In vitro Antidiarrheal and Antimicrobial Assessments of Phytofabricated Zinc Oxide Nanoparticles Through Curculigo Orchioides
R. Kanmani* , J. Felicita Florence
, J. Felicita Florence , J. Amala Infant Joice
, J. Amala Infant Joice , R. Megala
, R. Megala and M. Kavitha
and M. Kavitha
Department of Chemistry, Holy Cross College (Autonomous), Affiliated to Bharathidasan University, Tiruchirappalli, Tamilnadu, India.
Corresponding Author E-mail: kanmanichem@hcctrichy.ac.in
DOI : http://dx.doi.org/10.13005/ojc/390421
Article Received on : 08 Jul 2023
Article Accepted on : 10 Aug 2023
Article Published : 04 Aug 2023
Reviewed by: Dr. Hamid Reza Ghorbani
Second Review by: Dr. Javed
Final Approval by: Dr. Charanjit Kaur
The green approach was discovered to be a cost-effective and environmentally sustainable technique for the production of metal oxide and metallic nanoparticles. In this study, Curculigo orchioides' aqueous leaf extract was used to create zinc oxide nanoparticles (ZnO Nps) utilizing a plant-mediated component. The important medicinal herb Curculigo orchioides plant extract and zinc acetate were used to effectively prepare the ZnO Nps. For the manufacture of ZnO Nps, 0.1 M zinc acetate and plant extract were combined in various ratios of 5:5, 6:4, 7:3, 8:2, and 9:1. The 5:5 ratio was fixed. The subsequent yellow adhesive was fully dried, gathered, and packaged for further investigation. The UV-Vis spectroscopic absorption band, which is unique to ZnO Nps, was seen at 357 nm. The X-ray diffraction (XRD) pattern was utilized to assess the average size of ZnO Nps. Results from Energy Dispersive Spectrum (EDX) analysis revealed the constitution of zinc and oxygen, with values of 41.59% and 30.89%, accordingly. FT-IR spectroscopy study revealed the Zn-O bonding absorption peak to be around 400 and 600 cm-1. Additionally, the antimicrobial and antidiarrheal assessments of the produced ZnO Nps were examined through the disc diffusion technique. This research led to the conclusion that numerous biomedical activities are employed by this plant.
KEYWORDS:Antimicrobial Activity.; Curculigo Orchioides; Characterization; Invitro Antidiarrheal Activity; Phytochemical Screening; ZnO Nanoparticles,
Download this article as:| Copy the following to cite this article: Kanmani R, Florence J. F, Joice J. A. I, Megala R, Kavitha M. Invitro Antidiarrheal and Antimicrobial Assessments of Phytofabricated Zinc Oxide Nanoparticles Through Curculigo Orchioides. Orient J Chem 2023;39(4). |
| Copy the following to cite this URL: Kanmani R, Florence J. F, Joice J. A. I, Megala R, Kavitha M. Invitro Antidiarrheal and Antimicrobial Assessments of Phytofabricated Zinc Oxide Nanoparticles Through Curculigo Orchioides. Orient J Chem 2023;39(4). Available from: https://bit.ly/45OaCXZ |
Introduction
Diarrhea is among the primary reasons for illness and death in both kids and adults, owing to inadequate cleanliness and sanitation. The World Health Organization (WHO) describes diarrhoea as the passing of three or more watery or sloppy faeces per day or more often than is typical for the person. A number of bacterial, viral, parasitic, and virulent microorganisms, including Escherichia coli, Vibrio cholera, Shigella genera, and others, may cause this form of sickness, which often manifests as symptoms in the digestive system (protozoa and helminths). The antibiotic medicines may lessen the duration and intensity of diarrheal disease, according to earlier research. According to how long it lasts, diarrhoea is divided into three categories: acute, persistent, and chronic diarrhoea. While virions, germs, and pathogens are the main causes of acute diarrhoea, functioning or inflammation bowel problems, malabsorption disorders, and medications are the main causes of chronic diarrhoea.
The ayurvedic plant Curculigo orchioides, often called as Golden Eye Grass or Talamuli, is also described as “Kali Musli” in Hindi. Despite being widely utilised in Indian remedies, it is an important medicinal herb. Golden Eye Grass is a flowery rhizome, annual with many succulent root system growing from a short or elongated root stock. It can tolerate shade and has resilient leaf; In the shade, the leaf will only slightly lengthen compare to direct sunlight. Each day throughout the blooming season, a golden yellow blossom opens at the bottom of the leaves. This may create a nice little planter in your house. In the Ayurvedic medicine, rhizomes of Curculigo orchioides are employed to make Vrushya (aphrodisiac), Brimhana (improving weight), Rasayana (antiaging), etc. This herb has an important function in medicine because it contains a biological substance called curculigoside. The herb also includes additional bioactive chemicals like xylose, glucuronic acid, fat, starch, mucilage, and mannose in addition to the phenolic glycoside molecule known as curculigoside. From the entire plant, 61 phytoconstituents so far been extracted by investigators.
Materials and methods
Phytochemical analysis
Plant collection
The leaves of the herb Curculigo orchioides, a member of the Amaryllidacae family, were newly gathered in the Trichy region. The leaf was properly cleaned using regular water several times, then with distilled water to remove pollutants. They were then dried in a shaded area of the sunlight. Using mechanical crusher, the sun shade-dried leaf subsequently ground into powder.
 |
Image 1 |
Preparation of Plant Extract
At ambient temperature, 100 ml of several solvent, including methanol, ethyl acetate, chloroform, dichloromethane, and hexane in order of increasing polarity, were added to 20 g of powdered leaves. Grinded leaf components might be removed from the prepared solution by first filtering it over ordinary filter paper. The extracted material from plant source was further processed into filtration through Whatman No. 1 filter paper to produce a clear solutions. The supernatant was preserved at 4°C for additional experiments.
Phytochemical assessment of Different Solvents Extract of Curculigo Orchioides
To determine the different phytochemicals present in Curculigo orchioides preparations, a qualitative evaluation was carried out. Standard procedures were utilised for the phytochemical analysis, including the different assays and the relevant chemicals.
Phytofabrication of ZnO Nps
Preparation of Aqueous Leaf Extract Solution (ALE)
The microbes are killed by boiling a 20 gramme solutions of powdered leaf in 100 ml of deionized water for 20 minutes at 60 degrees Celsius. To obtain a clear solution, it is chilled and thereafter centrifuged at 1000 rpm. Following that, the aqueous leaf extract was stored at 4°C for future works.
Synthesis of ZnO Nps
Employing zinc acetate and plant extracts, ZnO Nps were prepared. Double distilled water was used to prepare 0.1 M zinc acetate. Plant extract and zinc acetate were combined in proportions of 5:5, 6:4, 7:3, 8:2, and 9:1. With a magnetic stirrer, the reaction mixture was constantly swirled at 800 rpm while being heated under the boiling point. Within an hour, the mixture had taken on a golden hue. The entire process took place in the darkness. The acquired yellow colour precipitate was repeatedly rinsed with distilled water to produce a white colour powder. The solution was then centrifuged at 4 °C for 15 minutes at 5000 rpm. The nanoparticles were gathered and heated at 60 °C for eight hours to dry.
Characterization Techniques
UV-Visible Analysis
The produced ZnO Nps were confirmed through UV-Visible spectrum. The produced particles were dissolved in deionized water. Utilizing Systronic Spectrophotometer, the nanoparticles were examined throughout the wavelength range of 190 to 1100 nm. The distinctive peaks were found by sequentially scanning those solutions at spacing of 50 nm. The UV-Visible’s value was noted.
Fourier Transform Infrared (FT-IR) Spectroscopic Analysis
Following earlier techniques using significant modifications, spectrums were acquired using an OMNI-samplerattenuated total reflectance (ATR) attachment on an FT-IR spectroscopy (Perkin Elmer Spectrophotometer system, USA) (Liuet al., 2006). Having steady pressure is exerted, a tiny volume of liquid was directly injected on the specimen holder of the infrared spectrometer. Measurements of infrared absorbance were then gathered and computerized for analysis utilizing the 21 CFR part 11software. According to the display of every specimen duplicate, the reference spectrum were obtained from the cleansed blank crystal. The FTIR peak values were noted. Twice-repeated analyses of each and every study supported the spectra.
SEM analysis of copper nanoparticles
While utilyzing VEGA3 LMU device, scanning electron microscopic (SEM) examination was carried out. A very tiny number of the zinc oxide nanoparticles were dropped onto a zinc grid that had been covered with carbon to create thin films of the material. Films on the SEM grid subsequently dried via placing it beneath a mercury lamp for five minutes after excess fluid was eliminated with blotting paper.
EDX spectrum
The EDX spectra of bio-synthesised zinc oxide nanoparticles showed their chemical make-up.
XRD Analysis
The X-ray diffraction data absolutely demonstrate that both types of leaf extracts produce crystalline zinc oxide nanoparticles. The Debye-Scherrer equation was utilized to determine the crystalline size of the synthesised ZnO Nps.
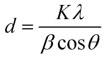
Where ϑ is the Bragg angle, λ is the wave-length of the X-Ray source used in XRD K is the Scherrer constant which ranges in value from 0.9 to 1, β is the whole width at half maximum of the diffraction peak, and D is the crystallite size of ZnO Nps.
Antidiarrheal Activity of Synthesized Zincoxide Nanoparticles
While using disc diffusion technique, the antidiarrheal efficacy of produced zincoxide nanoparticles was evaluated towards certain microorganisms.
In-vitro antidiarrheal activity
ZnO Nps were tested for their antidiarrheal properties using the disc diffusion technique by using various pathogens like B. subtilis, E. coli, S. aureus, and S. flexneri. In sterile petri plates with a diameter of 60 mm, 10 ml of Mueller-Hilton agar medium was added before the bacterial culture was added. Mueller-Hilton agar plates were covered using sterile filter paper discs containing 60, 80, and 100 µg/ml doses of zincoxide nanoparticles. As a positive control, 5 µg of amoxicillin was employed. The plates were kept at 37oC for 24 hours, and the assay was performed two times. The inhibition zone was measured in millimetres.
Antimicrobial Activity of Zincoxide Nanoparticles
This research used the disc diffusion technique to look at the antibacterial and antifungal activities of produced zincoxide nanoparticles against certain microorganisms.
In-vitro antibacterial activity
ZnO Nps were tested for their antibacterial properties using the disc diffusion technique through pathogenic bacteria P. vulgaris and P. aeruginosa. In sterile petri plates with a diameter of 60 mm, 10 ml of Mueller-Hilton agar medium was added before the bacterial culture was added. Mueller-Hilton agar plates were covered using sterile filter paper discs containing 60, 80, and 100 µg/ml doses of zincoxide nanoparticles. As a positive control, 5 µg of amoxicillin was employed. The plates were kept at 37 oC for 24 hours, and the assay was performed twice. The inhibition zone was measured in millimetres.
In-vitro antifungal activity
The disc diffusion technique was used to investigate the antifungal effect of zincoxide nanoparticles towards test microorganisms. In sterile petri dishes (60 mm) loaded with Sabouraud’s dextrose agar (SDA) and sown with 0.3 ml of the test organism. Zincoxide nanoparticles were applied to the sterile disc in 10 µl doses of 60, 80, and 100 µg/ml, correspondingly. Afterwards 24 hours of cultivation at 37°C, the zone of growth inhibition surrounding the disc were determined, and fluconazole was employed as a control sample.
Results and Discussion
Table 1 displays the outcomes of phytoconstituents testing performed on various solvent extracts of Curculigo orchioides leaves.
Table 1: Phytochemical Screening of Curculigo orchioides
|
S.No. |
Phytoconstituents |
Hexane |
Chloroform |
Ethyl Acetate |
Methanol |
DCM |
|
1 |
Alkaloids |
– |
– |
+ |
+ |
– |
|
2 |
Carbohydrates |
++ |
++ |
+ |
+ |
– |
|
3 |
Glycosides |
+ |
– |
++ |
+ |
– |
|
4 |
Phytosterols |
– |
– |
+ |
++ |
– |
|
5 |
Saponins |
– |
– |
+ |
++ |
+ |
|
6 |
Fixed oils & Fats |
+ |
+ |
– |
– |
+ |
|
7 |
Tannin Phenolic compounds |
+ |
– |
+ |
– |
+ |
|
– |
+ |
+ |
++ |
+ |
||
|
8 |
Proteins & Free amino acids |
– |
++ |
– |
– |
++ |
|
9 |
Flavonoids |
– |
+ |
+ |
+ |
– |
|
10 |
Terpenoids |
+ |
– |
– |
++ |
+ |
According to the findings, methanolic extract only contains more phytochemicals, like flavanoids and glycosides, than other solvent extracts, such as those made using ethyl acetate, DCM, chloroform, and hexane.
The herbs may act as an anti-microbial agent because of the existence of phenolic chemicals in the leaves. Terpenoids and alkaloids, the other secondary metabolites, were found in Curculigo orchioides. Despite its antispasmodic, antibacterial, and analgesic properties, pure isolated alkaloids and its synthetic analogues are utilised as fundamental medical agents. When given to animals, they display noticeable physiological responses. In contrast, flavonoids have substantial anticancer action and are powerful water-soluble free radical scavengers and antioxidants that prevent oxidative cellular injury. Gastrointestinal flavonoids reduce the risk of cardiovascular diseases. These herbs’ flavonoids, which serve as antioxidants and have anti-inflammatory properties. This might be the cause Curculigo orchioides has been utilised in herbal therapy to heal burns, wounds, and ulcers. Tannins contain astringent qualities that speed up the recovery of cuts and irritated mucous membranes.
Characterization of Zinc Oxide Nanoparticles
UV- Visible Spectroscopy
UV spectroscopy was utilized to analyze the production of ZnO NPs. The absorption peak captured by the spectrometer are shown in Figure (1). For produced ZnO NPs, the utmost absorption peak was measured at 357 nm. Furthermore, since all oxide substances devour broad band gaps and have a tendency to have smaller wavelength, these findings meet the normal ZnO absorption pattern. Additionally, materials with a nanometer likely to have even shorter wavelengths.
 |
Figure 1: UV- Visible Spectrum of Synthesized Zincoxide Nanoparticles. |
FT- IR
Employing FT-IR investigation, the purity and type of the nano particles as well as the several phytoconstituents presented were identified. Phytoconstituents that contact with the zinc surface, such as carboxylic acids, phenols, amines, and alcohols, stabilize the fabrication of ZnO Nps. The existence of -OH stretching vibrations caused the wide band to form at 3300 cm-1. At 1463cm-1, the C-O was shown to be extending. The vibrations of the zinc oxide stretching were revealed by 1641 and 653 cm-1. It is evident from the FT-IR spectra that phytoconstituents encircled the zinc oxide nanoparticles.
 |
Figure 2: FT -IR Spectrum of Synthesized Zincoxide Nanoparticles |
Scanning Electron Microscope (SEM)
The SEM examination provided information on the morphology of the surfaces of the zinc oxide nanoparticles. Figure 3 illustrates the dimensions, shapes, and variety of the nanoparticles. The biogenically created zinc oxide nanoparticles have a spherical form and are evenly dispersed.
 |
Figure 3: SEM image of Synthesized Zincoxide Nanoparticles |
Energy Dispersive X-Ray Spectrometer (EDAX)
The EDX spectra is performed to recognize the components that are available and the composition of the ZnO Nps. The creation of zincoxide nanoparticles was suggested by the zinc and oxygen high peak. The produced nanoparticles include 30% oxygen and 41% zinc.
 |
Figure 4: EDAX Spectrum of Synthesized Zincoxide Nanoparticles |
X-Ray diffraction studies (XRD)
Using XRD experiments at 20 to 80 degrees, the zincoxide nanoparticle’s size and structure were identified. The observed diffraction peaks belong to the crystal planes (100), (002), (101), (102), (110), (103), (200), (112), and (201) at 31.7°, 34.47°, 36.31°, 47.67°, 56.69°, and 67.9°, accordingly. This is consistent with earlier research on the production of ZnO Nps. In the figure 5, it was depicted.
 |
Figure 5: XRD Pattern of Synthesized Zincoxide Nanoparticles |
Invitro Antidiarrheal Activity of Synthesized Zincoxide Nanoparticles
The disc diffusion technique was used to investigate the in vitro antidiarrheal efficacy of produced zinc oxide nanoparticles towards several microorganisms, including B. subtilis, S. flexneri, S. aureus, and E. coli. The other bacteria were less active than S. aureus.
Table 2: In Vitro Antidiarrheal activity of Synthesized Zinc oxide nanoparticles
|
Samples
|
Concentrations (µg/ml) |
Organisms/Zone of inhibition (mm)
|
|||
|
Escherichia coli |
Staphylococcus aureus |
Shigella flexneri |
Bacillus subtilis |
||
|
Samples (Zinc |
60 |
3 |
3 |
3 |
2 |
|
80 |
5 |
5 |
4 |
4 |
|
|
100 |
9 |
10 |
6 |
6 |
|
|
Standard (Std) (Amoxicillin) |
10 µl/disc |
14 |
12 |
10 |
13 |
 |
Figure 6: In Vitro Antidiarrheal activity |
Antimicrobial Activity
Utilizing the disc diffusion technique, the produced nanoparticles are tested for anti-microbial properties towards gramme positive and gramme negative bacteria as well as fungus. As there would be a confluent principle of development, the findings were seen in perspective of an inhibition zone that is circular.
The disc diffusion technique’s findings demonstrated that the inhibition is dose-dependent, meaning that as the concentration rises, so does the inhibition. The antibacterial and antifungal properties of the zinc oxide nanoparticles is notable. With an ISD of 7 mm, the produced zinc oxide nanoparticles were more effective towards Pseudomonas aeruginosa. With an ISD of 5 mm, the zinc oxide nano particle suppresses Proteus vulgaris significantly.
The effectiveness was evaluated in comparison to the industry standard antibiotics Fluconazole and Amoxicillin, which are both used to treat fungus and bacteria, respectively. The IZD ranges between 8 and 9 mm for amoxicillin and 9 to 10 mm for fluconazole.
Table 3: In Vitro Antibacterial activity of Synthesized Zincoxide nanoparticles.
|
Samples CO zinc nano |
Concentrations (µg/ml) |
Organisms/Zone of inhibition (mm)
|
|
|
Pseudomonas aeruginosa |
Proteus vulgaris |
||
|
Samples |
60 |
3 |
2 |
|
80 |
5 |
3 |
|
|
100 |
7 |
5 |
|
|
Standard (Std) (Amoxicillin) |
10 µl/disc |
9 |
8 |
 |
Figure 7: In Vitro Antibacterial activity |
Table 4: In Vitro Antifungal activity of Synthesized Zinc oxide nanoparticles
|
Samples
|
Concentrations (µg/ml) |
Organisms/Zone of inhibition (mm) |
|
|
Aspergillus flavus |
Candida albicans |
||
|
Samples |
60 |
3 |
2 |
|
80 |
5 |
4 |
|
|
100 |
6 |
6 |
|
|
Standard (Std) (Fluconazole) |
10 µl/disc |
9 |
10 |
 |
Figure 8: In Vitro Antifungal activity |
Conclusion
In this work, Curculigo orchioides’ aqueous leaf extract was used to create zinc oxide nanoparticles. The existence of different phytoconstituents, which are in charge of reducing, stabilizing, and encapsulating zinc oxide nanoparticles, caused the precipitation to look black in color. The examination of the UV and FTIR spectrum provided additional proof of the production. According to FTIR data, proteins’ amine groups and phenolics are what reduce and encapsulate zinc oxide nanoparticles, prevent aggregation, and stabilize the medium for production. The SEM scans demonstrated the nanoscale size of the zinc oxide particles, which ranged from 20 to 100 nm. By using EDAX examination, the zinc and oxygen content of zincoxide nanoparticles was determined. After that, investigations on the nanoparticles’ anti-diarrheal and anti-microbial properties showed that they had excellent inhibitory properties towards a variety of microbes. Therefore, we came to the conclusion that the biogenically created ZnO Nps from the aqueous leaf extract of Curculigo orchioides are suitable and effective anti-microbial agents.
Acknowledgement
The authors would like to thank National College in Tiruchirappalli for allowing them to characterize the samples using EDX and XRD, as well as St. Joseph’s College in Tiruchirappalli for letting them utilise UV-visible spectroscopy, FT-IR spectroscopy, and SEM, and Bio – Techno Solution in Tiruchirappalli for letting them perform antimicrobial and antidiarrheal activities on the sample.
Conflict of Interest
The authors have declared no conflict of interest.
Funding Sources
There are no funding sources.
References
- Suriyakala G.; Sathiyaraj S.; Devanesan S.; AlSalhi M.; Rajasekar A.; Maruthamuthu, M.; Babujanarthanam R.; Saudi Journal of Biological Sciences, 2022, 29(2), 680-688.
CrossRef - Chetan Pandit ;Arpita Roy ; Suresh Ghotekar ; Ameer Khusro ; Mohammad Nazmul Islam; Talha Bin Emran ; Siok Ee Lam; Mayeen Uddin Khandaker; David Andrew Bradley; Journal of King Saud University – Science, 2022, 34 (3), https://doi.org/10.1016/ j.jksus.2022.101869.
CrossRef - Mohammad Oves; Mohd Ahmar Rauf; Mohammad Aslama Huda A Qari; Hana Sonbol; Irfan Ahmad; Gaffar Sarwar Zaman; Mohd Saeed; Saudi Journal of Biological Sciences, 2022, 29 (1), 460-471. https://doi.org/10.1016/j.sjbs.2021.09.007.
CrossRef - Tuğba Gur; Ismet Meydan; Hamdullah Seckin; Muhammed Bekmezci; Fatih Sen; Environmental Research, 2022, 204, https://doi.org/10.1016/ j.envres.2021.111897
CrossRef - Adrian Augustyniak; Joanna Jablonska; Krzysztof Cendrowski; Anna Głowacka; Dietmar Stephan; Ewa Mijowska; Pawel Sikora, Applied Nanoscience, 2022, 12, 489–502.
CrossRef - Kanimozhi, S.; Durga, R.; Sabithasree, M.; Kumar, A.; Vimal, Sofiavizhimalar, A.; Kadam, Avinash Ashok, Rajagopal, Rajakrishnan, Sathya, Rengasamy, Azelee, Nur Izyan Wan Journal of King Saud University – Science, 2022, 34(4), 10.1016/j.jksus.2022.101930
- Shah Faisal; Hasnain Jan; Sajjad Ali Shah; Sumaira Shah; Adnan Khan; Muhammad Taj Akbar; Muhammad Rizwan; Faheem Jan; Wajidullah; Noreen Akhtar; Aishma Khattak; Suliman Syed; ACS Omega, 2021, 6, 9709−9722.
CrossRef - Meron Girma Demissie; Fedlu Kedir Sabir; Gemechu Deressa Edossa; Bedasa Abdisa Gonfa; Journal of Chemistry, 2020, https://doi.org/10.1155/2020/7459042
CrossRef - Minha Naseer; Usman Aslam; Bushra Khalid; Bin Chen; Sci Rep., 2020, 10(1). https://doi.org/10.1038/s41598-020-65949-3
CrossRef - Ahmed S. Abdelbaky; Taia A. Abd El-Mageed;, Ahmad O. Babalghith;, Samy Selim; Abir M. H. A. Mohamed; Antioxidants, 2022, 11, 1444. https://doi.org/10.3390/antiox11081444.
CrossRef - Kanmani R.; IrudayaIrin Scleeva P.; Oriental Journal of Chemistry, 2021, 37(5) http://dx.doi.org/10.13005/ojc/370531.
CrossRef - Vinotha V.; Iswarya A.; Rajagopalan Thaya; Govindarajan M.; Alharbi N. S.; Kadaikunnan S.; Jamal M Khaled; Mohammed N. Al-Anbr; Vaseeharan B.; Journal of photochemistry and photobiology B: Biology, 2019, DOI:10.1016/j.jphotobiol.2019.111541.
CrossRef - Tu Uyen Doan Thi; Trung Thoai Nguyen; Y Dang Thi; Kieu Hanh Ta Thi; Bach Thang Phan; Kim Ngoc Pham; RSC Adv., 2020, 10, 23899–23907, DOI: 10.1039/d0ra04926c
CrossRef - Brintha S.; Rajesh S.; Renuka R.; Santhanakrishnan V.P.; Gnanam R.; Journal of Pharmacognosy and Phytochemistry, 2017, 6(4), 192-197.
- Anuj kumar Agrahari; Sanjaya Kumar Panda; Ashutosh Meher; Amiya Ranjan Padhan; Mohd Khaliquzzama; J. Chem. Pharm. Res., 2010, 2(2), 107-111.
- Harborne J.B.; Phytochemical methods, A guide to modern technique of plant analysis, Chapman and Hall, London, 2005,182-189.
- Raaman N.; Phytochemical Techniques, 2nd Edition, New Indian Publishing Agency, New Delhi, 2006,19-30.
CrossRef - Sharanya Kushalan; Undiganalu Gangadharappa Yathisha; Aloysius Khyahrii .; Smitha Hegde; In Vitro Cellular & Developmental Biology – Plant, 2022, 58, 382–391.
CrossRef - Elumalai Ananda kirouchenane; Irisappan Sarath Chandiran; Balamuthu Kadalmani; Journal of Pharmacy Research, 2013, 7(8), 692-696, https://doi.org/10.1016/j.jopr.2013.08.023
CrossRef - Sai Nandhini R.; Nirmala Nithya R.; Vidhya K.; Research Journal of Pharmacy and Technology, 2021, 14(8), 10.52711/0974-360X.2021.00756
- Kavitha, M.; Shenbagam, K.; Kanmani R.; Philomina Mary S.; Oriental Journal of Che,mistry, 2022, 38(5), http://dx.doi.org/10.13005/ojc/380533.
CrossRef - Hamid Reza Ghorbani, Chemical synthesis of copper nanoparticles, Oriental Journal of Chemistry, 2014, 30(2), 803-806. DOI: 10.13005/ojc/300254
CrossRef - Hamid Reza Ghorbani, Biological and Non-Biological Methods for Fabrication of Copper Nanoparticles, Chemical Engineering Communications, 2016, 202(11), 463-1467. DOI: 10.1080/00986445.2014.950732
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









