Cissus quadrangularis is a Potential Anticancer Herb - A Review
Arthi Rashmi D1,2 , Sangilimuthu Alagar Yadav1*
, Sangilimuthu Alagar Yadav1* , Harishchander A3
, Harishchander A3 , Karpagavalli M4
, Karpagavalli M4 and Ranjithkumar D5
and Ranjithkumar D5
1Department of Biotechnology, Karpagam Academy of Higher Education, Coimbatore, Tamil Nadu, India.
2Department of Bioinformatics, Sri Krishna Arts and Science College, Coimbatore, Tamil Nadu, India.
3Bioinformatics Division, Amrita School of Artificial Intelligence, Coimbatore, Amrita Vishwa Vidyapeetham, Tamil Nadu, India.
4Karpagam College of Pharmacy, Coimbatore, Tamil Nadu, India.
5Department of Pharmaceutical Analysis, PSG College of Pharmacy, Coimbatore, Tamil Nadu, India.
Corresponding Author E-mail: smuthu.al@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/390509
Article Received on : 28 Aug 2023
Article Accepted on : 14 Oct 2023
Article Published : 17 Oct 2023
Reviewed by: Dr. Weam Abdulrahim Abdulqadir Hussien
Second Review by: Dr. Sonia Yadav
Final Approval by: Dr. R. Kanmani
Cancer is uncontrolled growth of abnormal cells due to heredity, mutation, genetic factors, environment factors, chain smoking, alcoholic addiction and exposure of chemical carcinogen. Treating modern medicine for cancer are producing numerous adverse effects. Hence natural remedy is essential to combat disease with less side effects. Eighty percentage of the peoples are used the medicinal plants for various illness in developing countries asper the WHO survey. The present review was written extensively with past scientific reports on Cissus quadrangularis (CQ) and its anticancer properties. From the ancient times the various parts of CQ have used for various diseases and disorders as folk medicine by traditional healer and practice. Traditionally CQ has vital role in various disease management. Its active extracts and ingredients reported against various diseases such as inflammation, cancer, ulcer, bone fracture and epilepsy. Still its therapeutic potentials not yet fully explored to use common people after clinical trial. Mainly CQ and its active phytoconstituents contains potential action for the treatment of various types of cancer which is scientifically proven upto the lab scale that needs to be evolved as medicine for world threatened disease cancer.
KEYWORDS:Anticancer; Biological Properties; Cissus quadrangularis; Phytoconstituents
Download this article as:| Copy the following to cite this article: Rashmi D. A, Yadav S. A, Harishchander A, Karpagavalli M, Ranjithkumar D. Cissus quadrangularis is a Potential Anticancer Herb - A Review. Orient J Chem 2023;39(5). |
| Copy the following to cite this URL: Rashmi D. A, Yadav S. A, Harishchander A, Karpagavalli M, Ranjithkumar D. Cissus quadrangularis is a Potential Anticancer Herb - A Review. Orient J Chem 2023;39(5). Available from: https://bit.ly/3FnNnZL |
Introduction
The perennial species Cissus quadrangularis (CQ) is a member of the Vitaceae family. It is a common climber that can be found in the majority of India’s tropical regions. Cissus quadrangularis is a plant that grows majorly in the tropical areas of Asia and Africa. This plant mainly grows in the tropical regions of countries such as Nigeria, SriLanka, India, China, Uganda8. The entire herb is consumed because the root, stem, and leaves of the plant are employed for therapeutic purposes. This succulent, shrubby climber grows to a typical length of approximately 1.5 metres. The leaves are generally oval or kidney-shaped, 2.5–5 cm in length 5 cm wide, and rarely 3–7 lobed, dentate, and serrate, with a rounded base and no hairs. The small, bisexual, tetramerous flowers of the umbellate cymes are a greenish-white in colour. Short, cup-shaped, and deciduous describe the calyx. 4-5 petals make to a flower14. The seeds are noted to be ellipsoid to pyriform and a rich purple to black in hue. According to Ghouseet al. (2015), the fruits of a plant are globose or obovoid berries that are very acrid, succulent, and seedless. The dichotomously branched stem is subangular, smooth, fibrous, buff in colour with a tinge of green, and glabrous6. These stems, which measure 8–10 cm in length and 1–5 cm in width, have nodes and internodes that are joined (Figure 1)11. Dyspepsia, anorexia, flatulence, colic, seizures, tumours, epistaxis, asthma, irregular menstruation patterns, inflammation, antibacterial infections, and obesity have all been treated with it. Studies on the anticarcinogenic characteristics of CQ have revealed that it has the ability to fight cancer because it contains a variety of phytochemicals, including quercetin. Similar investigations have demonstrated that quercetin induces lethal effects on leukaemia, ovarian carcinoma, breast cancer, and colon cancer cells. According to toxicological studies, CQ extract has no harmful toxic effects.
Most of its anatomical parts have been researched and exploited for traditional medicinal purposes. In addition to ancient therapeutic purposes, recent research endeavors has also attempted to uncover any of its hidden potential to inhibit the growth of micro–organisms22. This plant is also known by various names around the world and known by different names in India, such as Pirandai in Tamil, Nalleruin Telugu, Changalam paranda in Malayalam, Hadjod in Hindi, and Asthisanghataor Kandavallior Vajrangi in Sanskrit3. It has various bioactive compounds such as triterpenoids, vitamins, enzymes, nicotinicacid, ascorbic acid, carotene, phyto sterol substances, calcium, flavonoids, alkaloids, resveratrol, piceatannol, pallidal, Partheno cissus, quadrangularins. Cissus quadrangularis comprises of aOnocer-7-ene-3, 21-diol and onocer-7-ene-3, 21-diol, along with sitosterol, -amyrin, and -amyrone, make up the unsymmetric tetracyclic triterpenoids2.CQ is extensively studies species in all aspects such as its potential action, species variation, taxonomic characteristics and genetic characteristics. CQ has the overall genome size is 312,971,854 base pairs in length, with 336,794 scaffolds and 753,355 cotyledons altogether. Totally there are 36,325 protein coding regions are present and the GC content is 35.90%4.
Biological properties of CQ
According to Kumar et al. (2014), CQ significantly reduced oxidative stress and inhibited the growth of the Ehrlich Ascites Carcinoma cell line. Although CQ has been utilised to treat a number of illnesses, research into its anticancer characteristics, particularly the molecular mechanisms behind its benefits, is still lacking10.
The various parts of CQ used to treat various diseases and disorders traditionally as well it has been scientifically proved its potential actions such as anti-helminthic, anti-ulcer, analgestic, anti-tumor, anti-inflammatory, anti-epileptic and bone fracture healing potential (Figure.1)18. Still its potential action needs to explore extensively for modern medicine.
 |
Figure 1: The Cissus quadrangularis Plant |
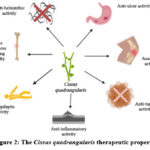 |
Figure 2: The Cissus quadrangularis therapeutic properties |
Phytoconstituents in CQ
Photochemical analysis of CQ extract showed the presence of metal ions, taraxerol, pallidol, resveratrol, piceatannol, methyl triacontanoic acid, parthenocissus, taraxeryl acetate, phenol, carotene, tannin, and vitamin. Additionally, it has brand-new flavonoids, indanes, phytosterols, and keto-steroids that are advantageous and function as powerful antioxidants24. Different parts of CQ contains numerous active ingredients including Daidzein, Quinine, Tetratriactanoic acid, Eicosyl Eicosanoate and Tetratriacotanol from leaves of CQ12,21. 24-methyl-dammara 20,25-diene-3β-yl acetate; 24-methyl-dammara 20,25-diene-3β-ylpalmitate; 24-methyl-dammara20,25-diene-3β-ylstearate; 24-methyl-dammara 2, 20, 25-triene-1-one; 24-methyl-dammara20,25-diene-3-One; Eugenol; Taraxerolacetate; Taraxerol; Isopentadecanoic acid; Cardiacglycosides; Cissusic acid; Cissuside; Cissusol; Saponins; Caffeine from stem of CQ (Table.1).
Table 1: Phytochemical compounds derived from Cissus quadrangularis
|
S.No., |
Type of compound |
Phytochemicals |
Source |
References |
|
1. |
Flavonoid and Flavonoid glycosides |
Daidzein |
Leaves |
Mukherjee et al.,2016 |
|
2. |
Alkaloids |
Quinine and Caffeine |
Leaves |
Tiwari et al.,2018 |
|
3. |
Terpenes and Terpenoids |
24-methyl-dammara20,25-diene-3β- yl acetate; 24-methyl-dammara20,25-diene-3β-ylpalmitate 24-methyl-dammara20,25-diene-3β-Ylstearate 24-methyl- dammara2,20,25-triene-1-one 24-methyl-dammara20,25-diene-3-One |
Stem |
Pathomwichaiwat, et al., 2015 |
|
Eugenol |
Stem |
Tiwari et al.,2018 |
||
|
Taraxerol acetate |
Stem |
Mishra et al.,2010 |
||
|
Taraxerol |
Stem |
Sen et al., 2012 |
||
|
4. |
Components in the form of fatty acids and Lipids |
Isopenta decanoic acid |
Stem |
Sen et al., 2012 |
|
Tetratriactanoic acid EicosylEicosanoate |
Leaves |
Mukherjee et al.,2016 |
||
|
5. |
Glycosides |
Cardiac glycosides |
Stem |
Das et al.,2018 |
|
6. |
Alcoholic compounds |
Tetra triacotanol |
Leaves |
Mukherjee et al.,2016 |
|
7. |
Phenolic glycosides |
Cissusic acid |
Stem |
Kumar et al., 2017 |
|
8. |
Lignan glycosides |
Cissuside and Cissusol |
Stem |
Kumar et al., 2017 |
|
9. |
Saponins |
Saponins |
Stem |
Tiwari et al.,2018 |
Cissus quadrangularis and its anti cancer activity
CQ against oral cancer
The CQ extracts and its active compounds have been shown to be effective in treating a variety of malignancies, including lung cancer, skin cancer, osteosarcoma, glioblastoma, breast cancer, and pancreatic and pancreatic cancer. (Figure. 3). The anti-cancer potential of an ethanolic extract of CQ stem against KB oral epidermoid cancer cells was assessed using morphological analysis, nuclei staining, reactive oxygen species (ROS) liberation, cell cycle arrest, mitochondrial membrane potential (MMP), and expression of p53and Bcl-2 proteins, which demonstrates induction of apoptosis. Ethanolic extract of CQ stem contains several bioactive agents that are responsible for cancer cell morphological changes, ROS liberation, G1phase cell cycle arrest, and reduced MMP, as well as up-regulation of p53 and down-regulation of Bcl-2. In KB oral cancer cells, p53 was up-regulated following treatment with CQ (200 g/ml for 24 h) as compared to control. As a result, CQ extract has an impressive influence on apoptosis in KB cell lines17.
 |
Figure 3: The Cissus quadrangularis anticancer activity |
CQ against cervical cancer
In a recent study, the formation of Reactive Oxygen Species (ROS), apoptosis, cell cycle analysis, and caspases-3 activation were evaluated in HeLa cells at various doses of CQ extract (25–300 g/ml). Cell survival of HeLa cells was significantly reduced (p<0.05) inadose-dependent manner, however, when tested on the normal renal epithelial NRK-52E cells, CQ extract proved non-toxic. With the generation of annexin V-FITC positive cells, CQ extract increased the nuclear condensation, intracellular ROS level, and decreased the mitochondrial membrane potential (MMP). CQ extract arrested the cell cycle progression in G0/G1 and G2/M check points and activated caspase-3 activity significantly in HeLa cells. From this study it can be stated that active constituents in CQ extract can be considered as potential chemo therapeutic candidates in the management of cervical cancer15. Another investigation was aimed to verify the anticancer activity of the plant’s methanolic and ethanolic extracts against the HeLa cell line. The IC50 concentration form ethanolic extract was 62.5µg/mland125µg/ml for ethanolic extract. When compared to the ethanolic extract, the methanolic extract of Cissus quadrangularis was clearly more active against the HeLa cell line5.
CQ against lung cancer
The apoptotic effects of Cissus quadrangularis ethanolic extract against human lung cancer cells was reported by Gurumurthy et al., 2021 and the results revealed that elevating the concentration of the plant extract lowered the vitality of the lung cancer cells considerably. Thus, Cissus quadrangularis ethanolic extract demonstrated strong cytotoxic effects on human lung cancer cells and can be used as a potent therapeutic strategy for the treatment7.
CQ against asciticlymphoma cancer
Ethanolic and aqueous concentrate of Cissus quadrangularis was proved to be more effective in the decrease of blood concentration of Packed Cell Volume, Alanine Transaminase, Aspartic acid, Alkaline phosphatase, Triglyceride Lipase, low density lipoproteins and rigid tumour volume with a significant decrease in the quantity of Red Blood Corpuscles, Haemoglobin and the quantity of platelets inascitic lymphoma cancer induced animal compared to control group. Simultaneously, both the chloroform and methyl alcohol extracts of CQ showed an increase in the cell life expectancy from 72% to up to 76%, in comparison to DLA (48%). These extracts obtained from methanol and chloroform have demonstrated their anti-tumour properties in other such studies as well. The presence of flavonoids and polyphenols was also detected in these extracts, in addition to various other molecules13. Many compounds have been identified from CQ and reported to be effective against a wide range of cancers, including carcinoma of squamous cells and ascites carcinoma as well as skin, oral, breast, colon, and bone cancers. The active molecules are gamma tocopherol, rutin, quercetin, Betulinic acid, epi-glut-5(6)-en-ol and lupeol (Table.2).
Table 2: Phytochemical compounds / extract and their activity against cancer derived from Cissus quadrangularis.
|
S.No |
Name of the phytochemical/ extract from CQ |
Parts used |
Active against |
Mode of action |
Reference |
|
1 |
Ethanolic extract |
Stem |
Skin cancer (A431 CELLS) |
CQ extract induced cytotoxicity in A431cells and induce apoptosis. |
Arshad et.al., 2016 |
|
2 |
Gamma-Tocopherol |
stem |
Oral cancer |
CQ induces apoptosis and cell cycle arrest at G1 phase on oral cancer cells |
Saba Sheik et.al., 2015 |
|
3 |
quercetin and rutin |
Aerial part |
Breast cancer |
Reduced the viability of breast cancer cells. |
Vijayalakshmi et.al., 2013 |
|
4 |
epi-glut-5(6)-en-ol and betulinic acid |
Stem |
Non-small-cell lung carcinoma (NCI-H226) and colon cancer (HCT-116) cell lines |
It acted against the colon cancer cells and lung cancer cells cause cytotoxicity. |
Pandey, Sandeep, et al., 2022 |
|
5 |
lupeol |
Stem |
MCF- 7 breast cancer cells |
exhibited a significant melanin promotion activity in increases the concentration of the extract. It arrest the cancer cells proliferations. |
Pandey, Sandeep, et al., 2022 |
|
6 |
Methanolic extract |
Aerial parts |
MG63 (Osteosarcoma cellline) |
Methanolic extract of CQ arrest the proliferation on MG63 cell in dose dependent manner. |
Suresh et al., 2019 |
|
7 |
Ethanolic extract |
stem |
Squamous cell carcinoma (KB oral epidermoid carcinoma cells) |
Membrane blebbing, cell shrinkage, loss of membrane asymmetry were seen on KB cells after the treatment. |
Rajamaheswari, et al., 2019 |
|
8 |
Chloroform extract And ethanol extract |
EAC (Ehrlich Ascites Carcinoma) cell line |
It acted against the Ascites Carcinoma cells and cause cytotoxicity. |
Rajamaheswari., et al., 2019 |
CQ against skin cancer (A431)
According to Arshad et al. (2006), excessive exposure to UV light is the cause of skin malignancies. Cissus quadrangularis extract was used to estimate the cytotoxicity and apoptotic effects of CQ extract in A431 cells over the course of 24 hours. Different quantities of CQ extracts (25, 50, 100, and 250 g ml1) were applied to A431 cells for 24 hours. A431 cells that had been exposed to CQ extract changed shape to a circle and lost contact with the surface. MTT results showed that CQ extract cytotoxicity in A431 cells was dose-dependent. Over the course of 24 hours, the CQ extract caused cell toxicity of 8.46% at 25 g/ml, 19.37% at 50 g/ml, 40% at 100 g/ml, & 59.26% at 250 g/ml. Using an MTT test, the cytotoxicity of a 24-hour CQ extract pretreatment on A431 cells was evaluated. ROS production and oxidative stress were produced by CQ extract. On a dose-dependent basis, CQ extract promoted the generation of intracellular ROS. In A431 cells, CQ extract stimulated the production of ROS1.
CQ against Colon cancer
The American Type Culture Collection provided two human cancer cell lines: colon cancer cell line (HCT116) and non-small cell lung carcinoma (NCI-H226). At 37 °C in a humid environment with 5% CO2, tumour cells were kept alive in appropriate media supplemented with 10% fetal bovine serum (FBS). The growth inhibition assay was carried out. The phytochemical actions of betulinic acid and epi-glut-5(6)-en-ol against these cell lines were discovered (Pandey, Sandeep et al., 2022).
CQ against brain cancer
Quercetin from CQ causes its toxic effects on breast cancer, colon cancer, ovarian carcinoma, and leukaemia cells, according to in vitro and in vivo research. According to toxicological studies, the CQ extract has no harmful toxic effects24,9,20. Numerous molecules possessing anti-tumour characteristics have been isolated from CQ and examined for its anticancer properties in human glioblastoma U87 MG cells. CQ treatment was shown to cause cytotoxicity, cell cycle arrest, and cell death inU87 MGcells23. The anticancer activity of CQ methanolic extract was investigated in humanosteosarcoma cell lines (MG63) cell lines by MTT assay. Cell viability of MG63 cells ranged from 29.65% to 73.59% at extract concentrations ranging from 1000g/ml to 7.8 g/ml. This cytotoxicity assay found that the extract’s IC50 was 100 mg/ml. Hence the anticancer activity of CQ was revealed against MG63cells19.MG63 is an osteosarcoma cell line. In a dose-dependent manner, the methanol extract of C. quadrangularis exhibited cytotoxicity against MG63 cells. Additionally, a cytotoxicity study using a methanolic extract from C. quadrangularis‘ aerial portions revealed antiproliferative effects against MG63 cells19.
CQ against Squamous cell carcinoma and EAC (Ehrlich Ascites Carcinoma) cell line
The ethanolic extract of C. quadrangularis stem inhibited the growth of cells of KB oral epidermoid carcinoma. Similarly, Hematoxylin and eosin staining revealed the morphological alterations such as cell shrinkage, membrane blebbing, and loss of membrane asymmetry were seen on KB cells following treatment with ethanolic extract of C. quadrangularis stem, confirming apoptosis. EAC cells are research tumour models that are employed in cancer research all around the world. A transplantable, undifferentiated malignant tumour that began in a mouse as a spontaneous breast cancer. Cissus quadrangularis chloroform extract exhibited 80.60% and ethanol extract showed 85.40% cytotoxic activity on EAC (Ehrlich Ascites Carcinoma) cell line16.
Conclusion
According to the findings of this review, all sections of CQ have increased cytotoxic potentials against many forms of cancer, including cancers of the bone, brain, breast, oral, lung, skin, cervix, and colon. CQ and its active components are actively eradicating cancer cells in the laboratory. CQ and its active constituents actively eradicate the cancer cells still it’s in laboratory level. Many reports are generated by scientific communities on CQ and its effectiveness in cancer treatment. Many active phytomolecules have reported from CQ against cancer but all reports are in laboratory level, which need to take further for clinical trial for effective natural based treatment.
Acknowledgment
The authors are grateful to the Karpagam Academy of higher education for providing a facility to comprise this review article.
Conflict of Interest
All the authors have no conflict of interest.
References
- Arshad, M.; Siddiqui, S.; Ali, D. Caryologia. 2016, 69(2), 128-132.
CrossRef - Bhutani, K. K.; Kapoor, R.; Atal, C. K. Phytochem. 1984, 23(2), 407-410.
CrossRef - Brahmkshatriya, H. R.; Shah, K. A.; Ananthkumar, G. B.; Brahmkshatriya, M. H. Ayu. 2015, 36(2), 169–173.
CrossRef - Gandhimathi, A.; Sunitha, S. M.; Gosh, P.; Harini, K.; Iyer, S. H. M.; Joshi, G. A.; Karpe, D. S.; Mahita, J.; Malini, M.; Mathew, K. O.; Mutt, E.; Naika, M.; Ravooru, N.; Nitish, S.; Pasha, S. N.; Raghavender, S. U.; Rao, M. R.; Shafi, K. M.; Shingate, N. P.; Sukhwal, A.; Upadhyay, K. A.; Vinekar, S. R.; Sowdhamini, R. Herb. Med. 2016, 2(1), 7-9.
- Geetha, S.; Vasuki, R. Int. J. Innov. Technol. Explor. Eng. 2019, 8(6), 2278-3075.
- Ghouse, M. S. Int. J. Res. Pharm. Biosci. 2015, 2(7), 28-35.
- Gurumurthy, K.; Raghunandhakumar, S.; Ezhilarasan, D.; Lakshmi, T. J. Complement. Med. Res. 2021, 12(3), 54-60.
CrossRef - Indran, S.; Raj, R. E.; Sreenivasan, V. S. Carbohydr. Poly. 2014, 110, 423-429.
CrossRef - Jadhav, A. N.; Rafiq, M.; Devanathan, R.; Azeemuddin, M.; Anturlikar, S. D.; Ahmed, A.; Ramchandran, S.; Babu, U. V.; Paramesh, R. Pharmacogn. Mag. 2016, 12(Suppl 2), S213-S217.
CrossRef - Kumar, A.; Deepa, B.; Saravanan, R.; Hameed, S. A. Int. J. Pharm. Pharm. Sci. 2014, 6, 269-274.
- Mishra, G.; Srivastava, S.; Nagori, B. P. Int. J. Pharmtech Res. 2010, 2(2), 1298-1310.
- Mukherjee, T.; Saha, N.; Palbag, S. Int. J. Res. Ayurveda Pharm. 2016, 7(4), 78-82.
CrossRef - Nalini, G.; Vinoth, P. V.; Chidambaranathan, N.; Jeyasundari, K. Int. J. Pharm. Sci. Rev. Res. 2011, 8(1), 75-79.
- Panda, S.; Das, A. BSMPS, Dehra Dun, 2004.
- Pandey, S.; Parmar, S.; Shukla, M. S.; Sharma, V. S.; Dwivedi, A.; Pandey, A.; Mishra, M. Herb. Med. J. 2022, 7(2), 1-7.
- Rajamaheswari, K.; Visweswaran, S.; Muthukumar, N. J.; Murugesan, M.; Banumathi, V. Int. J. Curr. Res. Chem. Pharm. Sci. 2017, 4(8), 1-3.
CrossRef - Sheikh, S.; Siddiqui, S.; Dhasmana, A.; Haque, E.; Kamil, M.; Lohani, M.; Arshad, M.; Mir, S. S. Pharmacogn.Mag. 2015, 11(Suppl3), S365–S374.
CrossRef - Sundaran, J.; Begum, R.; Vasanthi, M.; Kamalapathy, M.; Bupesh, G.; Sahoo, U. Bioinformation. 2020, 16(8), 579-585.
CrossRef - Suresh, P.; Xavier, A. S.; Karthik, V. P.; Punnagai, K. Biomed. Pharmacol. J. 2019, 12(2), 975-980.
CrossRef - Tamburaci, S.; Kimna, C.; Tihminlioglu, F. J. Bioact. Compat. Pol. 2018, 33(6), 629–646.
CrossRef - Tiwari, M.; Gupta, P. S.; Sharma, N. Res. J. Pharmacogn. Phytochem. 2018, 10(1), 81-90.
- Valli, J. S.; Vaseeharan, B. Mater. Lett. 2012, 82, 171-173.
CrossRef - Vijayalakshmi, A.; Kumar, P. R.; Sakthi Priyadarsini, S.; Meenaxshi, C. J. Chem. 2013, 150675, 1-9.
CrossRef - Zenebe, S.; Feyera, T.; Assefa, S. Biomed. Res. 2017, 1905987, 1-6.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









