Degradation of Biebrich Scarlet Textile Dye by using MBIR Dowex-1x8
Lal Chand Yadav , Hariom Jaimini
, Hariom Jaimini , R. C. Meena
, R. C. Meena , and S. L. Meena*
, and S. L. Meena*
Department of Chemistry, Jai Narain Vyas University, Jodhpur (Rajasthan), India.
Corresponding Author E-mail: slmeena.jnvu@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/390416
Article Received on : 22 May 2023
Article Accepted on :
Article Published : 10 Jul 2023
Reviewed by: Dr. Habib Md Ahsan
Second Review by: Dr. A.K Houssien
Final Approval by: Dr. Vijendra Singh
In the present study, Methylene blue immobilized resin (MBIR) Dowex-1x8, an effective heterogeneous photocatalyst, is used to perform the photocatalytic degradation of Biebrich Scarlet (BS) Textile dye. The degradation of the dye was systematically investigated under different parameters such as pH of the Biebrich Scarlet dye solution, different Photocatalyst doses, dye solution concentrations, and UV/Visible light intensity. Optimum results are shown for various parameters, such as temperature of 30oC, pH of 7.5, photocatalyst loading dose of 2.0 g, and light intensity of 10.5mW/cm2. Maximum degradation is shown on the above optimum condition and complete degradation was held in 210 minutes on the above optimum condition.
KEYWORDS:Biebrich Scarlet dye; Dowex-1x8; Methylene Blue; Optical density; Photocatalyst, Immobilization
Download this article as:| Copy the following to cite this article: Yadav L. C, Jaimini H, Meena R. C, Meena S. L. Degradation of Biebrich Scarlet Textile Dye by using MBIR Dowex-1x8. Orient J Chem 2023;39(4). |
| Copy the following to cite this URL: Yadav L. C, Jaimini H, Meena R. C, Meena S. L. Degradation of Biebrich Scarlet Textile Dye by using MBIR Dowex-1x8. Orient J Chem 2023;39(4). Available from: https://bit.ly/3D4zVbZ |
Introduction
The dyes are used in industries at different scales, such as textiles, paints, inks, plastics, cosmetics, etc. These are creating environmental problems. Dye is mainly used in the clothing industry1-2.In many countries, regulations have been adopted to discharge dye-containing dyed effluents. Azo dyes are the most prevalent dyes produced chemically. Azo dyes, including the dyes of the Biebrich Scarlet (BS), are particularly problematic due to their high prevalence. It is used in textiles (wool, silk, and cotton) where bright colours with a fluorescent effect are needed. Biebrich Scarlet is a common anionic azo dye. Biebrich Scarlet dyes include two groups of -N=N-, two groups of sulphonate (SO3–), and two sodium atoms3-4.The dye is non-biodegradable, its by-products are highly toxic, and poses a danger to human health and aquatic life5. Active carbon adsorption and chemical coagulation are commonly used to treat such dyes. However, these methods only transfer water dyes to solids, and the ideal solution requires additional treatment6-8.
Dyeing is a fundamental operation in textile fiber processing that result in the production of colored wastewater. Thus, the use and disposal of textiles and other industrial effluent is an important consideration in assessing the environmental impact of textile manufacturing. In the Textile industry, different types of dyes are used, in which azo dyes are the most common type of dye that contains an azo group (-N=N-)9. Textile effluent can be removed using physical and chemical methods such as absorption, precipitation, air removal, reverse osmosis, ultrafiltration, and flocculation. Hence, the need to create chemical treatments that are more successful at removing colors from wastewater10-15.
Applying advanced oxidation processes (AOPs) to totally remove colors has recently received much attention. AOPs works by producing reactive species such as OH radical, which have quick and no selective oxidation for various organic pollutants16-20. AOPs include photocatalysis system, such as semiconductor and oxidant combinations as well as semiconductor and light combinations. The majority of organic pollutants, including organic reactive colors, have been completely mineralized as a result of heterogeneous photo catalysis, which has become a significant destructive technique21-25. Supported photo catalysts have been developed as a solution to this issue. To efficiently use solar energy, the UV/Visible light induced photocatalyst MBIR Dowex-11 has completed a phenomenal success26.
The MBIR Dowex-1×8 photocatalysts has been developed as a solution to this issue 27,28.To our knowledge, no one has reported the photocatalytic degradation of the MBIR Dovex-1×8 dye to Bibrich Scarlet. Taking all the above factors into the account, the present study reported that it utilized photocatalytic Methylene Blue Immobilized Resin (MBIR) Dowex-1×8 with optimum values of various parameters affecting the degradation of Biebrich Scarlet dye. The dose of the photocatalyst, the pH, and the initial concentration of the sample has been analyzed.
Materials and Methods
The dye Biebrich Scarlet purchased from Loba chemie (India), the photocatalyst Dowex-1×8 resins, 20-50 mesh size (Sisco chem) and Methylene blue hydrate (Loba chemie, India). The different solution was prepared in the double distilled water. The desired pH conditions were adjusted using standardized solutions of 1N NaOH and 1N H2SO4. The pH of the prepared solution is determined with the help of a digital pH meter. All experiments were conducted at ambient temperature with magnetic stirrer. The structure (figure-1) and properties of Biebrich Scarlet dye are given in Table 1.
Table 1: Properties of Biebrich Scarlet (BS) dye
|
Dye Name : Biebrich Scarlet(BS) |
Molecular Weight : 556.5gm/mol |
|
C.I. Name : Acid Red 66 |
Class : Azo Dye |
|
C.I. No. : 26905 |
Nature : Acidic |
|
Other Name : Croceine Scarlet |
λmax : 507 nm |
|
Molecular Formula : C22H14N4O7S2Na2 |
 |
Figure 1: Structure of Biebrich Scarlet dye. |
Preparation of photocatalyst
The photocatalyst is prepared from Dowex-1×8 resin, 20-50 mesh sizes, and Methylene Blue hydrate. To immobilize, prepare the Methylene Blue hydrate solution in a concentration of M/100 in distilled water, add the Dowex-1×8 resin to the solution, and shake it well, immobilizing the spores for at least 24 hours. After this the solution is kept stable in a dark place. Then the prepared immobilized resin is filtered and used as a photo catalyst by washing thoroughly with double distilled water 2-3 times.
Purpose of Methylene Blue
Methylene Blue (MB) is a cationic photosensitive dye. Particles of MB are present in the pores of the immobilized resin. The excitation of MB dyes molecules by absorbing photons of light from radiation. When the photon falls, the electrons are excited and move from the electronic transition valence band (VB) to the conduction band (CB), allowing the electrons of Methylene blue to go from a single state to a triplet state via inter-system crossing (ISC). This study shows that the formation of •OH radical and superoxide ion (O2−) in the pores of the immobilization resin, they are highly oxidative in nature.
Experimental Setup and Degradation Process
Photodegradation experiments have been conducted in a glass reactor that contains a solution of Biebrich Scarlet dye and a photocatalyst. The solution is continuously stirred with the help of magnetic stirrer during the experiment. The solution is lit above the reactor with a 200-watt tungsten lamp (Indian Philips) and emits visible light-like radiation (Figure 2). The radiation lamp was surrounded by Aluminum reflectors to avoid any radiation loss. The mechanism of photocatalytic degradation systems under UV/Visible light is the reaction of extremely active oxygen species and electron excitation technology, which primarily react with the dye molecules that cause photodegradation.
 |
Figure 2: Experimental Setup of Photoreaction chamber |
Analytical Approach
The change in dye concentration was observed by the CSIM 500 microprocessor UV/Visible spectrophotometer at λmax. We eject 5 ml solution by pipette a time interval of 10 minutes, filter catalyst particles, record the absorbance spectrum of the dye solution and the rate of degradation was observed in terms of change in the light intensity at λmax of the dye. We calculated the degradation efficiency (%):

Where Co and C correspond to the initial and final concentrations of BS dye before and after photo irradiation.
So the above formula is related to the absorbance of BS dye (concentration). According to the concept of literature, absorbance is directly proportional to concentration. In this calculation, we are using absorbance with respect to the BS dye concentration. Absorbance is the quantity of light absorbed by a solution, also known as optical density.
|
Time |
Optical density |
Degradation efficiency % |
|
0 |
1.078 |
0 |
|
10 |
0.809 |
24.96 |
|
20 |
0.672 |
37.66 |
|
30 |
0.552 |
52.66 |
|
40 |
0.471 |
56.31 |
|
50 |
0.412 |
61.78 |
|
60 |
0.361 |
66.51 |
|
70 |
0.316 |
70.68 |
|
80 |
0.279 |
74.11 |
|
90 |
0.238 |
77.92 |
|
100 |
0.204 |
81.07 |
|
110 |
0.167 |
83.81 |
|
120 |
0.144 |
86.64 |
|
130 |
0.118 |
89.05 |
|
140 |
0.098 |
90.91 |
|
150 |
0.073 |
93.22 |
|
160 |
0.051 |
95.26 |
|
170 |
0.038 |
96.47 |
|
180 |
0.032 |
97.03 |
|
190 |
0.028 |
97.41 |
|
200 |
0.025 |
97.68 |
|
210 |
0.023 |
97.96 |
Result and Discussion
Effect of Photocatalyst amount
The amount of the photocatalyst affects the rate of photocatalytic degradation of BS dye. We find out change in the amount (from 1.0 gm to 2.5gm) of photocatalyst on the rate of degradation at constant other parameters. We observed that as the amount of photocatalyst increases, the rate of degradation of BS dye also increases. The amount of photocatalyst is increases due to the availability of more surface area for the absorption of photons and the cooperation of molecules in the reaction mixture with the photocatalyst. The outcome is that the number of oxidizing intermediate species (holes, •OH radicals, and O2−ions) increased, which increased the rate of degradation. In figure-3 the graph between optical density and Time shows that the photocatalytic amount on degradation rate.
 |
Figure 3: Effect of photocatalyst amount on optical density |
Effect of pH
We found that the variation in pH of BS dye solution, the degradation rate of dye molecules is very interesting for us. The outcome shows that in highly acidic pH range below pH 5, the degradation rate is very slow. When the pH of the solution is highly basic, the rate of degradation increases rapidly, and the pH range from 7.3 to 11, the rate of degradation is very good. At a constant photocatalytic amount 2.0g/100ml, BS dye concentration 30mg/L, and light intensity 10.5mWcm-2, the rate of degradation begins to increase as pH increases. As a result, we concluded that the degradation rate of BS dye in highly basic medium is higher than that of the acidic medium. The increase in photocatalytic degradation is due to the increased availability of •OH radical in the pH range of 7.3 to 11. The formation of more hydroxyl radicals (•OH) that combine with holes formed by electronic excitation in photocatalysts. Super oxides (O2−) are less likely to cause photocatalytic degradation than hydroxyl radicals. As pH increases above 11, the degradation rate slows down. This effect may be caused by competition between •OH radicals and O2− for attachment to the active site of the photocatalyst. A graphical representation of the pH effect on the rate of degradation is shown in figure 4.
 |
Figure 4: Effect of solution pH on Optical density |
Effect of initial BS dye Concentration
The degradation rate has been studied by varying the initial Biebrich Scarlet dye concentration from 10 to 50 mg/L based on a constant photocatalyst (2.0 g/100 ml), pH 7.5, and light intensity 10.5mWcm-2. As the dye concentration increases, the rate of degradation decreases (shown in Figure 5). The effect that may be caused by the degradation rate is due to the following reasons: the reduction in the number of photons striking the photocatalyst surface due to the increase in the dye concentration, and the reduction in the number of photocatalyst molecules by excitation. As a result, the rate of formation of •OH radical and super oxide ion (O2−) is reduced, also the rate of degradation is reduced. Because a limited amount of dye molecules is attached to the activated site of the photocatalyst and the remaining dye molecule remains present in solution until the previously attached molecule are degrade, the number of activated sites of the photocatalyst also decreases because of the excitation of the photocatalyst molecule. Less number of Photons is available at higher concentrations; the number of dye molecules is also greater, leading to greater competition between dye molecules attached to the activated photocatalyst site, thereby reducing the degradation rate.
 |
Figure 5: Effect of initial BS dye concentration on optical density |
Effect of UV/Visible light intensity
We observed the variation of light intensity on the rate of degradation. We conclude that if the light intensity increases, degradation rate of BS dye molecules increases to a lap of time, and after that time, invariable in the degradation rate of the dye is observed at that light intensity. This change in degradation rate of the dye molecule is due to the change in intensity of the light. As the intensity of light increases (from 5.4 mWcm-2 to15.5 mWcm-2), the number of photons reaching at the surface of the photocatalyst increases, the number of pores, hydroxyl radical (•OH), and super oxide ions (O2−) increases, and the degradation rate of dye molecules increases. This can result in the availability of the maximum number of photons required for the excitation within a constant range of photon light intensity after a significant change in the rate of degradation is observed because the excitation does not require more photons. Since all photocatalyst molecules are active in fixed light intensity ranges, the degradation rate remains unchanged when light intensity increases to any range (Figure 6).
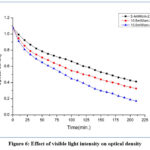 |
Figure 6: Effect of visible light intensity on optical density |
FTIR Analysis of BS Dye
FTIR plot (a, before degradation of dye) and (b, after degradation of dye) shows the comparative study of before and after the degradation of BS azo dye. In the plot (a) show IR frequencies of azo bond lie in between 1500-1600 cm-1(Alica Bartosova et al. 2017)29. But in the plot (b), there is no peak observed in between 1500-1600 cm-1. So below IR frequencies data shows that there is no azo bond is present after the degradation of BS dye. In figure 7 graph (b) show the IR study for the degradation of BS dye.
 |
Figure 7: FTIR analysis for BS dye (a) Before degradation, (b) After degradation. |
Conclusion
This study focuses on the evaluation of Biebrich Scarlet dye degradation with an MBIR Dowex-1×8. The system operates under different conditions (dye concentrations, amounts of photocatalyst, light intensities, pH). The change in the transparency of the solution from a colored dye solution to a transparent solution reflects the favorable outcome of the practical setup of the devices in implementing the MBIR Dowex-1×8 photocatalyst. The maximum degradation efficiency 98% is obtain at optimum condition (dye concentration 30mg/L, amount of photocatalyst 2.0g/100ml, light intensity 10.5mWcm-2, pH 10.5) These result shows that the new reactor setup using MBIR Dowex-1×8 photo catalysis textiles industry provide a prominent technology for improving the standard of wastewater from effluent degradation plants. We conclude, this photocatalyst (MBIR dowex-1×8) has a high ability to degrade simple azo dye molecules and purify textile effluent containing high levels of dye (mostly azo dyes) after extensive testing. We looked at the effects of different parameter:
Variation of dye concentrations: As the dye concentrations increase, dye degradation rates decrease.
Variation in the amount of photocatalyst: As the amount of the photocatalyst increases, the degradation rate of dye is also increases.
Change in pH: The degradation rate is very low in the acidic medium, but in the basic medium, the degradation rate is high.
Variation in Intensity of light: The degradation rate of dye molecule increases to a particular extent as the light intensity increases, and there is no further change in the degradation rate after that.
FTIR analysis of the degraded dye solution using immobilized Dowex-1×8 is carried out after a photo degradation process of 210 min. The dye was completely degraded in MBIR dowex-1×8, and no azo bonds were detected in the degraded dye. The decolorizing of the solution was due to degradation.
Acknowledgement
We are grateful to the Head, Department of Chemistry for providing the necessary facilities.
References
- Senthilraj, A.; Subash, B.; Krishnakumar, B.; Swaminathan, M.; Shanthi, M. Mater. Sci. Semicond. Process., 2014, 22, 83–91
CrossRef - Pyrzynska K.; J. Environ. Chem. Eng. 2019, 7, 102795
CrossRef - Kansal, S.K.; Ali, A.H.; Kapoor, S. Desalination, 2010, 259,147–155
CrossRef - Begum, S.; Mishra, S.R.; Ahmaruzzaman, M. Environ. Sci. Pollut. Res. 2022, 29, 87347–87360
CrossRef - Neppolian, B.; Choi, H.C.; Sakthivel, S.; Arabindoo B. Journal of Hazardous Materials., 2002, 89 303–317.
CrossRef - Quarta, A.; Novais, R.M.; Bettini, S.; Pullar, R.C.; Piccirillo, C. J. Environ. Chem. Eng., 2019, 7,102936
CrossRef - Koli, P.B.; Kapadnis, K.H.; Deshpande, U.G. Appl. Water Sci. 2018,8, 196
CrossRef - Patil, M.R.; Shrivastava,V.S. Desalination Water Treat. 2015, 57, 1-9
CrossRef - Attallah, O.A.; Ghobashy, M.A.; Nebsen, M. RSC Adv. 2016, 6, 11461–11480
CrossRef - Meena, V.K. International J. Adv. Sci. Res. 2016, 7, 58-62
- Ladakowicz, L.; Solecka M.; Zylla, R. J. Biotechnol. 2001, 89, 175-184
CrossRef - Robinson, T.; Marchant, R.; Nigam, P. Biores. Technol. 2001, 77, 247-255
CrossRef - Zamora, P.G.; Kunz, A.; Moraes, S.G.; Pelegrini, R.; Moleiro, P.C.; Duran. Chemosphere. 1999, 38, 835-852
CrossRef - Yang, W.; Ligang G.; Wanyong M.; Haihui J.; Xiangqian P.; Ind. Eng. Chem. Res. 2015, 54, 2279–2289
CrossRef - Khemani, L. D.; Srivastava, M. M.; Srivastava, S.; Chemistry of Phytopotentials: Health, Energy and Envi. Persp. 2012, ISBN: 978-3-642-23394-4
CrossRef - Georgiou, D.; Melidis, P.; Gimouhopoulos, K. Dyes Pigments. 2002, 52, 69–78
CrossRef - Tabrizi, G.B.; Mehrvar, M. J. Environ. Sci. Heal. A.2004,39, 3029-308
CrossRef - Sameiro, M.; Goncalves, T.; Pinto Elisa, M.S. Dyes and Pig. 2005, 64, 135-139
CrossRef - Poulios, I.; Tsachpinis,I. J. Chem. Tech. Bio. Technol. 1999,74, 349-357
CrossRef - Mantzavinos D.; Psillakis, E. J. Chem. Technol. Biot. 2004, 79, 431-454
CrossRef - Balanosky, E.; Fernadez, J.; Kiwi, J.; Lopez A. Water. Sci. Technol.1999, 40, 417-424
CrossRef - Konstantinou, I.K.; Albanis, T.A. Appl. Catalyst B: Environ. 2003, 42, 319-335
CrossRef - Meena, R.C.; Pachwarya, R.; Kumar V. American J. Environ. Sci. 2009, 5, 444-450
CrossRef - Meena, R.C.; Munesh.; Swati. Res. J. Chem. Sci., 2012,2, 56-62
- Murmat, S. Int. J. Adv. Res. 2017, 5, 1519-1528
CrossRef - Bartošová, A.; Blinová, l.; Sirotiak, M., Faculty of materials science and technology in trnava, 2017, 25, 40
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









