was properly titrated with 0.05N HCl standard stock solution using indicator (phenolphthalein). The equation was used to calculate the newly synthesized resin’s ion exchange ability.
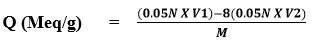
V1 Volume of 0.05N NaOH
V2 Volume of 0.05N HCl
M Wight of dry resin
Q (Meq/g) = (0.05x 200) – 8(0.05x 14.23) / 0.85
= (10-8) (0.05x 14.23) /0.85
= 1.67
FTIR Spectra of Gbsa Resin
FTIR spectra of GBSA show peaks at 3478 cm-1 and 3297 cm-1 due to OH ground and taking part in hydrogen bonding. Peak at 2874 due to sulphonic group and stretching due to S=O group in suphonic acid.1365 cm-1,1217 cm-1,1177 cm-1 due to stretching.
 |
Figure 3: FTIR of GBSA resin. |
SEM Characterization
SEM micrographs of surface of synthesized natural resin were presented in following figs no. 7, show that resins present the cavities to the level of their surface area. Surface show heterogeneity and varied structures. The adsorbent is porous in nature.
 |
Figure 4: SEM of GBSA resin. |
Factor effecting of kd on GBSA Resin
Effect of pH
pH is a critical metric for determining a synthetic resin’s exchange capability. The ion exchange capacity of a resin increases as the pH rises (pH 3-8), but when the pH rises above 8, the basic environment for metal ion absorption on the resin steadily decreases. At pH 5 the maximum value of Fe (II) metal adsorption on GBSA resin is 70.01% and at pH 6 for Co (II) metal ion is 57.77%.
Effect of contact time
The amount of metal ions that were adsorbed on GBSA resin increased when the contact time was extended from 40 to 160 minutes. While the temperature was held at 250°C, other variables including solution pH and agitation speed were kept at their ideal ranges. The findings indicate that there are two stages of heavy metal adsorption: one with a very high rate of adsorption percentage, with 56 to 75 percent of heavy metals adsorbed on GBSA resin in a contact time of 140 minutes; and another with a very low rate of adsorption percentage, with 56 to 75 percent of heavy metals adsorbed on GBSA resin in a contact time of 140 minutes. The next stage has a significantly decreased adsorption rate. The results of the treatment period demonstrate that as contact time increased until equilibrium was attained, the proportion of metal ions adsorption increased.
Effect of agitation speed
By increasing the agitation speed from 0 (no shaking) to 200 rpm while maintaining the other parameters constant, the impact of agitation speed on metal ion adsorption was examined. As the agitation speed increased, metal ion adsorption on ion exchange resin generally increased. The adsorption of Fe (II) and Co (II) metal ions on GBSA resin increased as the agitation speed was raised from 0 to 120 rpm. These results can be explained by the fact that agitation speed increases encourage metal ion migration to the surface of the adsorbents. This shows that the surface binding sites can all be made accessible for metal ion absorption at a shaking rate of 100–120 rpm.
Temperature Effects on GBSA resin
The amount of metal ions that adsorb on GBSA resin depends critically on temperature. As the treatment temperature increases from 250 to 750 degrees Celsius, the amount of metal ions as Fe (II) and Co (II) that is adsorbed decreases.
Results
Heavy metal ions are particularly selectively removed from aqueous solutions and industrial effluents by resin GBSA. According to the findings, the distribution coefficient (Kd) value for Fe (II) metal ion was found higher as compared to cobalt metal ion.
2897.89 > 2567.89
Fe (II) > Co (II)
Kd value from pH 3-8.
Adsorption studies of the metal ion Fe (II) using GBSA resin at their maximum adsorption 70.01% at pH 5 and Co (II) was 57.7% at pH 6.
Conclusions
The polysaccharide 20 utilized in the reagent production is essentially a flocculent. It is inexpensive and readily available. Heavy metal ion removal from stock solution of metal ions using resins made of guar gum polysaccharide has been proven to be successful such as Fe (II), and Co (II) metal ions. Guar gum 1-butane sulphonic acid (GBSA) resin is today regarded as one of the most effective and intriguing methods due to its eco-friendliness, low cost, and quickness. Di-vinyl benzene is used to create the majority of resins, but GBSA resin is now preferred because of its speed and low cost.
Acknowledgment
I am thankful to my supervisor professor Aresh Vikram Singh for providing me guidance and support during my research work.
Conflict of Interest
The author declared that no conflict of interest in my present work.
References
- ]. Kpor, O.B.; Ohiobor, G.O.; Olaolu, T.D. Advances in Bioscience and Bioengineering. 2014, 2, 37-43.
CrossRef - .Valko, M.; Morris, H; Cronin, M. Curr Med Chem.2005, 12 (10), 1161–1208.
CrossRef - Way, J.T. Journal of the Royal Agricultural Society. 1850, 11, pp 313–379.
- Gins, R. Jahrbuch der Königlich Preussischen Geologischen Landesanstalt.1905, 26, pp 179.
- Harper, T.R.; Kingham, N.W. Water Environ Res. 1992, vol. 64, No. 3, pp. 200-203.
CrossRef - Kiezyk,P.R.; Mackay,D. Canadian Journal of Chemical Engineering,1997, vol. 49, no. 6, pp. 747–752.
CrossRef - De Los, A.P.; Hernadez,F.J.; Lozano,L.J.; Sachez,S.; Moreno,J.I.; Gaoline,C. J.Chem.Eng, 2010,vol.55, pp.605-608.
CrossRef - Carlos, Barrera-Daz.; Ivonne Linare, Hernández.; Gabriela Roa,Morales.; Bryan, Bilyeu.; Patricia Balderas,Hernández.Ind. Eng.Chem.Res. 2008, vol. 48, August, pp. 1253–1258.
CrossRef - Kepa, U.; Stanczyk Mazanek, E.; Stepniak, L. Desalination, 2008, vol.233, no.1-3, pp.187-193.
CrossRef - Ei-Diwani, E.; Ei-Rafie, S.; Hewish’s. J. Agric. Environ Sci,2009, vol. 6, pp. 119-128.
- R, Jaya Santhi.; Vetriselvi, V. Water Res. and Ind.2015, vol. 10, pp. 39–52.
CrossRef - I, R. J. Appl. Polym. Sci. 2002, vol. 10, no. 3, pp. 1353-1357.
- C, R. Water Res. and Indus. 2015, vol. 11, pp. 46-57.
- C, Raman. International Journal of Polymer Materials, 2009. vol. 58, no. 4, pp. 499-508.
CrossRef - Das, D.; Das, A.K.; Sinha, C. Talanta, 1999.vol. 48, no. 5, pp. 1013-1022.
CrossRef - Kumar, I.R. J. Appl. Polym. Sci. 2003, vol. 43, no. 11, pp. 1159-1169.
- Nagar, Sarika. Research journal of chemical sciences, 2022, Vol 12(1), 1-10.
- Qiao-Hui, Zhong. Jie, Li. Journal of analytical atomic spectrometry, 2023, issue 4.
- Gopinath, V. Science direct, 2022, vol.146.
- Wenxu, Zhang. MDPI, 2023, vol.no.9 (3).

This work is licensed under a Creative Commons Attribution 4.0 International License.









