Synthesis and Application of Tamarind 4-Amino Butyric Acid (TABA) for Removal of Heavy Metal Ions from Industerial Effluent’s
Department of Chemistry, Jai Narain Vyas University, Jodhpur 342001, India.
Corresponding Author E-mail: reujoshi5000@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/390428
Article Received on : 09 Jun 2023
Article Accepted on : 17 Aug 2023
Article Published : 29 Aug 2023
Reviewed by: Dr. ALFA-SIKA M. Seyf Laye
Second Review by: Dr. Yashas S R
Final Approval by: Dr. S. A. Iqbal
4-Aminobutyric acid (TABA), a functional group that is incorporate in tamarind kernel powder to synthesized of TABA resin in laboratory. By employing batch approach and column separation experiment, their adsorption behavior for hazardous metal ion as Fe (II), Cr (III) and Cu (II) has been discovered. Various physiochemical properties as Ion exchange capacity, bulk density, SEM, and FTIR spectra were used to characterize the tamarind-based TABA resin. Industrial effluents which contaminated with heavy toxic metal ions are extracted using TABA resin. This paper emphasis that, the resin is used to remove heavy metal ions like Fe (II), Cr (II), and Cu (II) from aqueous solution and effluents of steel industry, Jodhpur. This paper present the highest % adsorption of Fe (II) metal ion and binary separation of Cr (III) and Cu (II) ions on TABA resin at pH 6. Maximum ion exchange capacity was found 3.38 milimol/gm and adsorption is 70.43% on TABA resin.
KEYWORDS:Contamination; Distribution coefficient; Hazardous metal ions; ion exchange capacity; TABA resin; Wastewater
Download this article as:| Copy the following to cite this article: Joshi R, Gaur S, Singh A. V. Synthesis and Application of Tamarind 4-Amino Butyric Acid (TABA) for Removal of Heavy Metal Ions from Industerial Effluent’s. Orient J Chem 2023;39(4). |
| Copy the following to cite this URL: Joshi R, Gaur S, Singh A. V. Synthesis and Application of Tamarind 4-Amino Butyric Acid (TABA) for Removal of Heavy Metal Ions from Industerial Effluent’s. Orient J Chem 2023;39(4). Available from: https://bit.ly/44tNJrO |
Introduction
Water contamination due to the dumping of heavy metals continues to be a major concern round the world. The treatment of unclean business waste water consequently continues to be a supply of world situation because waste reclaimed water from municipalities, communities, and enterprise need to finally be back to receiving or the land 1.
Diverse commercial waste waters, inclusive of those produced by means of steel plating operations, mining companies, battery production strategies, paint and pigment manufacturers, as well as the ceramic and glass industries, can comprise heavy metal infection. Cd+2, Pb+2, Zn+2, Ni+2, and Cr+2 are extensively found in business waste water2. When unsafe heavy metals are launched into the environment, steel ions can input people’s bodies immediately or not directly thru food.
As a result, heavy metals need to not be allowed to enter the natural surroundings 3. Chemical precipitation, coagulation, ion trade, solvent extraction, filtering, evaporation and membrane techniques 4 have all been used to do away with hazardous heavy metals from water systems.
Effect of Heavy Metal Polluted Industrial Effluent
When released into the environment, untreated wastewater containing toxic heavy metals reasons a whole lot of fitness and environmental troubles. The most harmful heavy metals consist of the ones like copper, nickel, and chromium which can be poisonous to human fitness. Fish and aquatic flowers are immediately impacted by using heavy metals considering the fact that they are extremely soluble in liquid environments and are therefore without difficulty absorbed via living things.
As those species pass up the food chain, people emerge as the very last clients and our our bodies begin to gather huge concentrations of heavy metals. Even even as heavy metals are sometimes essential for human health, ingesting them at uncontrolled ranges can have fundamental negative results on our health. For instance, if ate up in big doses, arsenic is a dangerous detail that could result in cancer, organ damage, stunted increase, or even loss of life. Heavy metals like lead or mercury can cause autoimmunity, a circumstance wherein the frame’s immune system attacks its personal cells.
This also reasons fundamental health conditions like kidney failure, arthritis, and nervous system disintegrate, and mind failure in the foetus of a pregnant woman. In reality, developing youngsters honestly consume large concentrations of heavy metallic thru ingestion than adults due to the fact they eat extra and soak up extra from their food on the way to gain weight even as they are growing,
Arsenic, for instance, is a risky substance which can purpose cancer, organ harm, stunted increase, and even loss of life if consumed in huge quantities. A sickness called autoimmunity, wherein the body’s immune system destroys its own cells, can be delivered on via heavy metals like lead or mercury.
This results in critical fitness troubles for the unborn toddler of a pregnant mom, inclusive of kidney failure, arthritis, frightened machine damage, and mind failure. Due to the truth that developing youngsters devour and soak up more in their food so one can benefit weight than adults, children sincerely consume a higher concentration of heavy metals than adults.
According to a examine, heavy metals in business effluent represent a substantial risk to the soil and the vegetation that develop there. When humans or animals eat these polluted plants, the heavy metals can then input the meals chain and reason considerable damage 5.
Kidney failure is regularly caused by heavy steel ion overdoses from veggies or land-grown end result that builds up in the kidneys.
For instance, high copper concentrations in water our bodies, especially drinking water, can lead to anemia, vomiting, diarrhea, and in extreme instances, kidney and liver harm, no matter the truth that copper is a vital nutrient for people. Copper toxicity has been connected to gastro-intestinal problems, hepatotoxic results, and nephron poisoning 6. Copper’s excessive toxicity reasons irritation of the primary fearful device, as well as damage to blood capillary mucosa.
Toxicity of Heavy Metal Ions
Heavy metals, similarly to posing huge dangers to people, additionally directly harm soil ecosystems because of their toxicity. Plants developing in polluted soils may additionally enjoy slowed increase, photosynthetic inhibition, decreased chlorophyll manufacturing, and reduced enzyme hobby. Heavy metals in excessive concentrations within the soil impair plant vitamins absorption, metabolic sports, chlorosys, and other procedures. Additionally, the great of receiving water our bodies, consisting of rivers and seas; will be impacted through heavy metal-polluted commercial effluent. This impact varies and is largely based on the chemical composition of the effluent launched via industries 7.
In aquatic ecosystems, dissolved heavy metals reduce the dissolved oxygen degree, ensuing in a drop in aquatic population 8.
In current years, there was a lot of interest in the removal of risky heavy steel ions from sewage, business, and mining waste effluents 9.
It turned into found that their presence in lakes and streams produced a wide range of health troubles in animals, flora, and people. Due to their bioaccumulation, non-biodegradability, and carcinogenic characteristics 10.
Cadmium toxicity
Cadmium can be present in electroplating wastewater, nickel manufacturing, cadmium batteries, fertilizers, insecticides, pigments and dyes, and textile activities 11, 12.
It has a prolonged existence cycle inside the food chain and isn’t biodegradable. At 15 mg Cd2+/L in humans, nausea and vomiting were visible, whereas at zero.05 mg Cd2+/L there were no terrible effects. There had been reviews of severe toxic however non-deadly signs at cadmium concentrations of 10–326 mg Cd2+/L.
Copper toxicity
A high fever is normally present with excessive ingestion of copper, which causes some of acute and chronic ailments in humans, along with hemochromatosis, gastrointestinal catarrh, clavicular cramps, and skin rashes brass chills 13, 14. Some of the biggest assets of copper in waste water encompass pulp and paper mills, fertilizers, petroleum refineries, primary metal paintings foundries, nonferrous metal industries, motors, and air craft plating and finishing 15-17.
Nickel poisoning
Even in very low quantities, nickel (II) may be located within the mining, electroplating, pigments, and ceramics sectors, as well as inside the manufacturing wastes from batteries and accumulators18. Nickel is toxic to a number of aquatic life. The Environmental Protection Agency (EPA) units a restriction of 0.04 mg/L for nickel in ingesting water because lung and nasal sinus cancer are the maximum not unusual destructive health results of nickel19.
Toxicity of Iron
Iron is a crucial element in lots of soils, specially clay soils wherein it’s far commonly a widespread component. The United States Geological Survey (USGS) reviews that the amount of dissolved iron in Lowa’s ground water can variety from much less than 1 mg/L to more than 20 mg/L (USGS Ground Water Monitoring Data).
Both domestic and business water resources include an unpleasant substance referred to as iron. When iron is laundered, it can discolour garb and plumbing substances and exchange the flavor of beverages. The EPA Red Book20 promoted a 0.3 mg/L widespread for iron in ingesting water.
Even though diffused iron is extra bioavailable and toxic to aquatic life, particle iron, when suspended in water, can be dangerous to fish and other aquatic lifestyles. Particulate iron can shape flocculants at the bottom of streams, killing bottom-dwelling organisms, plant life, and fish eggs that are incubating. Therefore, the whole iron requirement of 1 mg/L is implemented.
Ion Exchnage Adsorption Technique
Ion change reactions do away with dissolved elimination from aqueous solution and update them with different identically charged ions in a reversible chemical response. It is usually used for water softening in water remedy, in which Water, however, is depleted of calcium and magnesium ions. Other dissolved ionic species are increasingly being removed via ion change techniques.
Background
The ion alternate phenomena are a completely vintage incidence. This prevalence was first found numerous hundreds of thousands of years ago in many parts of the world. The earliest references can be observed inside the Bible, in which Moses is credited with performing an ion exchange method to transform brackish water into potable water and Aristotle with coming across that saltwater loses some of its salt content when percolated thru positive sand. Ancient Egyptians, Greeks, and Chinese people used soils, sands, natural zeolites, and flowers as equipment for improving the first-class of the water, desalting, and softening without having a systematic knowledge of the phenomenon21, 22.
There are guidelines inside the Bible and historical Greek resources that the historic Greeks knew how to desalt brackish water via ion change. It wasn’t until the early 1900s that the primary reputable studies at the mechanism of ion exchange become posted. In 1850, Harry Thompson and John Way, two agricultural chemists, combined a soil pattern with a liquid fertilizer answer that contained ammonia. The soil absorbed the ammonia even as discarding the calcium23, 24.
They said a number of vital observations in their reviews to the Royal Agricultural Society, which function a basis for information the ion exchange mechanism.
In soil, ion trade entailed the interchange of similar ions.
Some ions interchange extra easily than others.
The aluminum silicates in the soil supplied it with change properties.
Ion exchange was not the same as actual physical absorption.
In 1858, German chemist Eichorn verified that ion change procedures in soil are reversible25. In 1876, Lemberg mounted the reversibility of the system and predicted its stoichiometry. The implementation of ion exchange for sensible methods commenced at the flip of the 20th century.
Dr. Gans of Germany changed into the primary to apply an ion change (processed natural zeolite) on a big scale, based totally on the clinical expertise and technological maturity. In 1905, he commercialized a technique of softening water (through replacing magnesium and calcium ions for sodium ions) the usage of cation alternate materials.
Gans hired cation exchangers fabricated from artificial sodium aluminosilicates, which he dubbed zealots26. Around hundred years ago, a Swedish geologist named Gonstedt coined the time period zeolites to describe clearly produced siliceous minerals that have become dehydrated whilst heated. The word zeolite comes from the Greek words zein and lithos, which imply “boiling rock” in English. Although real sodium aluminositecates minerals at the moment are every so often applied, the phrases zeolite and sodium zeolite softener are nevertheless extensively used today. In 1913, the Permuitt Company brought the first synthesized zeolites to the American market.
In 1917, Follin and Bell27 used a artificial zeolite to accumulate and separate ammonia from urine for the primary time. Strong proponents of ion change, however, originated from the industrial facet, no longer the scientific community.
Polysaccharides
Polymeric carbohydrates referred to as polysaccharide molecules are composed of lengthy chains of monosaccharide units connected by means of glycosidic linkages, which, upon hydrolysis, launch monosaccharide. They may have linear or appreciably branched structures, amongst others.
Polysaccharides are often heterogeneous, with functional organizations which have been somewhat changed. Polysaccharides are modified to get a mix of features which can be appropriate for a sure software and to boom their versatility.
Ion exchange method28–33 has acquired numerous interests lately and has emerged as one of the most popular techniques for eliminating heavy metals from business effluent. A strong and insoluble matrix is required for green adsorption, and newly invented polysaccharide substances have been shown to be such materials34.
The fictionalization of a polysaccharide matrix with exclusive chelating features has been used to demonstrate the removal of metallic ions from aqueous answer35, 36. Utilizing chelating ion trade resins for metallic ion adsorption is a green system.
It is a great analytical strategy since it avoids the use of heavy chlorinated natural solvents, which are regularly utilized in traditional liquid-liquid extraction strategies and different procedures. Polysaccharides, which might be hydrophobic functionalized polymers, have been the focal point of research on cation and anion exchange resin. These resins provide a extensive range of operating conditions as well as accurate metallic ion stability. Polysaccharide-based resins are gaining popularity.
Higher selectivity in compared to other polymers due to the fast adsorption of metallic ions.
Materials and Methods
How is Tamarind Kernel Powder made?
Tamarind seed powder is created as a byproduct of tamarind seed processing, which incorporates the following steps:
Harvesting: The mature tree’s pods or kernels are harvested once they attain maturity.
Sorting: The seeds are removed from the harvested pods with the aid of breaking them apart and removing rotten seeds, debris, and stones, while preserving the brilliant seeds.
Roasting: The wholesome seeds are heated, causing the seed coat to emerge as brittle while having little have an effect on on the endosperm.
Stripping: After the seed coats have been roasted, it is simple to separate the endosperm from the seed coats the usage of a system referred to as stripping.
Grinding: The endosperm is beaten or powdered to create tamarind kernel powder after being separated from the germ.
Screening: To make tamarind gum powder, additionally called goma tamarind in Spanish, the tamarind seed powder is first screened over again to provide a quality powder. This great powder is then combined with a solvent.
Chemical composition and structure of Tamarind kernel powder, the supply of gum, replicates cereals with 12.7-15.4 percent protein, 3-7.5 percentage oil, 7-8.4 percent crude fiber, 61-72.2 percent carbohydrates, and 2.45-3.3 percentage ash. On a dry weight basis, all calculations had been made37. In phrases of chemistry, tamarind kernel powder is a branched chain carbohydrate polymer.
The predominant components of TKP, a polymer with a mean molecular weight of 52350 Daltons, are glucose, galactose, and xylose in a 3:2:1 molar ratio. A polymer is made of a cellulose-like spine with xylose and galactoxylose substituents. Xylose (1-6 linked) residues take the place of about eighty% of the glucose residues, and p-1–2 galactose replaces the ultimate 20%.
Synthesis of Tamarind 4-Amino Butyric Acid (Taba) Resin
Preparation of epoxy propyl ether of 4-amino butyric acid
5.1 gm of butyric acid (0.1 mole) was dissolved in minimum amount of methanol in a round bottom flas. 9.25ml (0.10 mole) of epichlorohydrine was added in the above solution and reaction mixture was stirred for 4 hrs on magnetic stirrer. 4 gm of sodium hydroxide (0.1mole) was added in reaction mixture and reaction mixture was further stirred for 5hrs on magnetic stirrer and left it overnight.
Preparation of tamarind 4-amino butyric acid resin
In a round bottom flask, 81 g of tamarind (0.5 mole) was combined with dioxane and 4 gm (0.1mole) NaOH solution added and stirred on magnetic stirred at 65OC. After this, butyric acid was added and mixture was stirred continuously for 4.5hrs at 65OC. After being washed with methanol and HCl to remove any remaining inorganic impurities and to neutralize any excess alkali. Tamarind 4-amino butyric Acid resin has a 116 gram yield.
 |
Figure 1: Reaction scheme of TABA resin synthesis |
Preparation of Fe (II) stock solution
The stock solution of Fe (II) metal ion was ferrous ammonium sulphate (NH4)2Fe (SO4)2.6H2O. To make 1000 ppm Fe (II) solution, 7.02 gm ferrous ammonium sulphate was mixed in 2 ml con. H2SO4 and the capacity were increased to 1000 ml.
Physiochemical Characterization
Physicochemical characterization Newly synthesized cellulose and tamarind based resin are characterized by various methods as followings:
Absorbance of Iron Metal Ion
Table 1: Absorbance of iron metal ion
|
S.NO. |
CONC.(ppm) |
ABSORBANCE |
|
1 |
2ppm |
0.123 |
|
2 |
4ppm |
0.222 |
|
3 |
6ppm |
0.323 |
|
4 |
8ppm |
0.434 |
|
5 |
10ppm |
0.555 |
|
6 |
12ppm |
0.676 |
|
7 |
16ppm |
0.785 |
|
8 |
18 ppm |
0.896 |
 |
Figure 2: Calibration Curve of Absorbance of Iron |
SEM Characterization of TABA resin
SEM micrographs of surface of synthesized ion exchange resin (TABA) were presented in following fig no. 3; show that resins present the Cavities to the level of their surface area. Surface show heterogeneity and varied structures. The adsorbent is porous in nature. Surface of resin show heterogeneity and varied structures. The adsorbent is porous in nature.
 |
Figure 3: SEM Characterization of TABA |
FTIR Spectra of Taba Resin
The characteristics bands observed in the FTIR spectra of TABA resin as 3276 cm-1 due to OH group and hydrogen bond, 3011 cm-1,2937 cm-1due to carboxylic acid group, -NH2 group show at 1900 cm-1, and 1572cm-1, 1428cm-1, 1338cm-1,1170cm-1 due to asymmetric and symmetric stretching.
 |
Figure 4: FTIR of TABA resin |
Determination of moisture content of TABA Resin
In a crucible, 1.0 gram of dry resin was placed and dried to a consistent weight in a vacuum desiccator at 80°C for 24 hours. The resin was then weighed, yielding the following result
The weight of dry resin = 0.88
Weight of the moisture = 0.12
% Moisture contain = 12 %
Ion exchange capacity of TABA resin
The total amount of charge or active ion groups per unit weight of material determines the resin’s ion exchange capacity. The ion exchange capacity of the exchanger increases as the number of active ions increases. In milli moles per gram of exchanger, the total ion exchange ability (IEC) of resin is stated.
The number of milli-moles of sodium ion absorbed by 1 gram of dry resin in hydrogen form can be used to determine a cation exchanger’s ion exchange capacity in the lab. The quantity of Cl– ion taken up by 1 gm of dry polymer in the OH– form is used to determine the anion exchange capacity of the anion exchanger.
Large ions may not be assimilated by moderate cross-linked.
Process
The resin capacity was determined using a back titration process. In an Erlenmeyer flask, 1.0g of resin was placed and 200 ml of standardized NaOH (0.05N) containing 5ml of 5% NaCl solution was then added and allowed to stand overnight. Using indicator, a 25ml aliquot of supernatant liquid was back titrated with 0.05N HCl standard solution (phenolphthalein). The formula was used to compute the ion exchange ability of the newly synthesized resin.
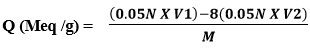
V1 volume of 0.05N NaOH (ml)
V2 Volume of 0.05N HCl (ml)
M Weight of dry resin (gm)
Q (Meq/g) =(0.05x 200) – 8(0.05×14.66) / 0.88
= 10-8(0.728) / 0.88
= 10-8(0.728)/ 0.88
= 3.38
Nitrogen content in TABA resin
The Kjeldahl’s method was used to estimate the amount of nitrogen in newly produced resins.
Procedure
In a dry kjeldahl’s flask, 0.25 gm of resin was added, along with 10 ml con. H2SO4 (18N), 0.5 gm of catalyst (homogeneous mix of 5 gm metallic selenium, 120 gm CuSO4, and 150 gm potassium sulphate). The mixture was heated or boiled until it became transparent, then left to cool for 2 hours. The solution was chilled and transferred to a distillation device with 30 mL of distilled water for ammonia measurement. After adding 12 mL of 10N NaOH, the flask’s total capacity was increased to 75 cm3. Ammonia-free steam was distilled for 5 minutes in a receiver holding 5 cm3 of 4% boric acid and 5-6 drops of indicator (75 grammes of bromocresol green and 50 grammes of methyl red diluted in 100 grammes of ethanol). 0.05M HCl was used to titrate the distilled ammonia.
1ml 0f 0.05N HCl = 0.7003 mg of N
Amount of resin = 0.25 gm
Volume of 0.05N HCl = 6.9x 0.7003 = 4.83
= 4.83 mg N / 0.25 g resin
= 1.93%
pH Titration of TABA resin
The tamarind derivative products were first converted to H+ ion form, then rinsed with water in order to remove excess acid, and dried overnight at 55-60 OC. 0.1 gram of resin was obtained from each and placed in one of seven flasks, with a reducing amount of 1 N NaCl solution and an increasing amount of 0.1N NaOH added to each flask. Finally, de-ionized water was added to each flask to make the total capacity of each flask 25 ml.
The magnetic stirrer was used to equilibrate all firmly closed flasks until the solution reached the final pH value. The pH value from each was determined using a digital pH meter, and the results are listed in the table below. pH vs. meq of alkali /g of resin curve was plotted for each resin.
Table 2: pH Titration of Tamarind 4-Amino Butyric Acid (TABA) Resin
|
flask no. |
0.1 N NaOH (ml) |
1M NaCl (ml) |
deionized water (ml) |
pH |
|
1 |
0.0 |
3.2 |
20.9 |
2.4 |
|
2 |
0.4 |
3.1 |
20.6 |
3.7 |
|
3 |
0.8 |
2.5 |
20.2 |
4.3 |
|
4 |
1.2 |
1.8 |
19.9 |
4.8 |
|
5 |
1.6 |
1.5 |
19.5 |
5.7 |
|
6 |
1.8 |
1.1 |
19.2 |
6.8 |
 |
Figure 5: pH Titration curve of TABA resin |
Observation
Distribution Coefficient
The following formula was used for calculating the distribution coefficient:

Method of Batch
The resins were placed in a stopper conical flask with 1.0 ml per liter analyze (equal to 1.0 mg metal ions) and an adequate buffer volume for pH adjustment throughout the batch operation. In a temperature-controlled shaker, the mixture was shaken continuously for 4-5 hours at 24-30 OC, and the containments were equilibrated. To filter the solution, what man filter paper no. 40 or 42 was used.
The mixture was filtered by what man filter paper no. 42 after the residual on the filter paper was equilibrating with 4N HCl. A Perkin-Elmer model 2380 atomic absorption spectrophotometer was used to calculate the amount of hazardous metal ions in the filtrates and residue.38 By examining a number of standard solutions of different metal ions using AAS, the calibration curve for distinct metal ions was plotted.
Adsorption % = ( Ci – Cf ) / Ci x 100
In this formula, Ci stands for the metal ion’s initial concentration in solution and Cf for the metal ion’s final concentration in solution once the resin is in equilibrium.39 Plots were made of the calibration curves for various metal ions.
TABA resin chelation of Fe (III) metal ion
To generate buffers with the required pH range of 2 to 8, applicable quantities of 0.2 M sodium acetate and 0.2 M acetic acid were added to numerous glass stopper conical flasks. Other glass stopper conical flasks received a suitable amount of 0.2 M NH4OH and 0.1 M NH4Cl to achieve a pH of 8.0. Each flask was filled with 0.070g dry TABA resin and 1 ml Fe (III) solution at 1000 ppm. After 1 hour of magnetic stirrer equilibration, the contents were filtered. AAS was used to determine the iron concentration of the filtrates. The findings are summarized in Table 3.
Table 3: Kd Value of Iron Metal Ion on Taba Resin
|
pH |
Absorbance |
concentration (ppm) |
metal ion in filtrate(mg) |
metal ion in resin(mg) |
Kd |
% absorbance |
IEC(mg/g) |
|
2 |
0.543 |
8.67 |
0.311 |
0.6889 |
2672.90 |
68.89 |
9.12 |
|
3 |
0.435 |
8.55 |
0.301 |
0.6990 |
2634.90 |
69.90 |
9.34 |
|
4 |
0.213 |
6.89 |
0.301 |
0.6998 |
2546.87 |
69.98 |
9.56 |
|
5 |
0.112 |
6.97 |
0.2989 |
0.7011 |
2343.89 |
70.11 |
9.89 |
|
6 |
0.034 |
6.65 |
0.2957 |
0.7043 |
3112.89 |
70.43 |
10.23 |
|
7 |
0.045 |
7.89 |
0.299 |
0.7010 |
2786.34 |
70.10 |
10.11 |
|
8 |
0.321 |
7.998 |
0.299 |
0.7001 |
2676.45 |
70.01 |
9.78 |
Binary Seperation of Cu (Ii) nd Cr (Iii) Mixtures using Taba Resin
Metal ions are removed from solutions through ion exchange. The majority of ion exchange operations take place in a column. We attempted to separate a combination of inorganic metal ions on resin using the distribution coefficient values acquired by batch technique at various pH levels.
A glass tube with an internal diameter of 1.6 cm is inserted into the column.
DMF was used to swell the resin. To make a homogenous layer, the swelling resin was allowed to settle 40. The height of the column was about 4-5cm, and the resin utilised was roughly 5.0 gm.
Table 4: Absorbance of Copper Metal Ion
|
S.NO. |
CONCENTRATION |
ABSORBANCE |
|
1 |
2 |
0.122 |
|
2 |
3 |
0.232 |
|
3 |
4 |
0.322 |
|
4 |
5 |
0.422 |
|
5 |
6 |
0.522 |
|
6 |
7 |
0.612 |
 |
Figure 6: Calibration curve of copper metal ion |
Table 5: Absorbance of Chromium Metal ion
|
S.NO. |
CONCENTRATION |
ABSORBANCE |
|
1 |
2 |
0.323 |
|
2 |
3 |
0.443 |
|
3 |
4 |
0.543 |
|
4 |
5 |
0.654 |
|
5 |
6 |
0.763 |
|
6 |
7 |
0.874 |
 |
Figure 7: Calibration Curve of Chromium Metal ion |
Table 6: Concnetration of Copper Metal ion
|
S.NO. |
VOLUME OF ELEUNT |
ABSORBANCE |
CONCENTRATION |
|
1 |
2 |
0 |
0 |
|
2 |
3 |
0 |
0 |
|
3 |
4 |
0 |
0 |
|
4 |
5 |
0.11 |
0.056 |
|
5 |
6 |
0.12 |
0.054 |
|
6 |
7 |
0.23 |
0.778 |
|
7 |
8 |
0.33 |
1.66 |
|
8 |
9 |
0.15 |
1.56 |
|
9 |
10 |
0.023 |
1.44 |
|
10 |
11 |
0.22 |
0.87 |
|
11 |
12 |
0 |
0.33 |
|
12 |
13 |
0 |
0 |
Table 7: Concentration of chromium metal Ion
|
S.NO. |
VOLUME OF ELEUNT |
ABSORBANCE |
CONCENTRATION |
|
1 |
2 |
0 |
0 |
|
2 |
3 |
0.002 |
0.33 |
|
3 |
4 |
0.003 |
0.45 |
|
4 |
5 |
0.675 |
0.76 |
|
5 |
6 |
0.777 |
1.56 |
|
6 |
7 |
0.876 |
1.78 |
|
7 |
8 |
1.233 |
1.99 |
|
8 |
9 |
0.055 |
1.33 |
|
9 |
10 |
0.342 |
0.54 |
|
10 |
11 |
0.11 |
0.23 |
|
11 |
12 |
0 |
0.1 |
|
12 |
13 |
0 |
0.1 |
Table 8: Binary Seperation of Mixture of Copper and Chromium Metal Ion on TABA Resin
|
volume (ml) |
Cu(ppm) |
Cr(ppm) |
|
3 |
0 |
0 |
|
6 |
0 |
0.33 |
|
12 |
0 |
0.45 |
|
15 |
0.056 |
0.76 |
|
18 |
0.054 |
1.56 |
|
21 |
0.778 |
1.78 |
|
24 |
1.66 |
1.99 |
|
27 |
1.56 |
1.33 |
|
30 |
1.44 |
0.54 |
|
33 |
0.87 |
0.23 |
|
36 |
0.33 |
0.1 |
|
39 |
0 |
0.1 |
 |
Figure 8: Binary separation of Cu and Cr metal ion on TABA resin |
Results
Fe (III) metal ions have a molar distribution coefficient of 3112.89 with TABA resin and a percentage absorbance of 70.43 at their maximum pH of 6. Chromium metal ion exhibits greatest% removal as compared to copper in binary separation of two metal ions like copper and chromium on TABA resin.
Discussions
To investigate the effects of pH on metal ion adsorption, the pH was changed from 2.0 to 8.0. Free metal ion absorption is pH-dependent, with optical heavy metal adsorption41-43 beginning at pH 6 and decreasing as pH rises. When the pH was raised from 2.0 to 6.0, metal ion adsorption on TABA resin increased.
The adsorption of metal ions on TABA resin improved when the contact time was increased from 20 to 120 minutes. Other variables including the pH of the solution and the spinning speed were kept at optimal values, while the temperature was fixed at 250 degrees Celsius. The findings reveal that heavy metal adsorption occurs in two stages: one in which the rate of adsorption % is very high (56 to 75 percent of heavy metals adsorbed on TABA resin in 60 minutes) and the other in which the rate of adsorption % is very low (56 to 75 percent of heavy metals adsorbed on TABA resin in 60 minutes). After then, there is a second stage with a much lower adsorption rate.
TABA resin is treated at temperatures ranging from 25 to 60 degrees Celsius.
Conclusions
Heavy metal ion removal from industrial effluents using resins made of tamarind polysaccharide has been proven to be successful such as Fe (III), Cu (II), and Cr (III) metal ions. Tamarind 4- amino butyric acid (TABA) resin is today regarded as one of the most effective and intriguing methods due to its eco-friendliness, low cost, and quickness. Di-vinyl benzene44 is used to create the majority of resins, but TABA resin is now preferred because of its speed and low cost.
Acknowledgement
I would like to express my gratitude to Dr. Aresh Vikram Singh, professor in the department of chemistry at Jai Narain Vyas University in Jodhpur, for his wonderful guidance, motivation, and recommendations. Beyond words, he is good and kind. He has always supported my scholarly efforts.
Conflict of Interest
There are no conflict of interest
References
- Weber, W.J.; Gintely, P.M.; Katz, L.E. J. Water Res., 1991, 25, 499-599.
CrossRef - Argun, M.E.; Dussuh, S. Bioresource Tech., 2008, 7, 2516-2527.
CrossRef - Meena, A.K.; Kadirvely, K.; Mishra, G.K.; Rajagopal, C.; Nagar, P.N. Hazard Mater.,2008,150,604-611.
CrossRef - Panayotora, M.; Velkov, B. Environ Sci. Health a Toxic Hazard Subst. Environ Eng., 2003, 38,545-554.
- Saidi, M. International Journal of Environmental Sciences. 2010, 1,666-676.
- Shrivastava, A.K.Indian J Environ Protect. 2009,29,552-560.
- Kpor, O.B.; Ohiobor, G.O.; Olaolu, T.D. Advances in Bioscience and Bioengineering. 2014, 2, 37-43.
CrossRef - Moore, J.W.; Ramamoorthy, S. Springer verlag, New York., 1984, 252-254.
- Lement, R.E.; Eiceman, G.A.; Koester, C. J. Environmental Analysis. 1995,67.
CrossRef - Cimino, G.; Caristi, C. Biol. Waste., 1990, 33, 201-210.
CrossRef - Cheung, C.W.; Porter, J.F.; Mckay, G. J. Chem. Technol. Biotechnol., 2000, 75, 963-970.
CrossRef - Mukherjee, A.G.Galgotia Publication., New Delhi 1986.
- Comp. T.R. Reinhold, New York. 1964.
- Dalang, F.; Buffle, J.; Haerdle, W. Environ. Sci. Technol., 1984, 18,134-140.
CrossRef - Forstner, U.; Wittman, T.W. Springer., 1981.
- Jenkins, D.W.Willey Interscience, New York, 1976, 1.
- Parab, H.; Joshi, S.; Shenoy, N.; Lali, A.; Sarma, U.S.; Sudersanan, M. Process Biochem.,2006, 41, 609-615.
CrossRef - Cheng, P.X.; Ting, Y.P.; Chen, J.P.; Hong, L. J. Colloid Interf. Sci., 2004,275, 131-141.
CrossRef - Gerhardt, A. Hydrobiologia., 1995, 306, 229-240.
CrossRef - Laura, A. J. Chemical Engineering. 1927., 25,7.
- Thompson, H.S.; Roy I. Agr. Soc. Engl., 1850, 11, 68.
- Way, J. T.; Roy, I. J. Agr. Soc. Engl., 1850, 11,313.
- Eichorn H. J.Ann. Physic. Chem., 1858,105,126.
- Gans R. Jahrb. Press. Geol. Landesantalt (Berlin). 1905, 26,179.
- Harms, F.; Rumpler A. Pure Appt. Chern., 1903, 50.
- Adams, B.A.; Holms, E. L. J. Soc. Chem. Ind., 1935, 54, 11.
- Sharma, R.K. Pure Appli. Chemi. 2001, 73,181-186.
CrossRef - Singh, A.V.; Soni, D.K.; Kumawat, I.K. Water and Environ. J., 2012, 26, 371-380.
CrossRef - Roy, P.K.; Rawat, A.S.; Choudhary, V. J. Appl. Polym.Sci., 2004, 94, 1771-1779.
CrossRef - Porath, I.; Fornstedt, N. J. Chromatog A., 1970, 51, 479-489.
CrossRef - “Guar Industry Outlook 2015”, Report, (NIAM) and (NCDX), November.
- Santosh, K.GAIN Report Number: IN4035, USDA Foreign Agricultural Services 2014.
- Undersander, D.J.; Putnam, D.H.; Kaminski, A.R.; Kelling, K.A.; Doll, J.D.; Oplinger, E.S.; Gunsolus J.L. Alternative Field Crops Manual, Accessed on October 7, 2015.
- Sharma, P.; Gummagolmath, K.C.Agricultural Economics Research Review. 2012, 25, 37-4.
- Chowdhary, M. Patent 20020052298, U.S, 2002.
- Sano, M.; Miyata, E.; Tamano, S.; Hagiwara, N.; Shirai, T. Food and Chem Toxicol. 1996,, 463-7.
CrossRef - Nagar, S.; Singh, A.V. int j of scientific research. 2021,10,1-6.
- Nagar, S.; Singh, A.V. Research journal of chemical sciences., 2022, 12(1), 1-10.
- Bargujar, S. Polimetry, 2022, 67 (5).
CrossRef - Fernado, S. The royal society publishing, march, 2023.
- Zhiying, B. Journal of Ethnopharmacology, 2023,305.
- Fadel, D.A.; Bahy, S.M.EI.; Abdelaziz, Y.A. Desalination and Water Treatment. 2016,57, 53, 25718-25728.
CrossRef - Meyyappan, R. Desalination and water treatment. 2016,57,43.

This work is licensed under a Creative Commons Attribution 4.0 International License.










