Impedance Analysis on Interaction Between of Loxacin and Supported BLM
K. Poornima1 , A. Sankar2
, A. Sankar2 , S. Rameshkumar3
, S. Rameshkumar3 and M. Periasamy4*
and M. Periasamy4*
1Department of Chemistry, Government College of Engineering, Salem -11, Tamilnadu, India.
2Department of Chemistry, Kandaswami Kandar’s College, Velur, Namakkal, Tamilnadu, India.
3Department of Chemistry, Sri Vasavi College of Arts and Science, Erode, Tamilnadu, India.
4Department of Chemistry, Thiruvallur Government Arts College, Rasipuram, Namakkal, Tamilnadu, India.
Corresponding Author E-mail: chem.gce@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/390426
Article Received on : 12 Jul 2023
Article Accepted on : 15 Aug 2023
Article Published : 18 Aug 2023
Reviewed by: Dr. Anand Balu
Second Review by: Dr. P. Sutharsan
Final Approval by: Dr.Thailan Vaithi
Using the EIS method (Electrochemical Impedance Spectroscopy), the interaction of the BLM (lipid bilayer membrane) system with Ofloxacin in NaCl bath solutions was studied. The BLM system shows electrical properties and stability, which mainly depend on the concentration of the sodium chloride bath solution. The stability of the BLM system increased due to the increase in the concentration of NaCl. On the surface of BLM, a fixing impact has been created due to the cations. The Ofloxacin atoms get divided into the BLM stage and display the fluidization impact. The resistance of the membrane decreases with the concentration of Ofloxacin. To identify Ofloxacin in the arrangement, an impedimetric sensor was created. The level of Ofloxacin detected mainly depends on the sodium chloride concentration present in the bath solution.
KEYWORDS:Bilayer lipid membrane; Biomembrane; EIS; Ofloxacin; NaCl bath
Download this article as:| Copy the following to cite this article: Poornima K, Sankar A, Rameshkumar S, Periasamy M. Impedance Analysis on Interaction Between of Loxacin and Supported BLM. Orient J Chem 2023;39(4). |
| Copy the following to cite this URL: Poornima K, Sankar A, Rameshkumar S, Periasamy M. Impedance Analysis on Interaction Between of Loxacin and Supported BLM. Orient J Chem 2023;39(4). Available from: https://bit.ly/3QJWDOQ |
Introduction
Biomembranes assume a significant role in the specific transport of atoms, receptor restriction, enzymatic movement, and control of cell-to-cell cooperation in every single organic framework 1. Biomembranes are made of lipids and proteins. Lipids are amphipathic molecules that self-assemble into a bilayer in aqueous electrolytic solutions. Different types of lipids, such as phospholipids, glycolipids, sphingolipids, etc., are found in the natural cell membranes. In living cells, the interface exists between the cell membrane surface and its surroundings. In addition to providing ample loci to carry out activities that are important for living systems; it also gives clues for understanding the mechanism of such biochemical activities 2. Many experimental membrane systems have been developed to understand the structure and functions of cell membranes. The first one developed is the black lipid membrane, or planar lipid membrane, which resembles the biomembrane and is useful for studying the properties of the plasma membrane. The model film frameworks, for example, lipid vesicles that uphold bilayer lipid layers and so on, which mirror the lipid bilayer design of the cell layers, were viewed as exceptionally valuable in concentrating on the collaboration of drugs with cell layers 3-6. The model film frameworks, for example, lipid vesicles, which uphold bilayer lipid layers, and so on, which mirror the lipid bilayer design of the cell layers, were viewed as exceptionally valuable in concentrating on the collaboration of drugs with cell layers.
The analysis of planar BLM can be done in many ways, and the use of electrochemical impedance spectroscopy was found to be a nondestructive and sensitive method for the investigation of lipid films on solid surfaces 7.
Certain contaminations like pneumonia, bronchitis, venereal sickness (VD), and prostate, skin, and urinary tract diseases are brought about by microorganisms, Ofloxacin has been used as an antibiotic to treat these infections 8,9. Ofloxacin exists in special preparations for the treatment of eye and ear infections. Inhibiting metabolic activities causes the death of the bacteria. Figure 1 shows the construction of Ofloxacin. Gastro-digestive problems are the most widely recognized unfavorable occasions related to the utilization of ofloxacin, including sickness, looseness of the bowels, and stomach uneasiness. Normal CNS grievances incorporate cerebral pain, wooziness, and anxiety. Tipsiness and rest aggravations might be risky, especially at higher doses. Mind flights and maniacal responses have likewise been accounted for. Looseness of the bowels might be brought about by the disposal of useful microorganisms ordinarily tracked down in the colon. The indications of a dangerous response include wheezing; snugness in the chest; fever; tingling; terrible hack; blue skin tone; fits; expanding of the face, lips, tongue, or throat 10.
 |
Figure 1: Structure of Ofloxacin. |
The present study investigates the interaction between Ofloxacin and planar lipid membrane using the electrochemical impedance spectroscopic method.
Materials and Methods
Phospholipid was extracted from hen eggs and purchased locally. The phospholipid was extracted using a procedure described by Ramesh et al. 11. Planar lipid membranes were formed by a method described in 12 across a 0.6 mm Teflon cup using phospholipid dispersion in n-decane. The extract was first dissolved in chloroform (50 mg/mL), from which 100 µL was transferred into a screw cap bottle. A chloroform solution of phospholipid lipid was made into this film by purging nitrogen. Using 200 µL of n-decane phospholipid film, it was dissolved. All the solutions were prepared using triple distilled water. Before adding the phospholipid dispersion in n-decane to the bath solutions in the Teflon chambers, the hole connecting the two chambers was preconditioned by placing 3 µL of phospholipid dispersion in n-decane and drying under nitrogen gas. A stabilization time of 30 minutes was given, after applying 5µL of lipid dispersion at the hole when the bath solutions are taken into the chambers; to self-assemble the phospholipid molecules into a bilayer.
Ofloxacin 3.5mM stock solution was prepared, and from this, suitable dilutions were made for studies. The EIS (Electron Impedance spectroscopy) was carried out using a potentiostat, GAMRY REFERENCE 3000, with two electrodes set up. Two Ag/AgCl electrodes were dipped in saturated KCl solutions taken in two different chambers, which are in turn connected to bath solutions through KCl salt bridge tubes. An amplitude of 25 mV of AC sinusoidal voltage was superimposed on open circuit potential at various frequencies to record the Impedance spectra.
For the impedance range, a buffering time of 30 minutes was given for the drug to equilibrate prior to recording between the lipid bilayer and fluid stage layer subsequent to adding it to the electrolytic arrangement.
Results and Discussion
Formation and Stability of Planar BLM
After applying lipid dispersion at the hole connecting two chambers, a self-assembly for a period of 30 min given to the lipid molecules which get arranged into the double layer spontaneously in the presence of an electrolytic bath and then impedance spectra were recorded in various concentration of Molarity (M) (1.0 M, 0.1 M and 0.01 M) Sodium Chloride immersion solutions. From the Bode Plots, the frequency domain in which the capacitive nature is observed is noted. The dispersion of phase angle and net impedance with frequency in Sodium Chloride bath solutions are shown in Figure 2.
 |
Figure 2: Dispersion of IZI and phase angle of planar BLMs forms in NaCl bath solutions. |
From the above Bode Plots; it is clear that at the uppermost frequencies, the impedance measured corresponds to solution resistance (Rs), which resistance increases and decrease in the addition concentration of NaCl in the immersion tank. With the decrease in frequency the contribution of the capacitive element to the net impedance increases and the contribution from Rs decrease. A straight line which is obtained with a negative incline (Slope) of -1 in the IZI vs. log (frequency) plot. In the bode phase diagram, this region is represented by a phase angle close to -90. With a further decrease in frequency, the contribution from membrane resistance (Rm) will be larger than the capacitive reactance of capacitive elements. This region is again portrayed by a straight line, parallel up with the recurrence hub at the most minimal frequencies..
The stability of BLMs was determined by measuring the phase angle and capacitance values at a particular frequency (1Hz) of the frequency domain at which the phase angle is very close to -90. The variation of capacitance and phase angle of BLMs formed in NaCl immersion bath arrangements are displayed in Figure-3 and 4 separately.
 |
Figure 3: Capacitance profile of BLM with time Click here to View Figure |
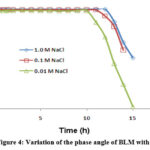 |
Figure 4: Variation of the phase angle of BLM with time. |
From Figure-3 and 4, it is clear that with rising NaCl concentration the stability of BLM increases. The phase angle and capacitance values are also higher in (1.0 Molar NaCl bath solutions than that in 0.1 Molar and 0.01 Molar NaCl bath solutions. In the present study, for about ten hours the BLM systems formed have stable electrical properties and hence drug membrane interaction can be studied within this period.
Electrochemical Impedance Concentrates on interaction of Ofloxacin with BLM in NaCl immersion bath arrangements
he Nyquist plots acquired for exposed and Ofloxacin entered BLMs in the recurrence range 10 MHz to 1 MHz in 1 M, 0.1 M and 0.01 M NaCl shower arrangements are displayed in Figures-5a, 5b and 5c separately. The trial bends seemed to be twofold crescents and the focuses of these semi-circles are viewed as on the genuine hub. The expansion of Ofloxacin to the shower arrangement diminished the distances across of these half circles. This shows a lessening in layer opposition or an expansion in film conductance with the expansion of Ofloxacin. This is a significant distinctive property of the bilayer lipid layer frameworks to turn out to be a lot leakier to more modest cations like Na+, and K+ because of fluidization of the film within the sight of medications infiltrated like Ofloxacin [12]. The twofold half circle nature of the trial impedance spectra likewise showed the dielectric idea of the lipid bilayer with spillage 13,14.
 |
Figure 5: (a, b and c). Nyquist plots for the interaction of Ofloxacin in NaCl bath solutions. |
Direct interpretations can’t be produced using the impedance spectra got, much of the time and the examination of impedance spectra is done utilizing an identical circuit15. The prevailing parts of biomimetic layers are addressed by associating the resistors and capacitors in serious and equal blends to address the different districts of the biomimetic
 |
Figure 6: Equivalent Circuit for Planar Lipid Membrane System |
layer. A biomimetic film can be considered to have pieces with shifted dielectric properties. An ionic current emerges because of the progression of particles and a capacitive current emerges because of the gathering of particles at the limits between the chunks under AC conditions across each slab16. In this way, every section in the layer can be reenacted by an equal mix of a resistor and a capacitor, specifically by a RC network, for ionic and capacitive flows separately. The electrochemical impedance boundaries were extricated from the exploratory bends by fitting them with a by and large acknowledged – R(RC)(RC)- model (Figure-6)17.
The film capacitance (Cm) and its thickness are interrelated by the articulation
Cm = εoεA/d (1)
Where εo is the permittivity of free space (εo= 8.854×10-14 F cm-1)
ε is the dielectric steady of the lipid bilayer (ε = 2.05) and ‘A’ is the surface area of BLM18.
The determined upsides of thickness of the BLM framed in 1.0 M, 0.1 M and 0.01 M NaCl shower arrangements separately are 4.5 nm, 5.1 nm and 5.6 nm in 1.0 M, 0.1 M and 0.01 M NaCl shower arrangements, which are exceptionally near twofold the thickness of the lecithin monolayer (2.5 nm)19,20. In this manner, the layer framework formed from the self-gathering of phospholipid particles is ended up being a bilayer film.
The capacitance (Cm) and conductance (Gm) values are scattered with recurrence and the dissemination of conductance (G) and capacitance (C) of polar (P) and hydrocarbon (H) locales of the bilayer stage are communicated as follows


The electrical properties of planar BLM obtained by fitting the experimental impedance spectra with an approximated circuit for dark lipid film in the presence and nonattendance of Ofloxacin in the NaCl shower arrangements are displayed in Table-1, 2 and 3.
Table 1: EIS- Parameters derived for the interaction of Ofloxacin in 1.0 M NaCl immersion bath.
|
S.No |
Concentration of Ofloxacin (micro molar) |
RP (kΩ) |
CP (nF) |
CH (nF) |
RM (109Ω) |
CM (nF) |
|
1 |
0 |
982 |
0.213 |
1.142 |
9.852 |
1.139 |
|
2 |
10 |
913 |
0.209 |
1.146 |
8.949 |
1.141 |
|
3 |
20 |
852 |
0.206 |
1.151 |
7.591 |
1.144 |
|
4 |
40 |
801 |
0.197 |
1.156 |
6.092 |
1.149 |
|
5 |
60 |
743 |
0.186 |
1.162 |
4.115 |
1.155 |
|
6 |
80 |
731 |
0.181 |
1.167 |
2.221 |
1.160 |
|
7 |
100 |
722 |
0.178 |
1.171 |
0.622 |
1.165 |
|
8 |
200 |
308 |
0.092 |
1.342 |
0.114 |
1.322 |
Table 2: EIS- Parameters derived for the interaction of Ofloxacin in 0.1 M NaCl immersion bath.
|
S.No |
Concentration of Ofloxacin (micro molar) |
RP (Ω) |
Cp (pF) |
CH (nF) |
RM (109Ω) |
CM (nF) |
|
1 |
0 |
1312 |
197 |
1.011 |
6.23 |
1.006 |
|
2 |
10 |
1304 |
182 |
1.018 |
5.41 |
1.012 |
|
3 |
20 |
1210 |
164 |
1.024 |
4.89 |
1.016 |
|
4 |
40 |
1105 |
151 |
1.031 |
3.37 |
1.021 |
|
5 |
60 |
933.7 |
133 |
1.038 |
2.01 |
1.027 |
|
6 |
80 |
822.4 |
75.2 |
1.236 |
0.332 |
1.231 |
|
7 |
100 |
779.2 |
33.1 |
1.239 |
0.215 |
1.233 |
|
8 |
200 |
702.4 |
29.7 |
1.238 |
0.091 |
1.235 |
Table 3: EIS-Parameters derived for the interaction of Ofloxacin in0.01 M NaCl immersion bath.
|
S.No |
Concentration of Ofloxacin (micro molar) |
RP (Ω) |
CP (pF) |
CH (nF) |
RM (109Ω) |
CM (nF) |
|
1 |
0 |
1952 |
182 |
0.919 |
2.16 |
0.916 |
|
2 |
10 |
1661 |
176 |
0.924 |
1.69 |
0.921 |
|
3 |
20 |
1228 |
166 |
0.932 |
1.34 |
0.929 |
|
4 |
40 |
1142 |
159 |
0.939 |
0.483 |
0.937 |
|
5 |
60 |
829 |
142 |
1.124 |
0.311 |
1.119 |
|
6 |
80 |
651 |
102 |
1.129 |
0.116 |
1.117 |
|
7 |
100 |
507 |
89.3 |
1.131 |
0.083 |
1.121 |
|
8 |
200 |
429 |
66.7 |
1.128 |
0.005 |
1.118 |
It is seen that the capacitance (Cp)and opposition (Rp) of the polar district decline when the segment of Ofloxacin into the center of the BLM caused an extreme change in its electrical properties. At an exceptionally high centralization of Ofloxacin, its fluidization impact will be large and expands the vulnerability of more modest particles across the BLM stage, before which the more prominent measure of charged species cross the arrangement film interface and thus conductance at the layer arrangement interface increments. With the rising centralization of Ofloxacin, its adsorption onto the BLM surface likewise expanded, which diminishes the capacitance (Cp) at the polar district because of an adjustment of dielectric steady or expansion in thickness21.
The variety of capacitance of BLM stage with the grouping of Ofloxacin in NaCl shower arrangements is displayed in Table-1,2 and 3. From these tables, obviously there is a slow expansion in the capacitance of the BLM stage with the convergence of Ofloxacin and an unexpected leap. The penetration of Ofloxacin into the BLM stage is demonstrated by the underlying expansion in capacitance because of which the immovably bound phospholipid particles are isolated and the area of BLM increments.
The bilayer hydrophobic area (CH) and the two electrical twofold layers framed at the two film arrangement interfaces (CP) add to the all out layer capacitance (Cm), which expanded with the medication portion. This can be made sense of in view of the translation given by LiviuMovileanu et al for the connection of flavonoid quercetin with planar BLM22 as follows;
Two capacitors CP and CH are in series association,

Accordingly

The capacitance of the hydrocarbon or center of the BLMis associated with the thickness and the dielectric coefficient of the phospholipid acyl chains as

Where εH-relative permittivity of the core of BLM
dH – thickness of the hydrocarbon center of the film.
At the point when the Ofloxacin particles are apportioned into the hydrocarbon center of the lipid bilayer, the all out capacitance of the layer framework changes. Consequently, when the Ofloxacin particles are parceled into the lipid bilayer, eq.5 changes as

CmOflox can be communicated as a capability that likewise relies upon the capacitance of the film without any Ofloxacin, as follows

Where

(NOflox -average number of Ofloxacin molecules per unit area of the bilayer)
(VOflox – the molecular volume of Ofloxacin)
εOflox -the dielectric coefficient of Ofloxacin)
The capacitance of the Ofloxacin (COflox) is more modest than the capacitance of the hydrocarbon center (CH) and polar district CP) even at exceptionally high groupings of Ofloxacin. This is on the grounds that the cross-sectional region of the phospholipid-involved space is a lot higher than the cross-sectional region of the Ofloxacin-involved area.
In this condition,

and

Subsequently, the complete capacitance of the model layer framework (Cm) increments with the convergence of Ofloxacin. An unexpected ascent in the all out layer capacitance is because of the substitution of countless phospholipid particles with Ofloxacin particles.
Improvement of an Impedimetric sensor for the detection of Ofloxacin in Arrangement.
From Table.1,2 and 3, it tends to be seen that the layer obstruction diminishes with an expansion in the convergence of Ofloxacin in the NaCl shower arrangements. The variety of film opposition with the centralization of Ofloxacin in NaCl shower arrangements is addressed in Figure-7a,7b and 7c. From these Figures, it is seen that the variety of film opposition with the centralization of Ofloxacin follows a straight-line relationship up to a specific focus. This straight-line relationship diminished with a lessening in the convergence of NaCl in the shower. This can be made sense of as follows. At unbiased pH, the surface charge of the BLM s framed in the NaCl shower arrangements relies upon the convergence of NaCl 23. The BLM surface has both positive and negative charges because of nitrogen bases and phosphate bunches separately in a lipid bilayer. The surface positive charges are to a great extent killed by the adsorption of Cl-particles from the shower arrangement and the surface negative charges are to some degree killed by the adsorption of Na+ particles from the shower arrangement 23. Since the limiting steady of Cl-particles onto the layer surface is around two orders bigger than that of Na+ particles, there will be generally overabundance negative charges on the film surface and this makes BLM surface negative23. With the expansion in NaCl fixation in the shower arrangement the adsorption of Na+ particle additionally expanded and the negative charge on the BLM surface reductions. An expansion in NaCl fixation likewise expanded the adsorption of Cl-particles which applies a fixing impact on the film surface and diminishes the thickness of the layer and makes the film smaller and more steady. This additionally lessens the ionic conductance of the layer because of the development of more modest particles like K+ and Na+.
When Ofloxacin starts to get partitioned into BLM, i.e. enters into the core of BLM, it shows a fluidization effect and increases the membrane conductance. Moreover, the adsorption of Ofloxacin onto the membrane surface also develops some pores and surges ionic conductance across the BLM- bilayer membrane phase. Based on concentration of Sodium Chloride decreases in the electrolytic bath, the stability and density of the membrane decrease due to this reason resistance doesn’t follow a linear association with the absorption of Ofloxacin. Thus, in a 1.0 M NaCl bath Ofloxacin can be determined up to 100 µM. The bath detection limit decreases due to a decrease in Sodium Chloride concentration.
 |
Figure 7: (a,b and c). Variation of membrane resistance with the concentration of Ofloxacin. |
Conclusion
The segment of Ofloxacin molecules into the model bilayer lipid layer framework was explained by utilizing EIS-electrochemical impedance spectroscopy. Ofloxacin molecules are isolated into the BLM stage and caused fluidization of the hydrocarbon centre of the bilayer lipid film framework, which is reflected in the expansion in layer conductance or abatement in film obstruction. Sodium Chloride concentration in the bath impacted the electrical properties of the BLM. The layer capacitance and resistance diminished film thickness expanded with a decline in the convergence of NaCl in the electrolytic shower. An impedimetric sensor was made for the assessment of Ofloxacin without using catalysts. The recognizable proof scopes of Ofloxacin additionally rely upon Sodium Chloride fixation.
Conflict of Interest
There is no conflict of interest.
References
- Longzhen Zheng, LeyanXiong, Dan Zheng, Yindi Li, Qiang Liu, Kui Han, Wen Liu, Kun Tao, Shaoming Yang, Jian Xia and L. Zheng, Talanta, 2011, 85, 43–48.
CrossRef - Edward, T.; Castellana Paul .; Cremer, S. Surface Science Reports, 2006,61, Issue 10, 429-444.
CrossRef - Anna Puiggalí-Jou, Jan Pawlowski, Luis J. del Valle, Catherine Michaux, Eric A. Perpète, Slawomir Sek, Carlos Alemán. ACS Omega, 2018,3(8), 9003-9019.
CrossRef - H.Ti. Tien, “Bilayer Lipid Membranes (BLM) – Theory and Practice, Marcel Dekker Inc, New York, 1974.
- Jain, M.K. The Bimolecular Lipid Membrane: A System, Van Nostrand Reinhold, New York, 1972.
- Hanke, W.; Schlue, W.R. Planar Lipid Bilayers-Methods and Applications, Academic Press, 1993, pp. 1–78.
CrossRef - Gerald Wiegand, Noah Arribas-Layton, Heiko Hillebrandt, Erich Sackmann, and Peter Wagner. The Journal of Physical Chemistry B, 2002,106 (16), 4245-4254.
CrossRef - Alagappan, M.;.Kandaswamy, A.; .Kumaravel, M; Rameshkumar, S. Arabian Journal of Chemistry, 2020,13, 423-430.
CrossRef - Thévenot, D.R.; Toth, K.;. Durst, R.A.; Wilson, G.S. Biosens. Bioelectron., 2001,16 ,121
CrossRef - Steven J.Cooper. Brain Research Bulletin, 1986,17, 5, 627-637.
CrossRef - Naumowicz, M.; Figaszewski, Z.Bioelectrochemistry, 2003,61, 21-27.
CrossRef - Guidelli, R.; Becucci, L. Modern Aspects of Electrochemistry, 2012,53, 147–266.
CrossRef - Tien, H.T.; Ottova, A.L. J.Membrane.Sci, 2001,189,83-117.
CrossRef - Coster, H.G.L.; Smith, J.R. BiochemicaBiophysica Acta, 1974,37, 151-164.
CrossRef - Han, X.;Tong, Y.; Huang, W.; Wang, E. Journal of Electroanalytical Chemistry,2002, 523, 136-141.
- Watt Harlos, K.; Marsh, D. Biochim. Biophys. Acta,1981, 645, (1981) 91-96 (1981)
CrossRef - Favero, G.; D’Annibale, A.; Campanella, L.; Santucci, R.; Ferri, T. Analytica Chimica Acta,2002,460, 23-34.
CrossRef - Behpour, M.; Ghoreishi, S.M.; Gandomi-Niasar, A.;. Soltani, N.; Salavati-Niasari, M.J. Mater. Sci, 2011, 44(10), 2444-2453.
CrossRef - Franks, N.P.;. Lieb, W.R. J.Mol.Biol, 1979, 133 (4), 469-500.
CrossRef - LiviuMovileanu, IoanaNeagoe, Maria Luiza Flonta. Int. J. of Pharmaceutics, 2005, 205,135–146.
CrossRef - Kotynska,J.; Figaszewski,Z.A. Biochimica et BiophysicaActa(BBA) Biomembranes, 2005,1720,1-2,22-277.
CrossRef - Hideo Matsumura, Ken-ichi Watanabe, Kunio Furusawa. Colloids and Surfaces A, 1995, 98,175–184.
- .Chiu, S.W.; Jakobsson, E. J. Mashl,BioPhycs, 2002,83(4),1842-1853.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









