Synthesis, Characterization and Antimicrobial Evaluation of Amino Acid Derivatives of 1,3,4-Oxadiazole
Yudhishthir Vaishnav1 , Sangeeta Rajpurohit2*
, Sangeeta Rajpurohit2* and Kamalkant Vyas1
and Kamalkant Vyas1
1Department of Chemistry, J. N. V. U, Jodhpur, Rajasthan India
2Department of Chemistry, Lachoo Memorial College of Science and Technology, Jodhpur, Rajasthan, India.
Corresponding Author E-mail: rajpurohitsangeeta94@gmail. com/
DOI : http://dx.doi.org/10.13005/ojc/390422
Article Received on : 03 Jun 2023
Article Accepted on : 29 Jul 2023
Article Published : 16 Aug 2023
Reviewed by: Dr. Amrita Prasad
Second Review by: Dr. Chintan P. Somaiya
Final Approval by: Dr. Tawkir Sheikh
Several novel 1,3,4-oxadiazole compounds were synthesized for this investigation. NMR and IR spectrum analysis as well as carbon, hydrogen, and nitrogen studies were used to characterize these novel synthesized compounds. Antibacterial and antifungal tests were conducted on all of the newly synthesized compounds. Staphylococcus aureus and Bacillus subtilis, both Gram +ve bacteria, and Escherichia coli and Pseudomonas aureginosa, both Gram -ve bacteria, were utilised in antibacterial studies. Aspergillus niger and Candida albicans were used to test the efficacy of antifungal treatments. Ciprofloxacin and fluconazole were utilized as reference medications in antibacterial and antifungal research, respectively. The inhibitory effects ranged from mild to strong across all of the substances. The results of the screenings showed that several of the compounds had stronger antibacterial and antifungal properties than the standard medicines.
KEYWORDS:Antibacterial; Antifungal; Oxidiazole
Download this article as:| Copy the following to cite this article: Vaishnav Y, Vyas K, Rajpurohit S. Synthesis, Characterization and Antimicrobial Evaluation of Amino Acid Derivatives of 1,3,4-Oxadiazole. Orient J Chem 2023;39(4). |
| Copy the following to cite this URL: Vaishnav Y, Vyas K, Rajpurohit S. Synthesis, Characterization and Antimicrobial Evaluation of Amino Acid Derivatives of 1,3,4-Oxadiazole. Orient J Chem 2023;39(4). Available from: https://bit.ly/3sd3gPm |
Introduction
Oxadiazoles, sometimes known as furadiazoles are compounds with a five-membered ring consisting of one oxygen and two nitrogen atoms1, 2. Oxadiazoles have been shown to have a wide range of beneficial biological effects3. By replacing two of the furan’s methane (-CH=) groups with pyridine-type nitrogen atoms (-N=), oxadiazole is thought to be the outcome2. The synthesis of 1,3,4-oxadiazoles is a topic of much research and discussion. Typically, 1,3,4-oxadiazoles are synthesized by directly annulating hydrazides with methyl ketones. Using K2CO3 as a base was discovered to result in a very effective and unexpected C-C bond cleavage. It is hypothesized that oxidative breakage of Csp3-H bonds occurs first, then cyclization, and finally deacylation, in this process4. Antimicrobial,5 anti-inflammatory,6 antibacterial,7 anticancer,8 antifungal,9 tuberculostatic10, analgesic11, vasodilating agent12, anti-HIV agent13 and anti-hypertensive14,15 properties have been found for 1,3,4-oxadiazoles and their derivatives in recent investigations. In addition, amino compounds are widely used as a class of drugs with antibacterial and germicidal properties. Keeping the above in mind, it was proposed that a few substituted oxadiazoles derivatives produced from amino acid moieties may be synthesized. The synthesized chemicals were tested for their ability to kill bacteria and fungi.
Material and Method
Both the reagents and the solvents were obtained from commercial sources. The open capillary technique was used to get all of the melting points, without any adjustments being made. Recrystallization was used to improve the quality of the final products. Thin films mounted on KBr pellets were used to capture IR spectra using a PERKIN ELMER FT-IR Spectrophotometer. Chloroform was used to collect 1HNMR spectra by using Bruker NMR instrument, and the resulting chemical shift values are presented in ppm with respect to TMS (δ = 0) as the internal standard.
Experimental Section
Synthesis and Characterization
A new strategy for the synthesis of 1,3,4-oxadiazoles was established through direct annulation of hydrazides with methyl ketones. It was found that the use of K2CO3 as a base achieves an unexpected and highly efficient C−C bond cleavage. This reaction is proposed to go through oxidative cleavage of Csp3−H bonds, followed by cyclization and deacylation4.
 |
Figure 1 Click here to View Figure |
Three aromatic amino acids named Tyrosine, Phenylalanine and Tryptophan were used to synthesis of amino acid derivatives of 1,3,4-oxadiazoles. In first step acylation of amino acid has been done by using different organic acids named benzoic acid, succinic acid and pthalic acid. In second step acylated product reacted with differently substituted hydrazides in presence of 3.0 equiv K2CO3 over 20 h using 2.5 eqiv of I2 at 1000 C in DMSO which results in the formation of various 1,3,4-oxidiazole derivatives.
By using Tyrosine and Benzoic acid
 |
Scheme 1: By using Tyrosine and Benzoic acid |
By using Phenylalanine and Succinic acid
 |
Scheme 2: By using Phenylalanine and Succinic acid |
By using Tryptophan and Pthalic acid
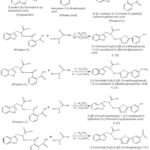 |
Scheme 3: By using Tryptophan and Pthalic acid |
In all total 12 derivatives were formed A (1-4), B (1-4) and C (1-4).
3-(4-hydroxyphenyl)-2-{[5-(3-methylphenyl)-1,3,4-oxadiazol-2-yl]amino}propanoic acid (A 1)
Yield 88.89%, M. P.-605.810C, Mol. Wt. 339.35, IR (cm-1) – 2800.82- (-NH) stretching, 2779.1- (Ar-CH) stretching, 1716.17- (C=O) stretching, 1699.09- (C=N) stretching, 1692.32- (Ar C=C) stretching, 1561.83 – (-NH) bent, 1145.81- (C-N) stretching, 1H-NMR (CDCl3, 500 MHz, ppm): 7.02 (2 CH), 7.02 (3 CH), 6.73 (4 CH), 6.73 (5 CH), -0.86 (15 OH), 2.73 (8a CH2), 3.16 (8b CH2), 4.49 (9 CH), -1.99 (10 NH), 9.61 (13 OH), 2.33 (19 CH3), Anal. calcd. for C18H17N3O4 : N, 12.38; H, 5.05; C, 63.71; O, 18.86.
3-(4-hydroxyphenyl)-2-{[5-(3-methoxyphenyl)-1,3,4-oxadiazol-2-yl]amino}propanoic acid (A 2)
Yield 79.84%, M. P.-628.040C, Mol. Wt. 355.34, IR (cm-1) – 2822.38- (-NH) stretching, 2775.19- (Ar-CH) stretching, 1711.42- (C=O) stretching, 1692.56- (C=N) stretching, 1676.79- (Ar C=C) stretching, 1553.59- (-NH) bent, 1182.17- (C-O-C) stretching, 1141.64- (C-N) stretching, 1H-NMR (CDCl3, 500 MHz, ppm): 3.8 (1 CH3), 6.93 (3 CH), 7.17 (4 CH), 7.37 (5 CH), 7.37 (5 CH), 7.18 (7 CH), 7.07 (10 CH), 7.07 (11 CH), 6.85 (12 CH), 6.85 (13 CH), 3.37 (7 OH), 2.95 (16a CH2), 3.17 (16b CH2), 4.43 (17 CH), -3.42 (18 NH), 9.54 (21 OH), Anal. calcd. for C18H17N3O5 : N, 11.83; H, 4.82; C, 60.84; O, 22.51.
3-(4-hydroxyphenyl)-2-{[5-(3-hydroxyphenyl)-1,3,4-oxadiazol-2-yl]amino}propanoic acid (A 3)
Yield 81.79%, M. P.-693.740C, Mol. Wt. 341.32, IR (cm-1) – 3021.34- (-OH) stretching, 2811.74- (-NH) stretching, 2769.17- (Ar-CH) stretching, 1781.42- (C=O) stretching, 1691.48- (C=N) stretching, 1644.79- (Ar C=C) stretching, 1551.49- (-NH) bent, 1139.17- (C-N) stretching, 1H-NMR (CDCl3, 500 MHz, ppm): 6.97 (2 CH), 6.97 (3 CH), 6.72 (4 CH), 6.72 (5 CH), 3.49 (7 OH), 3.12 (8a CH2), 2.92 (8b CH2), 4.52 (9 CH), -3.37 (10 NH), 9.67 (13 OH), 3.88 (25 OH), Anal. calcd. for C17H15N3O5 : N, 12.31; H, 4.43; C, 59.82; O, 23.44.
3-(4-hydroxyphenyl)-2-{[5-(3-nitrophenyl)-1,3,4-oxadiazol-2-yl]amino}propanoic acid (A 4)
Yield 71.74%, M. P.-738.150C, Mol. Wt. 370.32, IR (cm-1) – 2872.86- (-NH) stretching, 2797.68- (Ar-CH) stretching, 1738.52- (C=O) stretching, 1699.9- (C=N) stretching, 1679.32- (Ar C=C) stretching, 1549.49- (-NH) bent, 1451.16- (NO2) stretching, 1153.18- (C-N) stretching, 1H-NMR (CDCl3, 500 MHz, ppm): 7.04 (2 CH), 7.04 (3 CH), 6.75 (4 CH), 6.75 (5 CH), 3.26 (7 OH), 3.18 (8a CH2), 2.85 (8b CH2), 4.5 (9 CH), -2.03 (10 NH), 9.7 (13 OH), Anal. calcd. for C17H14N4O6 : N, 15.13; H, 3.81; C, 55.14; O, 25.92.
2-{[5-(3-methylphenyl)-1,3,4-oxadiazol-2-yl]amino}-3-phenylpropanoic acid (B 1)
Yield 81.79%, M. P.-494.090C, Mol. Wt. 323.35, IR (cm-1) – 2779.46- (-NH) stretching, 2769.43- (Ar-CH) stretching, 1710.62- (C=O) stretching, 1698.96- (C=N) stretching, 1686.01- (Ar C=C) stretching, 1551.89- (-NH) bent, 1141.02- (C-N) stretching, 1H-NMR (CDCl3, 500 MHz, ppm): 7.16 (2 CH), 7.16 (3 CH), 7.24 (4 CH), 7.24 (5 CH), 7.14 (6 CH), 3.17 (7a CH2), 2.97 (7b CH2), 4.43 (8 CH), -3,61 (9 NH), 9.66 (12 OH), 2.33 (18 CH3), Anal. calcd. for C18H17N3O3 : N, 13.00; H, 5.30; C, 66.86; O, 14.84.
2-{[5-(3-methoxyphenyl)-1,3,4-oxadiazol-2-yl]amino}-3-phenylpropanoic acid (B 2)
Yield 69.75%, M. P.-516.320C, Mol. Wt. 339.35, IR (cm-1) – 2772.07.34- (-NH) stretching, 2765.55- (Ar-CH) stretching, 1711.73- (C=O) stretching, 1692.0- (C=N) stretching, 1578.9- (Ar C=C) stretching, 1556.13- (-NH) bent, 1186.57- (C-O-C) stretching, 1146.12- (C-N) stretching, 1H-NMR (CDCl3, 500 MHz, ppm): 3.79 (1 CH3), 6.99 (3 CH), 7.15 (4 CH), 7.43 (5 CH), 7.1 (7 CH), 7.12 (10 CH), 7.12 (11 CH), 7.21 (12 CH), 7.21 (13 CH), 7.21 (14 CH), 3.18 (15a CH2), 2.97 (15b CH2), 4.49 (16 CH), -3,71 (17 NH), 9.63 (20 OH), Anal. calcd. for C18H17N3O4 : N, 12.38; H, 5.05; C, 63.71; O, 18.86.
2-{[5-(3-hydroxyphenyl)-1,3,4-oxadiazol-2-yl]amino}-3-phenylpropanoic acid (B 3)
Yield 91.05%, M. P.-582.020C, Mol. Wt. 325.32, IR (cm-1) – 2799.11- (-OH) stretching, 2792.46- (-NH) stretching, 2771.13- (Ar-CH) stretching, 1715.09- (C=O) stretching, 1696.92- (C=N) stretching, 1573.36- (Ar C=C) stretching, 1544.89- (-NH) bent, 1150.9- (C-N) stretching, 1H-NMR (CDCl3, 500 MHz, ppm): 7.15 (2 CH), 7.15 (3 CH), 7.23 (4 CH), 7.23 (5 CH), 7.13 (6 CH), 3.16 (7a CH2), 2.96 (7b CH2), 4.42 (8 CH), -3.67 (9 NH), 9.67 (12 OH), 3.93 (24 OH), Anal. calcd. for C17H15N3O4 : N, 12.92; H, 4.65; C, 62.76; O, 19.67.
2-{[5-(3-nitrophenyl)-1,3,4-oxadiazol-2-yl]amino}-3-phenylpropanoic acid (B 4)
Yield 69.95%, M. P.-626.430C, Mol. Wt. 354.32, IR (cm-1) – 2857.87- (-NH) stretching, 2769.48- (Ar-CH) stretching, 1711.93- (C=O) stretching, 1695.56- (C=N) stretching, 1577.64- (Ar C=C) stretching, 1546.5- (-NH) bent, 1441.39- (NO2) stretching, 1140.63- (C-N) stretching, 1H-NMR (CDCl3, 500 MHz, ppm): 7.17 (2 CH), 7.17 (3 CH), 7.24 (4 CH), 7.24 (5 CH), 7.14 (6 CH), 3.27 (7a CH2), 2.74 (7b CH2), 4.35 (8 CH), -3.67 (9 NH), 9.66 (12 OH), Anal. calcd. for C17H14N4O5 : N, 15.81; H, 3.98; C, 57.63; O, 22.58.
3-(1H-indol-3-yl)-2-{[5-(3-methylphenyl)-1,3,4-oxadiazol-2-yl]amino}propanoic acid (C 1)
Yield 76.91%, M. P.-634.510C, Mol. Wt. 362.38, IR (cm-1) – 2821.34- (-NH) stretching, 2779.57- (Ar-CH) stretching, 1711.62- (C=O) stretching, 1693.59- (C=N) stretching, 1674.39- (Ar C=C) stretching, 1559.59- (-NH) bent, 1143.14- (C-N) stretching, 1H-NMR (CDCl3, 500 MHz, ppm): 7.21 (3 CH), 7.23 (4 CH), 7.19 (5 CH), 7.21 (6 CH), 6.97 (8 CH), 7.33 (9 NH), 3.08 (10a CH2), 3.34 (10b CH2), 4.5 (11 CH), 18.17 (14 OH), -2.89 (15 NH), 2.32 (21 CH3) Anal. calcd. for C20H18N4O3 : N, 15.46; H, 5.01; C, 66.29; O, 13.25.
3-(1H-indol-3-yl)-2-{[5-(3-methoxyphenyl)-1,3,4-oxadiazol-2-yl]amino}propanoic acid (C 2)
Yield 86.27%, M. P.-648.460C, Mol. Wt. 378.38, IR (cm-1) – 2832.38- (-NH) stretching, 2773.36- (Ar-CH) stretching, 1711.9- (C=O) stretching, 1688.95- (C=N) stretching, 1675.69- (Ar C=C) stretching, 1562.77- (-NH) bent, 1182.91- (C-O-C) stretching, 1143.9- (C-N) stretching, 1H-NMR (CDCl3, 500 MHz, ppm): 3.8 (1 CH3), 7.21 (4 CH), 7.23 (5 CH), 7.19 (6 CH), 7.21 (7 CH), 6.97 (9 CH), 7.33 (10 NH), 3.34 (11a CH2), 3.08 (11b CH2), 4.5 (12 CH), 18.13 (15 OH), -2.89 (16 NH), Anal. calcd. for C20H18N4O4 : N, 14.81; H, 4.79; C, 63.48; O, 16.91.
2-{[5-(3-hydroxyphenyl)-1,3,4-oxadiazol-2-yl]amino}-3-(1H-indol-3-yl)propanoic acid (C 3)
Yield 68.72%, M. P.-636.430C, Mol. Wt. 364.35, IR (cm-1) – 3393.49- (-OH) stretching, 2828.65- (-NH) stretching, 2772.68- (Ar-CH) stretching, 1712.29- (C=O) stretching, 1687.4- (C=N) stretching, 1672.42- (Ar C=C) stretching, 1547.73- (-NH) bent, 1133.05- (C-N) stretching, 1H-NMR (CDCl3, 500 MHz, ppm): 7.21 (3 CH), 7.23 (4 CH), 7.19 (5 CH), 7.21 (6 CH), 6.97 (8 CH), 7.33 (9 NH), 3.08 (10a CH2), 3.34 (10b CH2), 4.5 (11 CH), 18.21 (14 OH), -2.89 (15 NH), 3.62 (27 OH), Anal. calcd. for C19H16N4O4 : N, 15.38; H, 4.43; C, 62.63; O, 17.56.
3-(1H-indol-3-yl)-2-{[5-(3-nitrophenyl)-1,3,4-oxadiazol-2-yl]amino}propanoic acid (C 4)
Yield 78.63%, M. P.-658.090C, Mol. Wt. 393.35, IR (cm-1) – 2838.89- (-NH) stretching, 2773.79- (Ar-CH) stretching, 1712.27- (C=O) stretching, 1695.04- (C=N) stretching, 1674.96- (Ar C=C) stretching, 1555.49- (-NH) bent, 1441.23- (NO2) stretching, 1143.14- (C-N) stretching, 1H-NMR (CDCl3, 500 MHz, ppm): 7.35 (3 CH), 7.21 (4 CH), 7.2 (5 CH), 7.2 (6 CH), 6.96 (8 CH), 7.26 (9 NH), 3.37 (10a CH2), 3.03 (10b CH2), 4.48 (11 CH), 16.7 (14 OH), -3.29 (15 NH), Anal. calcd. for C19H15N5O5 : N, 17.80; H, 3.84; C, 58.01; O, 20.34.
Antimicrobial Evaluation
For antibacterial investigations, two Gram +ve i.e. Staphylococcus aureus and Bacillus subtilis while, two Gram -ve bacteria i.e. Escherichia coli and Pseudomonas aureginosa were used.Two fungal species i.e. Aspergillus niger and Candida albicans were taken for antifungal examinations. The concentrations 100 µg/mL of the compounds were used. For antibacterial and antifungal studies, Ciprofloxacin and Fluconazole were used as standard drugs separately. The 20 µg/mL of concentration of each standard drug was used.
Table 1: Antibacterial activity of oxodiazole derivatives (Zone of inhibition, ZOI and % inhibition).
|
S. No |
Comp Code |
S. aureus |
|
B. substilis |
|
E. coli |
|
P. aureginosa |
|||||
|
ZOI ± SD |
% |
t-value# |
ZOI ± SD |
% |
t-value# |
ZOI ± SD |
% |
t-value# |
ZOI ± SD |
% |
t-value# |
||
|
Inhib |
Inhib |
Inhib |
Inhib |
||||||||||
|
1 |
A (1) |
18.83±0.80 |
93.4 |
2.728 |
19.91±1.07 |
91.8 |
2.774 |
18.10±0.60 |
81.1 |
9.670* |
14.27±0.62 |
70.2 |
15.52* |
|
2 |
A (2) |
14.17±0.81 |
70.3 |
12.08* |
15.10±0.40 |
69.7 |
24.12* |
14.20±0.70 |
63.6 |
18.58* |
12.33±0.52 |
60.6 |
23.65* |
|
3 |
A (3) |
18.33±1.53 |
90.9 |
2.047 |
19.93±1.15 |
92 |
2.561 |
18.87±1.53 |
83.6 |
3.848* |
14.33±0.70 |
70.5 |
13.85* |
|
4 |
A (4) |
12.67±0.58 |
62.8 |
20.03* |
13.83±0.25 |
63.8 |
38.41* |
11.67±0.42 |
52.3 |
24.07* |
11.00±0.40 |
54.1 |
33.49* |
|
5 |
B (1) |
18.70±0.87 |
92.7 |
2.776 |
20.50±0.70 |
94.6 |
2.726 |
20.00±1.07 |
89.6 |
2.594 |
18.87±0.93 |
92.8 |
2.611 |
|
6 |
B (2) |
18.80±0.86 |
93.2 |
2.615 |
20.13±0.95 |
92.9 |
2.715 |
16.87±0.66 |
75.5 |
13.12* |
15.60±0.67 |
76.7 |
11.34* |
|
7 |
B (3) |
19.17±0.62 |
95 |
2.53 |
20.15±0.95 |
92.9 |
2.68 |
15.73±0.62 |
70.4 |
16.70* |
12.43±0.70 |
61.1 |
18.24* |
|
8 |
B (4) |
12.07±0.36 |
59.8 |
30.35* |
13.27±0.42 |
61.2 |
29.77* |
12.77±0.35 |
57.2 |
36.43* |
13.33±0.45 |
65.6 |
23.10* |
|
9 |
C (1) |
12.27±0.58 |
60.8 |
21.10* |
13.53±0.29 |
62.4 |
36.82* |
11.80±0.60 |
52.8 |
27.37* |
9.33±0.33 |
45.9 |
44.68* |
|
10 |
C (2) |
11.47±0.65 |
56.9 |
21.17* |
13.87±0.25 |
64 |
38.21* |
11.43±0.42 |
51.2 |
36.99* |
10.91±0.37 |
53.7 |
35.62* |
|
11 |
C (3) |
18.95±0.73 |
94 |
2.69 |
20.10±0.95 |
92.8 |
2.768 |
17.27±1.53 |
77.3 |
5.628* |
18.70±1.20 |
91.9 |
2.295 |
|
12 |
C (4) |
18.87±0.81 |
93.5 |
2.617 |
20.17±0.91 |
93.1 |
2.753 |
20.90±0.90 |
93.6 |
2.619 |
18.97±0.81 |
93.3 |
2.759 |
|
13 |
Cipro |
20.17±0.29 |
100 |
0.000 |
21.67±0.25 |
100 |
0.000 |
22.33±0.29 |
100 |
0.000 |
20.33±0.27 |
100 |
0.000 |
|
14 |
DMF |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
Table 2: Antifungal activity of oxodiazole derivatives (Zone of inhibition, ZOI and % inhibition)
|
S. No |
Compound |
A. niger |
|
C. albicans |
|||
|
ZOI ± SD |
% Inhib |
t-value# |
ZOI ± SD |
% Inhib |
t-value# |
||
|
1 |
A (1) |
16.27±0.66 |
71.3 |
15.40* |
15.33±0.75 |
63 |
15.60* |
|
2 |
A (2) |
11.10±0.70 |
48.6 |
26.25* |
12.17±0.66 |
50 |
22.56* |
|
3 |
A (3) |
11.33±0.96 |
49.6 |
19.62* |
10.50±0.40 |
43.2 |
31.04* |
|
4 |
A (4) |
9.83±0.81 |
43.1 |
25.74* |
9.50±0.70 |
39 |
26.70* |
|
5 |
B (1) |
15.63±0.25 |
68.5 |
30.12* |
16.20±0.27 |
66.6 |
19.75* |
|
6 |
B (2) |
12.50±0.41 |
54.8 |
34.00* |
14.43±0.66 |
59.3 |
18.37* |
|
7 |
B (3) |
13.40±0.29 |
58.7 |
37.18* |
13.27±0.81 |
54.5 |
18.33* |
|
8 |
B (4) |
20.50±1.50 |
89.8 |
2.628 |
21.93±1.53 |
90.1 |
2.495 |
|
9 |
C (1) |
21.33±0.91 |
93.4 |
2.633 |
22.55±0.95 |
92.7 |
2.665 |
|
10 |
C (2) |
20.45±1.45 |
89.6 |
2.772 |
22.07±1.15 |
90.7 |
2.636 |
|
11 |
C (3) |
15.50±0.30 |
67.9 |
28.47* |
14.73±0.66 |
60.5 |
17.81* |
|
12 |
C (4) |
12.43±0.33 |
54.4 |
38.60* |
13.57±0.81 |
55.8 |
17.84* |
|
13 |
Fluconazole |
22.83±0.33 |
100 |
0.000 |
24.33±0.66 |
100 |
0.000 |
|
14 |
DMF |
– |
– |
– |
– |
– |
– |
Results and Discussion
The primary focus of this study was the synthesis of a number of substituted 1,3,4-oxadiazole derivatives (Scheme 1, 2, and 3). Excellent yields of 60-90% were obtained for all of the synthesized compounds. Spectroscopic (IR and 1 H-NMR) approaches were used to verify the structures of all freshly synthesized derivatives. All synthesized derivatives had analytical and spectral data that agreed completely with their postulated structures.
Antibacterial and antifungal properties were tested for all synthesized compounds. The inhibitory effects ranged from mild to strong across all of the substances. From what we can tell from the screening findings, several of these compounds have far stronger antibacterial and antifungal properties than the standard medications.
When compared to Ciprofloxacin, the inhibitory activity of compounds A (1), A (3), B (1), B (2), B (3), C (3), and C (4) was shown to be between 75% and 95% more effective against Staphylococcus aureus and Bacillus substilis. The inhibitory effects of compounds A (2) were moderate, at 65-74%, while those of compounds A (4), B (4), C (1), and C (2) were weak, at 50-64%.
Inhibitory action against Escherichia coli was increased by 75-95% when comparing compounds A (1), A (3), B (1), B (2), C (3), and C (4) to Ciprofloxacin. Compound B (3) exhibited moderate activity (66-77 percent inhibition), whereas compounds A (2), A (4), B (4), C (1), and C (2) all shown inhibitory effects in the 50-64 percent range.
Inhibitory activity against Pseudomonas aeruginosa was greatest for compounds B (1), B (2), C (3), and C (4) (Table 1), whereas the other compounds exhibited moderate to weak activity.
Aspergillus niger and Candida albicans were shown to be susceptible to killing by some of the chemicals in the aforementioned series. Compounds B (4), C (1), and C (2) showed the greatest effectiveness (75-95% inhibition). Inhibition rates between 50% and 74% were observed with other drugs against the aforementioned fungal species (Table 2).
According to the results shown above, antimicrobial activity are modified by the presence of a heterocyclic nucleus.
Acknowledgement
We are thankful to HOD, Chemistry, Lachoo Memorial College, Jodhpur, India for providing us with valuable lab facilities to conduct our research work.
Conflict of Interest
The authors declare no conflict of interest in the present work.
References
- Sengupta P.; Mal M.; Mandal S.; Singh J.; Maity T. K., Iran. J. Pharma. Therap., 2008, 7(2), 165.
- Bhardwaj N.; Saraf S. K.; Sharma P.; Kumar P.; E-J. Chem., 2009, 6 (4), 1133.
CrossRef - Kadi A. A.; El-Brollosy N. R.; Al-Deeb O. A.; Habib E. E.; Ibrahim T. M.; El-Emam A. A., Eur. J. Med. Chem., 2007, 42 (2), 235.
- Gao Q.; Liu S.; Wu X.; Zhang J.; Wu A., Org. Lett., 2015, 17, 2960.
CrossRef - Ingole S. P.; Mohane S. R.; Berad B. N., Asian J. Chem., 2007, 19, 2683.
- Amir M.; Javed S. A.; Kumar H., Ind. J. Chem., 2007, 46B, 1014.
- Siddiqui N.; Khan S. A.; Bhat M. A., Ind. J. Het. Chem., 2005, 14, 271.
- Shivarama H. B.; Poojary K. N.; Subrahmanya B. K.; Ashok M.; Poojary B., Ind. J. Chem., 2005, 44B, 1669.
- Mishra A.; Singh D. V.; Mishra R. M., Ind. J. Het. Chem., 2005, 14, 289.
- Franski R., Asian J. Chem., 2005, 17, 2063.
- Reddy V. M.; Reddy P. S.; Reddy P. C.; Ratmanc V., Ind. J. Het. Chem., 1997, 7, 17.
- Schlecker R.; Thieme P. C., Tetrahedron 1988, 44, 3289.
CrossRef - Erik S.; Srinivas O.; Kavya R.; Neamati N., Retrovirology, 2009, 6, 25.
- Partyka R. A.; Crenshaw R. R. U. S., Pat., 1977, 4001238.
- Vardan S.; Mookherjee S.; Eich R., Clin. Pharm. Therp., 1983, 34(3), 290.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









