Experimental investigation of Inhibition on Mild Steel Corrosion in two Different Acid Mediums by Synthesized Piperazin Derivatives
1Department of Chemistry, Bharathiyar University, Coimbatore-641046, Tamilnadu, India.
2Department of Chemistry, Government Art's College (Autonomous), Salem-636007, Tamilnadu, India.
Corresponding Author E-mail: vimmahes@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/390425
Article Received on : 09 Jun 2023
Article Accepted on : 17 Jul 2023
Article Published : 01 Aug 2023
Reviewed by: Dr. Anand Balu
Second Review by: Dr.V.Collins Arun Prakash
Final Approval by: Dr. Tanay Pramanik
Corrosion prevention studies show a predominant trend in the recent era. Especially mild steel material was used in many industrial sectors because it has specific properties like malleability and ductility. Corrosion inhibition action of a novel synthesized N-(1,3-benzothiazol-2-yl)-2-[4-furan-2-carbonyl)piperazin-2-yl]acetamide (1,3BFCPA) compound was performed on MS-mild steel material in 1 N HCl and 1 N H2SO4 medium. Using the mass loss data, the inhibitor efficiency has been evaluated on both the acidic and alkaline mediums. The inhibitor shows a maximum inhibitor efficiency on both acidic mediums, but when comparing 1N sulfuric acid, it shows a better efficiency of 90%. Based on surface coverage analysis (ϴ) from mass loss data, adsorption isotherms were interpreted to fit the behavior of the inhibitor over the surface of MS material, which shows a Langmuir adsorption isotherm.
KEYWORDS:Adsorption; Corrosion Inhibition; Langmuir; Mild Steel; Novel inhibitor
Download this article as:| Copy the following to cite this article: Vimala M, Chandrasekaran V. Experimental investigation of Inhibition on Mild Steel Corrosion in two Different Acid Mediums by Synthesized Piperazin Derivatives. Orient J Chem 2023;39(4). |
| Copy the following to cite this URL: Vimala M, Chandrasekaran V. Experimental investigation of Inhibition on Mild Steel Corrosion in two Different Acid Mediums by Synthesized Piperazin Derivatives. Orient J Chem 2023;39(4). Available from: https://bit.ly/3rUttCg |
Introduction
To remove the scale deposited over the metal surface, it has been treated in acid pickling baths. The choice of inhibitors generally depends on the type of acid medium used, concentration, temperature, and nature of the metal chosen 1–3. Organic-based inhibitors are known to be the best at protecting metals and equipment made of metal during transportation and storage 4–7. Many researchers investigate corrosion inhibitor studies by using various synthesized organic compounds that contain heteroatoms, oxygen and sulphur atoms in their rings 8–11. On a report of consumption productivity on copper and its combination, the benzotriazole compound shows a superior inhibition proficiency 12,13. Three salts of Morpholine in particular Morpholine carbonate, phosphate, and borate, displayed 90% hindrance productivity, while outstanding salts showed an extremely low rate of 40% restraint proficiency 14,15. In 1.11N Hydrochloric acid medium at three different temperatures, the synthesized Formazan derivative (FD) product was used as an inhibitor 61. Using organic-based inhibitors that attribute an interaction with the surface of the metal via adsorption, either physical adsorption or chemical adsorption, which forms a coordinate bond between metal and inhibitors 17, Therefore, in my investigation, (1,3BFCPA) was used as a corrosion inhibitor in 1N HCl and 1N H2SO4 medium at room temperature at estimated two-hour time intervals. Inhibitor studies were performed using weight loss methods, and results were confirmed by electrochemical studies. Adsorption studies also reveal a better result when using (1,3BFCPA) corrosion inhibitor on mild steel material.
Materials and Methods
Chemistry
Synthesis of Primary Compound N-1, 3-benzothiazole-2-yl-2-chloro-acetamide
2-amino benzothiazole (1.5g, 0.01 mole) and potassium carbonate (4.14, 0.030 mol) in 25 mL dimethylformamide chloroacetyl chloride (1.03 mL, 0.013 mol) was added slowly at Zero degree Celsius. The reaction was processed at room temperature (RT) for six hours with constant stirring. The reaction mixture was slowly added to ice water (150 mL) and extracted with acetooxyethane (2x 150 mL). The combined layer of acetooxyethane was washed with water (1×200 mL) and saturated NaCl (1×200 mL). The concentrated acetooxyethane layer was carried out under reduced pressure. After the extraction, the solid product was thoroughly washed with diethyl ether to get the pure product N-1, 3-benzothiazol-2-yl-2-chloro-acetamide. Light brown solid; Yield 91%; M.P. 241-242OC.
Synthesis of N-(1,3-benzothiazol-2-yl)-2-[4-furan-2-carbonyl)piperazin-2-yl]acetamide
To the mixture of N-1, 3-benzo-thiazol-yl-2-chloro-acetamide (1g, 0.0044 moles) and 1-(2-furoyl) piperazine (0.864, 0.0048 moles) in Dimethylformamide potassium carbonate (1.52 g, 0.011 moles) was added. For eight hours at 700 C, the resulting reaction mixture was heated. The hot reaction mixture was processed in a controlled manner using ice cooling and filtered. To the extracted filtrate, water (100 mL) was added and extracted with acetooxyethane (2×150 mL). The combined acetooxyethane was washed with brine solution (1×200 mL), desiccated on sodium sulfate and under reduced pressure it was concentrated. The resulting products were recrystallized from ethyl alcohol. Pale yellow solid; Yield 88%; M.P. 265-266OC.The synthetic route for the preparation of (1,3BFCPA) is shown in Scheme 1. To get N-1, 3-benzothiazol-2-yl-2-chloro-acetamide, 2-aminobenzothiazole was reacted with 2-chloroacetyl chloride in the presence of Potassium carbonate.
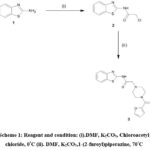 |
Scheme 1: Reagent and condition: (i).DMF, K2CO3, Chloroacetyl chloride, 0°C (ii). DMF, K2CO3,1-(2-furoyl)piperazine, 70°C |
Various concentration of inhibitor such as 10, 20, 40, 60, and 80,100ppm was prepared from the Stock solutions.
Specimen Preparation (MS)
Mild steel material (MS) was cut into coupons of 5 ´ 1 cm2 having the percentage composition Iron -99.718, Nickel- 0.012, Molybdenum – 0.016, Chromium- 0.038, Silicon- 0.014, Phosphorus- 0.011, Silicon- 0.006, Manganese- 0.171, Carbon- 0.014.
Mass Loss Data
In 1N HCl and 1N H2SO4 at room temperature for two hours in various inhibitor concentrations, MS specimens were immersed. After the completion of two hours, the coupons were removed from the immersed medium, and washed with doubled distilled water and acetone. From the initial weight and final weight difference mass loss data was calculated. Mass loss data such as the rate of corrosion and the efficiency inhibitor was calculated using the formula,
Corrosion Rate (CR)= (87.6 x Difference in Weight) / (Density x Area x Time)
The efficiency of the Inhibitor (Inhibition efficiency),
Inhibition efficiency (IE )% = [(Wo–Wi)/Wo] x 100
Where (Wi) and (Wo) are the initial and final weight loss (in g) of MS in the inhibitor presence and absence respectively
Adsorption parameters
Langmuir adsorption isotherm was calculated using the parameters of adsorption such as ΔGads – free energy of adsorption, ΔHads – enthalpy of adsorption and ΔSads – adsorption entropy, which is presented in the following equation.
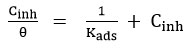
Where Cinh is the concentration of the inhibitor used, Kads is the Equilibrium Constant, and (θ)-Theta is surface coverage.
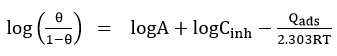
Where A is a constant, Qads is the adsorption of heat or enthalpy of adsorption.
Kads -Equilibrium constant was calculated from the intercepts value of (1/Kads) of the straight line received from the plot of Concentration of inhibitor / Surface coverage (θ) Vs Concentration of inhibitor. Adsorption of free energy was calculated using equilibrium constant value (Kads ) to the below
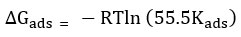
From the value, a direct line is obtained from the graph which is plotted between log (θ /1-θ) Vs 1000 / T at many concentrations of inhibitor used. The slope is formed from the instant line which is -∆Hads / 2.303R. Enthalpy of activation (ΔHads) calculated from the slop formed. The entropy of activation (ΔSads) is arrived from the equation.
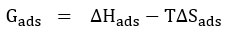
Result and Discussion
Mass Loss Data
After the preliminary process, MS specimens are polished with various ranges of emery sheets and punched with numbers at the top. Numbered plates were immersed in different concentrations of the inhibitor in 1N HCl and 1N H2SO4 medium for two hours at room temperature. From the weight loss data, the difference in weight of the plate before and after dipping it in the test solution was calculated and shown in Table 1.
Table 1: Corrosion parameter in the Presence and Absence of (1,3BFCPA) inhibitor on mild steel in different acidic medium solutions.
|
S.No |
Conc. of inhibitor PPM |
Acid Medium |
|||
|
1N HCl |
1N H2SO4 |
||||
|
Rate of Corrosion (mmpy) |
The efficiency of the inhibition (%) |
Rate of Corrosion (mmpy) |
The efficiency of the inhibition (%) |
||
|
1. |
blank |
19.8380 |
— |
26.6366 |
— |
|
2. |
10 |
7.1334 |
64.04 |
7.0231 |
73.63 |
|
3. |
20 |
6.4643 |
67.41 |
6.0183 |
77.40 |
|
4. |
40 |
5.4610 |
72.47 |
5.2380 |
80.33 |
|
5. |
60 |
4.1232 |
79.21 |
4.7922 |
82.00 |
|
6. |
80 |
2.8971 |
85.39 |
2.6743 |
89.96 |
|
7. |
100 |
2.5633 |
87.07 |
2.0061 |
92.46 |
 |
Figure 1: Corrosion rate Comparison of (1,3BFCPA) inhibitor on mild steel in both acidic Click here to View Figure |
Medium for two hours
The maximum inhibition efficiency of (1,3BFCPA was 87.07% in 1N HCl and 92.46 % in 1N H2SO4 obtained at the optimum concentration (100 ppm). With the addition of the inhibitor, a strong protective layer is formed on the surface of the metal. The rate of corrosion and efficiency of the inhibitor (1,3BFCPA) are represented in Figs. 1 and 2.
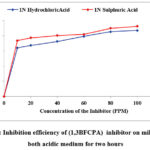 |
Figure 2: Inhibition efficiency of (1,3BFCPA) inhibitor on mild steel in both acidic medium for two hours |
Adsorption isotherm
The mass loss data calculated, which gives information about the interaction of the inhibitor (1,3BFCPA) on the surface of the metal coverage (θ) in 1N HCl and 1N H2SO4 for two hours at room temperature, is given in Table 2. A barrier is created for mass and rate change during the adsorption of (1,3BFCPA) on the MS surface. The situation prompted the protecting nature of a steel surface from the movement of serious particles in 1N HCl and 1N H2SO4 arrangement. The level of protection expanded with surface assurance by utilizing the adsorbed particles.
Table 2: Adsorption isotherm parameters of (1,3BFCPA) on mild steel in both acidic medium
|
Inhibitor |
(1,3BFCPA) |
|||
|
Concentration (M) |
Acid medium |
MS-Surface coverage(θ) |
∆Gads (KJ / mol-1) |
[K x(10-2)] |
|
Optimum Concentration (100 PPM) |
1N HCl |
0.87 |
-6.056 |
1.80 |
|
1N H2SO4 |
0.92 |
-5.031 |
1.79 |
|
 |
Figure 3: Langmuir adsorption isotherm for adsorption of (1,3BFCPA) on mild steel in both acidic mediums. |
From Table-2 the data shows that there is no chemisorption, but there is physisorption between the inhibitor and metal surface in both acidic mediums. Fig-3 clearly shows the fascination between the surface of the metal and inhibitor molecules. Adsorption of loose power (ΔGads), in 1N HCl and 1N H2SO4 became observed to be -6.056 kJmol−1 and -5.031 kJmol−1. The values suggest physisorption takes place in the inhibitor structures.
Conclusion
Synthesized organic inhibitor synthesized N-(1,3-benzothiazol-2-yl)-2-[4-furan-2-carbonyl)piperazin-2-yl]acetamide (1,3BFCPA) shows better efficiency on both the acid medium 1N HCl and 1N H2SO4. On comparing the acidic medium efficiency of the inhibitor, it shows 92.46% on 1N H2SO4 compared to 1N HCl medium. Based on mass loss data the surface coverage (ϴ) is calculated Langmuir adsorption isotherm is obtained which shows a strong attraction between inhibitor and metal surface on both acidic mediums.
Acknowledgement
The author gratefully thanks Dr.Ganavel, Director, CHEMKOVIL Lab, Mettur, Tamilnadu for providing Lab facilities to carry out the process.
Conflict of Interest
There is no conflict of interest.
Funding sources
There is no funding source.
Reference
- Dharuman, S.; Wallace, M.J.; Reeve, S.M.; Bulitta, J..B.; Lee, R.E. Molecules. 2022, 27(5),1518.
CrossRef - Ameya, A.; Chavan.; Nandini, R. Molecules. 2007, 12, 2467- 2477.
CrossRef - Quraishi, M.A.; Rawat, J.; Ajmal, M. J Appl Electrochem. 2000, 30,745-751
CrossRef - Hammouti, B.; Aouniti, A.; Taleb, M.; Brighli, M.; Kertit, S. Corrosion. 1995, 51,411
CrossRef - Tebbji, K.; Aouniti, A.; Benkaddour, M.; Oudda, H.; Bouabdallah, I.; Hammouti, B.; Ramdani, A. Prog Org Coat. 2005, 54,170.
CrossRef - McConnell, R. Metal Finishing. 2008, 106, 23-27.
CrossRef - Andreev, N. N.; Kuzentsov, Y. I.; Fedotova, T. V.Protect. Metals. 2001, 37, 1-8.
CrossRef - Furman, A. Proceedings 9th Europ Symp on Corrosion Inhibitors. Ferrara. 2000, 465-479.
- Batidas, D. M.; Cano, E.; Mora, E. M. Anti-Corrosion Meth. Mater. 2005, 52, 71-77.
CrossRef - Sastri, V. S. Corrosion Principle and Application. John Wiley and Sons., New York. 1998, p. 787.
CrossRef - Gao, G.; Liang, C. H. Corrosion Sci. 2007, 49, 3479-3493.
- Subramanian, A.; Sathiya Priya, A. R.; Vasudevan, T. Mater. Chem. Phys. 2006,100, 193-197.
CrossRef - Zang, D. Q.; Gao, L. X.; Zhou, G. D.Mater. Corrosion. 2008, 58, 594-598.
CrossRef - Rammelt, U.; Koehler, S.; Reinhard, G. Corrosion Sci. 2009, 51, 921-925.
CrossRef - Bellakhal, N.; Dachraoui, M. Mater.Chem. Phys. 2004, 85, 366-369.
CrossRef - Subramanian, A.; Gopalakrishnan, R.; Boopathi, C.S.; Balakrishnan, K.; Vasudevan, T.; Natesan, M.; Rangaswami, N. S. Bull. Electrochem. 1998,14, 289-290.
- Vuorimen, E.; Skinner, W. Br. Corrosion J. 2002, 37, 159-160.
CrossRef - Venkatesan, P.; Anand, B.; Matheswaran, P. e- Journal of Chemistry. 2009, 6(S1), S438-444
CrossRef - Suroor Athar, S.M.; Ali. H.; Quraishi, M.A. Anti-Corros Methods Mater. 2001, 48,251
CrossRef - Abboud, Y.; Abourriche, A.; Saffaj,T.; Berrada, M.; Charrouf, M.; Bennamara ,A.; Hannache,H. Desalination.2009,237, 175.
CrossRef - Ozcan ,M.; Dehri,J.;M Erbil, M.Appl Surf Sci,2004,236, 155.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.










