Sunlight Induced Photogalvanics for Conversion and Storage of Solar energy: Coomassie Brilliant Blue-Isopropyl Alcohol-Sodium Lauryl Sulphate System
Photochemistry Laboratory, Department of Chemistry, Faculty of Science, Jai Narain Vyas University, Jodhpur, Rajasthan, 342001, India.
Corresponding Author E-mail: photochemistrylabjnvu@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/390432
Article Received on : 13 Feb 2023
Article Accepted on :
Article Published : 19 Jul 2023
Reviewed by: Dr.Ghaida Salman
Second Review by: Dr. Erum hussain
Final Approval by: Dr. K. R. Genwa
Research plan was proposed for systematic observation with scientific way in the solar cell field of photogalvanics. It was analysis of experimental work under the solar energy output. The study of photogalvanic was done for solar energy conversion and storage by using of dye as Coomassie Brilliant Blue CBB), reductant as Isopropyl alcohol (IA), and surfactant as Sodium Lauryl Sulphate (SLS). For this purpose, a specially designed H shaped photogalvanic system was used under investigation for innovative results. Different scientific instruments were used for methodology set up i.e., pH meter (digital), microammeter, and 200 Wt. W bulb (As light source), multi-meter, two electrodes (one was calomel and another was Pt), carbon pot 450 k, resistance key. Findings: The photogalvanic cells were studied using different parameters via photo potential, photocurrent, conversion efficiency, fill factor and cell performance. The above values are as follows: 533.0 mV, 201.0 uA, 0.8796 %, 0.3066 and 114.0 minutes. These cells were studied for the good results in solar energy field. Novelty: The observed results are very good over previously obtained results with respect to Coomassie brilliant blue, reductant as Isopropyl alcohol, and surfactant as sodium lauryl sulphate system.
KEYWORDS:Coomassie Brilliant Blue; Fill factor; Solar energy; Sunlight; Photosensitiser; Photocurrent; Photopotential
Download this article as:| Copy the following to cite this article: Sharma P, Rathore J. Sunlight Induced Photogalvanics for Conversion and Storage of Solar energy: Coomassie Brilliant Blue-Isopropyl Alcohol-Sodium Lauryl Sulphate System. Orient J Chem 2023;39(4). |
| Copy the following to cite this URL: Sharma P, Rathore J. Sunlight Induced Photogalvanics for Conversion and Storage of Solar energy: Coomassie Brilliant Blue-Isopropyl Alcohol-Sodium Lauryl Sulphate System. Orient J Chem 2023;39(4). Available from: https://bit.ly/3Q6emj1 |
Introduction
Energy is unique key for scientific development of biosphere. Renewable and non-renewable sources of energy are under limitations. Depletion factor for wood, coal, kerosene, etc are responsible for next searching way for energy demand. The scientific groups are on way to search out the alternative source of energy to fulfil the whole world with eco-friendly nature and commercially viability. Thus, the solar energy is the best option to fulfil the energy demand. Solar energy is based on photogalvanic cell and photovoltaic cells for energy transformation. With respect to storage capacity photogalvanic cells are best over photovoltaic cells and due to this scenario, research work has been taken under investigation. First of all, Rideal and Williams1 were studied on FeI and Rabinowitch2 was studied on the photogalvanic effect about photochemical reactions. Peter3, Hall4, Ameta5, Bhimwal and Gangotri6, Mohan Lal and Gangotri7, Gangotri KM and Lal Mohan8, Lal Mohan and Gangotri9, mixed surfactant10, Saini et al.11, Tartrazine12, Sudan-I dye13, Main Progress14, transition15 has been studied. Later on, silver nanoparticles16, Indigo carmine17, cell dimensions18, Xylose + MB + Brijj -35 + NaLS19,fructose20, and single surfactant21 for solar energy conversion and storage. The observed result are good over previous published results in solar conversion and storage, Gangotri and Lal, 2013; Lal and Gangotri, 2013). It is very cleared from above literature survey that different group of researchers worked on photogalvanic cells but on one worked on Coomassie Brilliant Blue, Isopropyl alcohol, and sodium lauryl sulphate system for better electrical output and due to reason, the present research work was undertaken for scientific investigation.
Materials and Methods
Material required
Solutions used
Dye- Coomassie Brilliant Blue, reductant – Isopropyl alcohol, Surfactant- sodium stearate, NaOH (1N), Oxalic acid, doubly distilled water.
Scientific instruments
Especially designed H glass tubes,Saturated calomel electrode (SCE), Pot for Carbon function, Multi – meter, 250 k Rheostat, Digital pH meter, Platinum electrode, Microammeter, Resistance key, and 200 W tungsten bulb.
Experiments
First of all, we have designed the specific photogalvanic cell for solar transformation of photochemical conversion and storage (kindly see fig.1). Specially designed photogalvanic cell having two lobs named as dark chamber and illumination chamber. Saturated calomel electrode connected with dark chamber and platinum electrode connected with illumination chamber. The photochemical electrical circuit was completed by using of required scientific instrumentations i.e., Especially designedH glass tubes,SC electrode, Pot for carbon, Multi – meter, 450 k carbon pot, Digital pH meter, Platinum electrode, Microammeter, Resistance key, and 200 W tungsten bulb. We have kept volume of solution up to 30 ml during electrochemical and photochemical process of photogalvanic cell. Water filter was used for ultra-filtration of radiations. Nature of solution was alkaline for pH measurement during experiment. The electrolytical configuration was as fellow: Dye- Coomassie Brilliant Blue, reductant – Isopropyl alcohol, Surfactant- sodium lauryl sulphate, NaOH (1N), Oxalic acid, doubly distilled water. The strength of electrolytical solution were as follow: dye M/5000, Reductant M/2000, Surfactant M/200 and 1N NaOH. Figure 1 represented the photochemical set up for energy conversion in Coomassie Brilliant Blue-Isopropyl Alcohol-Sodium Lauryl Sulphate System.
 |
Figure 1: Photogalvanic cell: Experimental set up. |
Result and Discussion
Variation of surfactant strength (sodium lauryl sulphate concentration) on the photogalvanics
On initial stage of photogalvanic experiment, electrical outcomes were increased on increasing of strength of surfactant and after particular range of strength (ongoing experiment) it reached at optimum position. After optimum position of electrical outcomes its decreased continually. above variation was obtained due to electron transfer process in hydrophilic hydrophobic interaction of large number of surfactant molecule in electrochemical process. At optimum position, three will be required number of surfactants molecules are responsible for results. The observed result are good over previous published results in solar conversion and storage, Gangotri and Lal, 2013; Lal and Gangotri, 2013). The photochemical outcomes of energy conversion and storage are given in Table 1 to 5.
Variation effect of photosensitizer strength (Coomassie Brilliant Blue concentration) on the photogalvanics
On initial stage of photogalvanic experiment, electrical outcomes were increased on increasing of strength of photosensitizer and after particular range of strength (ongoing experiment) it reached at optimum position. After optimum position of electrical outcomes its decreased continuously. above variation was obtained due to electron transfer process in hydrophilic hydrophobic interaction of large number of photosensitizer molecule (dye molecule) in electrochemical process. At optimum position, three will be required number of photosensitizer molecule (dye molecule) molecules are responsible for results. The photochemical outcomes of energy conversion and storage are shown in Table 1 to 5.
Variation of reductant strength (Isopropyl alcohol concentration) on the photogalvanics
On initial stage of photogalvanic experiment, electrical outcomes were increased on increasing of strength of reductant and after particular range of strength (ongoing experiment) it reached at optimum position. After optimum position of electrical outcomes its decreased continuously. above variation was obtained due to electron transfer process in hydrophilic hydrophobic interaction of large number of reductant molecule (Isopropyl alcohol molecule) in electrochemical process. At optimum position, three will be required number of reductant molecule (Isopropyl alcohol molecule) molecules are responsible for results. The observed result are good over previous published results in solar conversion and storage, Gangotri and Lal, 2013; Lal and Gangotri, 2013). The photochemical outcomes of solar energy conversion and storage are reported in Table 1 to 5.
Table 1: Effects of CBB on electrical output of photogalvanic
|
CBB X 10-5 M) |
Photo potential (mV) |
Photocurrent (µA) |
|
1.20 |
834 |
372 |
|
1.21 |
883 |
401 |
|
1.22 |
931 |
443 |
|
1.23 |
889 |
414 |
|
1.24 |
832 |
381 |
Table 2: Effects of isopropyl alcohol on electrical output of photogalvanics
|
(IA ×10-4) |
Photopotential (mV) |
Photocurrent (µA) |
|
2.10 |
819 |
380 |
|
2.11 |
887 |
411 |
|
2.12 |
933 |
432 |
|
2.13 |
885 |
404 |
|
2.14 |
813 |
376 |
Table 3: Effects of NaLS on electrical output of photogalvanics
|
(NaLS X 10-4) |
Photopotential (mV) |
Photopotential (mV) |
|
1.79 |
812 |
381 |
|
1.77 |
883 |
414 |
|
1.93 |
931 |
447 |
|
1.72 |
875 |
417 |
|
1.61 |
827 |
373 |
Table 4: Photogalvanic cell electrical output
|
S. No. |
Time (Min.) |
Power (ìW) |
|
1 |
34.0 |
127.72 |
|
2 |
36.0 |
123.11 |
|
3 |
38.0 |
139.36 |
|
4 |
40.0 |
134.49 |
|
5 |
42.0 |
129.97 |
Table 5: Comparison of present study with Previous reports
|
S. No. |
Parameters |
Coomassie Brilliant Blue, Isopropyl alcohol, sodium lauryl sulphate system |
NaLS, Tween-80, Methylene blue, Xylose system |
NaLS, CTAB, Methylene blue, Xylose system |
DSS, Tartrazine ISOPROPYL ALCOHOL system |
|
|
|
Present Study |
Past studies |
||
|
1 |
Conversion efficiency |
0.8796% |
0.5313% |
0.4326% |
0.6163% |
|
2 |
Storage capacity |
114.0 minutes |
100.0 minutes |
90.0 minutes |
100.0 minutes |
|
3 |
Fill factor |
0.3066 |
0.3024 |
0.2770 |
0.2800 |
|
4 |
Photopotential |
533.0 mV |
645.00 mV |
655.00 mV |
493.00 mV |
|
5 |
Photocurrent |
201..0 uA |
210.0 uA |
190.0 uA |
130.0 uA |
(i-V) Current and voltage characteristics of photogalvanics
The observed fill factor of photogalvanic was calculated by using photochemical values i.e., Photopotential at = 533 mV, power point current (ipp) = 201 µA, open circuit Potential (Voc) = 734 mV, Current at short circuit (isc) = 140 µA, and fill factor value = 0.3066, The power point of photogalvanics (pp) = 118, (see the Figure 2).
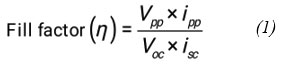
 |
Figure 2: Current and voltage curve of the cell. |
Cell Photogalvanic performance and efficiency of conversion
Efficiency of conversion in photogalvanics has calculated by using photochemical values i.e., Power point Photopotential (Vpp,) = 323 mV, Photocurrent at power point (ipp) = 201 µA, Electrode area for photogalvanics (A) and obtained values was 0.8796% (See the figure 3).
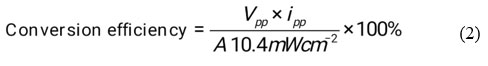
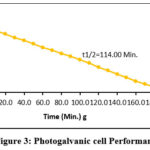 |
Figure 3: Photogalvanic cell Performance |
Photochemical reaction Mechanism of current generation in the photogalvanics Illuminated chamber (at platinum electrode)
Photochemical reaction at illuminate chamber and photochemical reaction at platinum electrode as below
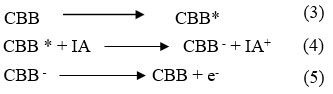
Dark Chamber: At counter electrod
During the photochemical process, CBB molecule gain an es from electrode and converted into CBB– and at ending stage, CBB– converted into CBB molecule and oxidized form of Isopropyl alcohol combine with CBB molecule to give original dye and reductant molecule and the cycle will continue.
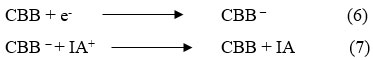
Where: CBB = Coomassie Brilliant Blue dye molecule, CBB* = Excited Coomassie Brilliant Blue molecule, CBB– = Semi form of Coomassie Brilliant Blue molecule, IA = reductant molecule, IA+ = Oxidized form of the reductant,
Conclusion
On basis of observed electrical output, we are scientifically concluded that the Coomassie brilliant blue dye molecules are affected the photogalvanic cell more than existing dyes. The surfactant has also enhanced the efficiency of conversion and storage capacity of photo galvanic cells. Our recent reported results on photogalvanic cell about conversion efficiency and storage capacity, 0.8796% and 114.0 minutes respectively. These values are relatively higher in comparison to previously reported i.e. (0.6163% and 100.0 minutes), (0.4326% and 90.0 minutes), (0.5313% and 100.0 minutes), (0.1469% and 20.0 minutes) developed by Rathore Jayshree and Lal Mohan (2018), Gangotri KM and Mohan Lal (2013), Lal Mohan and Gangotri KM (2012) and Gangotri and Gangotri (2010), respectively. These observed outcomes (0.8796% and 114.0 minutes) are relatively lower in conversion efficiency but higher in storage capacity in comparison to recently reported photogalvanic cells i.e. (27.79% and 115.0 minutes), (9.02% and 70.0 minutes), developed by Koli et al. (2021), and Koli et al. (2022), respectively. The conversion efficiency, t1/2 and fill factor are recorded as 0.8796%, 114.0 min. and 0.3066 respectively in PG system. Potential at power point, Potential at open circuit, power point of cell (pp) and current at short circuitwere also studied. The obtained values are as follows:734mV, 533 mV, 201 and 140 µA. Therefore, the photogalvanic cell containing Coomassie brilliant blue, sodium lauryl sulphate Isopropyl alcohol system is more efficient than existing cells selecting suitable substances. Present study in photogalvanic as limitation and future scopes as becoming cost competitive with solar power.
Acknowledgement
Both authors are thankful to Professor Sangeeta loonker, Head, Department of chemistry, JNVU Jodhpur Rajasthan, INDIA for necessary facilities and Pratibha Sharma (one of the authors) is thankful to Dr Jayshree Rathore for scientific analysis during research work.
Conflicts of interest
Authors have no any conflict of interest.
References
- Keightley, RE.; Gardner, WE., J. Chem. Soc., 1925, 127,258-269.
CrossRef - Rabinowitch, E., J. Chem. Phy., 1940, 8(7),551-559.
CrossRef - Peter, D.; David, R.; Hobart, N.; Hall, A. Solar Energy., 1977, 19(5),567-570.
CrossRef - Hall, DE.; Wildes, PD.; Lichtin, N.; J. Electrochem Soc., 1978, 125(9),1365-1371.
CrossRef - Gangotri, P.; Gangotri, KM., Arab J. Sci. Engineering., 2010, 35(1A),19-28.
- Bhimwal, MK.; Gangotri, KM., Energy., 2011, 36,1324-1331.
CrossRef - Mohan, L.; Gangotri, KM., Res. J. Rec. Sci., 2013, 2(12),19-27.
- Gangotri, KM.; Lal, M., Res. J. Chem. Sci., 2013, 3(3),20-25.
- Lal, M.; Gangotri, KM., Res. J. Rec. Sci., 2013, 2(ISC2012),76-81.
- Mohan, L.; KM, Gangotri., J. Solar Energy Res., 2022, 7(3),1095-1103,
- Saini, S.; Meena, S., Meena, R., 2017, 07,125-136.
CrossRef - Rathore, J.; Lal, M., Res. J. Chem and Environ., 2018, 22(6),53-57.
- Pooran, K., Arabian J. Chemistry. 2021, 14,232-240.
- Wu, T.; Qin. Z.; Wang, Y., Nano-Micro Lett., 2021, 13,152-158.
CrossRef - Zhao, C., Tang, CG., Seah, ZL., Nat Commun., 2021, 12,2250-2258.
CrossRef - Chen, Y.; Du, C.; Sun, L., Sci Rep., 2021, 11,14550.
CrossRef - Koli, P.; Pareek, RK.; Dayma, Y.; Jonwal, M., Energy Reports., 2021, 1(7),3628-38.
CrossRef - Koli, P.; Dayma, Y.; Pareek, RK.; Jonwal, M., J. Electrochem., 2022, 1,904-1159.
CrossRef - Rathore, J.; Rakesh, K.; Sharma, P.; Lal, M., Ind. J. sci. Tech., 2022,15,1159-1165.
CrossRef - Lal, M.; Gangotri, KM., Int. J. Energy Res., 2022, 46(14),19538-19547.
CrossRef - Rathore, J.; Arya, RK.; Sharma, P.; Lal, M., Res. J. Chem. Environ., 2022, 26(6),24-29.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.










