Correlation Between Structure and New Anti-Cancer Activity of Some Antioxidants Following Chemical Structure Modification. Does the Evidence Support this Correlation?
Weiam A. Hussein1,2* , Mohammed Khaled Bin Break1
, Mohammed Khaled Bin Break1 , Ahmed Alafnan3
, Ahmed Alafnan3 , Bader Huwaimel1
, Bader Huwaimel1 , Weaam M. A. Khojali1,4
, Weaam M. A. Khojali1,4 , Nasrin Khalifa5,6
, Nasrin Khalifa5,6 , Farhan Alshammari5
, Farhan Alshammari5 , Tahani S. Albalawi7, Ghaliah Alshammary7, Zhawah F. Alshammary7, Haya O. Almutairi7, Reem M. Alrasheedi7 and Shahad Fayad7
, Tahani S. Albalawi7, Ghaliah Alshammary7, Zhawah F. Alshammary7, Haya O. Almutairi7, Reem M. Alrasheedi7 and Shahad Fayad7
1Department of Pharmaceutical Chemistry, College of Pharmacy, University of Ha’il, 2240/Hail, Saudi Arabia.
2Department of Pharmaceutical Chemistry, College of Pharmacy, Aden University, 6075/Aden, Yemen.
3Department of Pharmacology and Toxicology, College of Pharmacy, University of Ha’il, 2240/Hail, Saudi Arabia.
4Department of Pharmaceutical Chemistry, College of Pharmacy, Omdurman Islamic University, 14415/ Al Khartoum, Sudan
5Department of Pharmaceutics, College of Pharmacy, University of Ha’il, 2240/Hail, Saudi Arabia.
6Department of Pharmaceutics, Faculty of Pharmacy, University of Khartoum, 11115, Khartoum, Sudan.
7College of Pharmacy, University of Ha’il, 2240/Hail, Saudi Arabia.
Corresponding Author E-mail: w.hussein@liveuohedu.onmicrosoft.com
DOI : http://dx.doi.org/10.13005/ojc/390401
Article Received on : 01 Jun 2023
Article Accepted on :
Article Published : 10 Jul 2023
Reviewed by: Dr. Yahya Albayati
Second Review by: Dr. Rahmat Unissa Syed
Final Approval by: Dr. S. A. Iqbal
In medicinal chemistry, the link between structure and activity is essential. We are seeking to relate chemical structure and reactivity to medicinal properties, which has gained popularity recently. In this study, scaffolds from three antioxidants were changed to new derivatives to show that their biological effects as antioxidants would change. We also reviewed the anticancer effects of these medications (based on the SRB test) to find other biological effects that may be related to their chemical structural modifications. Moreover, SWISS ADME software was used to determine further ADME characteristics. Compound 2C had the highest cytotoxicity (1.2 μM) against lung cancer cell lines, whereas 1C had good cytotoxicity (87.66 μM). Compound 2C also demonstrated excellent cytotoxicity against the other three cell lines with IC50 values of 5.049, 6.26, and 9.71 μM, respectively. After its antioxidant structure was tweaked, 1C might be a critical molecule for building a novel treatment for lung cancer.
KEYWORDS:Antioxidants; Cytotoxicity; In-Silico ADME analysis; Lung cancer; Structure-activity relationship
Download this article as:| Copy the following to cite this article: Hussein W. A, Break M. K. B, Alafnan A, Huwaimel B, Khojali W. A. M, Khalifa N, Alshammari F, Albalawi T. S, Alshammary G, Alshammary Z. F, Almutairi H. O, Alrasheedi R. M, Fayad S. Correlation Between Structure and New Anti-Cancer Activity of Some Antioxidants Following Chemical Structure Modification. Does the Evidence Support this Correlation?. Orient J Chem 2023;39(4). |
| Copy the following to cite this URL: Hussein W. A, Break M. K. B, Alafnan A, Huwaimel B, Khojali W. A. M, Khalifa N, Alshammari F, Albalawi T. S, Alshammary G, Alshammary Z. F, Almutairi H. O, Alrasheedi R. M, Fayad S. Correlation Between Structure and New Anti-Cancer Activity of Some Antioxidants Following Chemical Structure Modification. Does the Evidence Support this Correlation?. Orient J Chem 2023;39(4). Available from: https://bit.ly/46IuPzW |
Introduction
Structure-Activity Relationship (SAR) is an area of medicinal chemistry. It has changed from focusing on making, isolating, and describing drugs to paying more attention to the biochemistry of disease states and making drugs to avoid diseases. Finding a link between the structure of a drug and how it works in the body has been an important part of medical chemistry. In recent years, there has been more talk about how chemical structure relates to chemical reactivity or real qualities, and these links can be used to figure out how they work as medicines. Despite the fact that there has been a lot of accomplishment in understanding the connection between chemical structure and biological activity in various aspects, particularly for antioxidants medications, there are yet numerous human burdens that require better than already available drugs. Most medications act at a particular site like a protein or receptor, comparable chemical structures are typically associated with comparable pharmacological effects. However, they often display varying degrees of efficacy and toxicity, as well as occasionally engaging in distinct activities1. Structure-activity relationships (SAR) are used to describe these variations that arise from underlying structure. The pharmacophore and off-target effects of a lead chemical may be isolated by comparing it to similar compounds, a process known as studying the Structure- Activity Relationships (SAR) of the lead and its analogues. This data is then utilized to create a new medication with enhanced efficacy (by optimizing its SAR), a different mechanism of action than the original, fewer adverse effects, or an easier route of administration for the patient.2. Therefore, understanding the SARs between a given set of molecules allows researchers to rationally explore chemical space and design a series of compounds with desirable pharmacological, toxicological, and biochemical profiles 3-4.
Thus, with accelerating trends towards a better understanding of the SAR, it is important for both those who plan to use SAR models and those who plan to develop or change them to have a basic understanding of how to construct or change a SAR model, which is what we plan to implement in this paper. Therefore, in this paper, the chemical structures of three antioxidants (Hydroquinone, diphenylamine, and ascorbic acid) has been changed in some important parts, in an attempt to prove that, their biological effect as antioxidants will change due to this transformation. Likewise, the anticancer effects of these drugs will be discussed in an effort to identify other biological effects that may be associated with the transformation of the chemical structures of these compounds. Furthermore, the relationship between the modified molecular structure (SAR) and anticancer activity of newly synthesized derivatives will also discuss in this study. SwissADME software also, was used to conduct the ADME analysis. The BBB permeability, bioavailability, and solubility of our newly synthesized chemicals are all provided via this search engine. It was used to analyze various pharmacokinetics parameters and drug likeness, among other things. Therefore, the goal of this work was also to conduct an in-silico ADME analysis.
Materials and Methods
Chemistry
Sigma-Aldrich Chemical Co. supplied all of the chemicals. Uncorrected melting points were calculated using the capillary tube technique and the Stuart® Analogue Melting Point Apparatus. The following instruments were used to collect spectroscopic data: MS-FAB, VG Quattro Mass spectrometer; 1HNMR, Bruker 400 MHz spectrometer. Chemicals will be produced in batch reactors (glass flasks), then purified using the appropriate process, such as recrystallization. Thin layer chromatography on commercially available silica plates will be used to monitor the reactions. Solvents will be dried and purified using normal medicinal chemistry methods.
 |
Figure 1: Proposed general synthesis design strategy |
General procedure for the synthesis of amino-1,4-naphthoquinone derivatives (1C-3C)
First In a beaker (25 ml), combine 0.5 g hydroquinone and 5 mL pure water. To get a clear solution, heat on a wire gauze. In a conical flask, dissolve 1 g K2Cr2O7 in 10 mL water and add 1 mL conc. H2SO4. Shake the conical flask and immerse it in cold water to cool. Dropwise add hydroquinone solution (made above) to this very cold solution for 30 minutes with steady shaking. Allow the temperature to increase no higher than 20o C. Continue shaking for another 10 minutes once the addition is complete. Quinone crystals separate as yellow crystals. Filter through a Buchner funnel and thoroughly dry it. (Be aware that the product is water-soluble and should not be washed). Crystallize again from ethyl alcohol. The resultant (1,4-Naphthoquinone) (1 mmol) chemical was diluted in ethanol (20 mL). The solution was put in a round-bottom flask with a volume of 100 mL. A solution of suitable anilines (1 mmol) in ethanol (20 mL) was progressively added, along with (0.5 mmol) of Fecl3. For 3 hours, the reaction mixture was agitated and refluxed. A solvent condenser was installed in the reaction vessel to reduce ethanol losses during the experiment. When the equivalent 2-(phenylamino)-1,4- naphthoquinone was produced, the solution became purple, red, or orange. The resultant solid was filtered and rinsed with 5 mL of cold ethanol. The product was filtered and rinsed with cold ethanol (5 mL). All of the products were crystalline solids that did not need to be purified further (Figure 2).
1C: “methyl (3,6-dioxocyclohexa-1,4-dien-1-yl)glycinate”
FTIR (ATR, cm-1): 3255 (N–H), 1650 (C=O), 1511 (C–C aromatic), 1423 (N–H bend), 1178 (C–N).
1H-NMR (400 MHz, DMSO-d6; δ, ppm): 3.44 (2H, s), 3.87 (3H, s), 6.89-6.98 (2H, m), 7.48-7.51 (1H, m). 13C NMR: δ 10.13 (1C, s), 21.16 (1C, s), 50.42 (1C, s), 123.15 (1C, s), 134.84 (1C, s), 136.66 (1C, s), 140.63 (1C, s), 167.42 (1C, s), 180.15 (1C, s), 194.98 (1C, s). HR-MS: (m/z) EI+: 196.8639.
2C: “2-(2-(4-isopropylphenyl)hydrazineyl)cyclohexa-2,5-diene-1,4-dione”.
FTIR (ATR, cm-1): 3246 (N–H), 1653 (C=O), 1510 (N–H bend), 2995 (C-H stretching), 1215 (C–N).
1H-NMR (400 MHz, DMSO-d6; δ, ppm): 3.08 (6H, s), 6.85-6.87 (2H, d), 7.47-7.50 (1H, m), 7.61-7.62 (1H, t), 7.74-7.76 (3H, t). 13C NMR: δ 31.77 (2C, s), 34.0 (1C, s), 114.68 (1C, s), 122.29-122.36 (2C, s), 124.94-125.03 (2C, s), 126.61-126.83 (1C, s), 135.16 (1C, s), 143.57 (1C, s), 146.71-146.80 (2C, s), 168.59 (1C, s), 183.40 (1C, s). HR-MS: (m/z) EI+: 256.9066.
3C: “2-(2-(2-nitro-5-(trifluoromethyl)phenyl)hydrazineyl)cyclohexa-2,5-diene-1,4-dione”
FTIR (ATR, cm-1): 3257 (N–H), 1653 (C=O), 1508, 1333 (N-O stretching), 1029 (C-F stretching), 1271 (C–N). 1H-NMR (400 MHz, DMSO-d6; δ, ppm): 7.52 (2H, s), 8.09 (2H, s), 8.57 (2H, s). 13C NMR: δ 112.31 (1C, s), 123.16(1C, s), 125.36 (1C, s), 126.24 (1C, s), 126.26 (1C, s), 129.60 (1C, s), 130.93 (1C, s), 137.29 (1C, s), 140.57 (1C, s), 149.38 (1C, s), 154.16 (1C, s), 167.33 (1C, s), 195.41 (1C, s). HR-MS: (m/z) EI+: 327.0000.
General procedure for the synthesis of sodium n-substituted diphenylamine dithiocarbamates salt (1E)
A solution of sodium hydroxide (10 mmol, 0.4 g) in ethanol (10 mL) was added to a solution of diphenylamine (10 mmol, 10 mL). The mixture was allowed to cool in an icy solution, and 100 mmol (6.0 mL) of carbon disulfide was added drop by drop while being stirred for 1 hour at room temperature. The precipitates were filtered and cleaned with diethyl ether to get a 70–90% yield of a product that was white to pale yellow 5. (Figure 2).
1E: “sodium diphenylcarbamodithioate”
FTIR (ATR, cm-1): 3220 (aromatic C-H Stretch), 1550 (aromatic C=C Bending), 1270 (C=S). 1H-NMR (400 MHz, DMSO-d6; δ, ppm): 7.52 (2H, s), 8.39 (5H, s), 8.50 (3H, s). 13C NMR: δ 123.15 (4C, s), 129.57 (2C, s), 130.91 (4C, s), 149.38 (2C, s), 195.01 (1C, s). HR-MS: (m/z) EI+: Salt is incompatible with MS.
General procedure for the synthesis of general procedure for the synthesis of (5, 6-dioxolan ascorbic acid) (1G)
40 mL of high-purity acetone and 0.5 mL of conc. sulphuric acid were put into a reaction bottle with a magnetic stirrer. The mixture was stirring at room temperature for a maximum of one hour. Then, 1 mol (0.176 g) of ascorbic acid was added and stirred. The temperature of the mixture is then kept at 70°C in an ice bath. Thin-layer chromatography (TLC) with (25%:75%) dichloromethane:ethanol was used to keep track of the process. (Figure 2).
1G: “5-(2,2-dimethyl-1,3-dioxolan-4-yl)-3,4-dihydroxyfuran-2(5H)-one”
FTIR (ATR, cm-1): 1651 (C=O Stretch), 1509 (C=C double bond), 1311 (C–O stretch), 2891 (alkyl C-H Stretch). 1H-NMR (400 MHz, DMSO-d6; δ, ppm): 1.31 (6H, s), 3.77-3.83 (3H, s), 5.31 (1H, s). 13C NMR: δ 25.68-26.26 (2C, s), 50.90 (1C, s), 52.19 (1C, s), 62.73 (1C, s), 123.15 (1C, s), 125.36- 126.20 (1C, s), 149.37 (1C, s), 167.44 (1C, s). HR-MS: (m/z) EI+: 216.8610.
A:K2Cr2O7/B:conce. H2SO4/0O C; C:EtOH/FeCl3-reflux; D:Etoh/NaOH- E:CS2/ 0OC; F:Acetone, conce. H2SO4, at R.T for one hour, then G:the mixture temperature is maintained at (70 ° C) in an ice bath.
 |
Scheme 1: Proposed routes for the synthesis. |
Cell culture
Nawah Scientific Inc. (Mokatam, Cairo, Egypt) supplied the HCT-116 colorectal cancer cell line. At 37 degrees Celsius in a humidified 5% (v/v) CO2atmosphere, cells were grown in RPMI medium containing 100 mg/mL of streptomycin, 100 units/mL of penicillin, and 10% of heat-inactivated fetal bovine serum.
Cytotoxicity assay
The SRB test was used to determine the cell viability. This test is used to determine cell density by measuring cellular protein concentration. The approach presented here has been refined for screening drug toxicity to adherent cells in a 96-well format 6. Aliquots of 100 μL cell suspension (5×10^3 cells) were incubated in full medium in 96-well plates for 24 hours. A further portion of 100 L media containing drugs at varying doses was used to treat the cells. Cells were fixed by changing the medium with 150 L of 10% TCA and incubating the dish at 4 °C for 1 hour after 72 hours of drug exposure. After removing the TCA solution, the cells were rinsed with distilled water five times. The mixture was then incubated at room temperature for 10 minutes in the dark with aliquots of 70 L SRB solution (0.4% w/v). After being rinsed three times in 1% acetic acid, the plates were dried overnight at room temperature. The protein-bound SRB stain was dissolved by adding 150 L of TRIS (10 mM), and absorbance was measured at 540 nm using a microplate reader from BMG LABTECH® (Ortenberg, Germany) 7, 8.
ADMET evaluation
Using the SwissADME web tool, the ADMET study of recently synthesized compounds was done. Chemsketch was used to draw the structures of the molecules, and their pharmacokinetics and drug-likeness were calculated. All of this information was subsequently organized into a table to show the results 9.
 |
Figure 2: Confirmed reaction mechanism for the reaction of 1,4-quinone & amines |
 |
Figure 3: The Proposed reaction mechanism suggested for dithiocarbamates sodium salt |
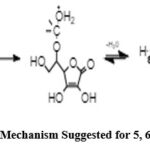 |
Figure 4: The Proposed Reaction Mechanism Suggested for 5, 6-dioxolan ascorbic acid derivative |
Results and Discussion
The syntheses derivatives are illustrated in Schemes 1 . A synthesis has been designed wherein in case of hydroquinone will converted first to quinone. The product from this reaction (1,4-quinone) is then expected to react with several primary amines under special conditions, to produce the final products 10 (Mechanism in figure II). In case of Diphenylamine it will reacts additionally with carbon disulfide to form dithiocarbamates sodium salt 11, in accordance with literatures method in figure III, which shows the expected reaction mechanism, given for dithiocarbamates sodium salt. Finally, structural changes in ascorbic acid itself will be done by the reaction with acetone in acidic solution to form 5, 6-dioxolan ascorbic acid derivative 12. After submitting to biological evaluation procedures, all novel compounds (Table 1) revealed logical analytical and spectroscopic data that was highly congruent with their structures.
For instance, the (1C, 2C, 3C) compounds exhibited a distinctive absorption band at 3246-3257 cm-1 due to the (N-H) group in infrared examination. In addition, 1H-NMR spectra showing a downfield emergence of a singlet peak provided further confirmation of N-H production. Hydrogen bonding (-NH) can occur with protons from anywhere in the proton NMR spectrum. They might be absent at times. It’s not always easy to observe since they’re so sensitive to solvation, acidity, concentration, and temperature 13. Thio-carbonyl (C=S) bands were detected at 1270 cm-1. The phenyl ring aromatic protons and all the others were found to be within the predicted ranges. The protons of dimethyl groups were also detected at the predicted ppm levels 14. Conclusions concerning their structure-activity connections based on the findings of biological tests include the following: (a) the presence of quinone ring in both 1C & 2C appears to boost anticancer activity; compared to that of other derivatives. Thus, other studies 15 support our findings and suggest that quinone-based heterocycles have broad-spectrum anticancer activity. (b) Secondary amines were shown to be required for anticancer activity. In fact, secondary amines are not only shown to be a possible anticancer candidates, but they also have minimal adverse effects 16. (c) Addition of nitro & trifluoromethyl groups in the phenyl ring has no effect as anticancer. (d) No effect against cancer was produced when ascorbic acid was converted into 5, 6-dioxolan ascorbic acid. Furthermore, it was revealed that the salt of dithiocarbamates has no anti-cancer effects. Even though some research has suggested that dithiocarbamates have anti-cancer properties, most of these studies synthesized dithiocarbamates in a complex form rather than a salt 17-18.
 |
Table 1: Some properties of the synthesized compounds. |
Using SRB assay method, the anticancer properties of our synthesized derivatives were examined. It has been observed that exhibits significant anticancer activity when compared to standard drug in terms of percent viability. Table 2, 3 & figure 5 is a list of the outcomes from anticancer action. Compound 2C showed more activity than cisplatin against the four cancer cell lines tested HCT-116 (Colorectal Cancer), MCF-7 (Breast Adenocarcinoma ), HepG2 (Hepatocellular carcinoma) & A549cells (Lung Cancer) with IC50 values of 5.049 µg/ml, 6.26μM , 9.71μM & 1.2μM respectively, while compound 1C exhibited acceptable activity against A549cells (Lung Cancer) with IC50 value of 87.6μM. The remaining compounds exhibited no activities. This demonstrates that substance 2C is more selectively toxic to lung cancer cell lines. To clarify its mechanism of action, more research might be done, which might result in the creation of new anticancer drug.
Table 2: IC50 values of compound 1C & 2C against HepG2, A549, HCT116 and MCF7 cells.
|
Compound |
IC50 (μg/ml)* |
|||
|
HepG2 |
A549 |
HCT116 |
MCF7 |
|
|
1C |
>100 |
87.6± 5.1 |
>100 |
>100 |
|
2C |
9.71± 6.4 |
1.2± 5.1 |
5.049± 5.2 |
6.26± 9.2 |
|
Doxorubicin |
0.7 ± 0.02 |
0.009 ± 0.001 |
0.06 ± 0.0007 |
0.3 ± 0.03 |
*IC50 values are reported as the mean (IC50 ±SD) of at least three independent experiments.
Table 3: Percentage viability of active derivatives against selected cell lines
|
Compound |
% viability (100 mcg) |
|||
|
HepG2 |
A549 |
HCT116 |
MCF7 |
|
|
1C |
93.7584 |
46.3851 |
92.3156 |
98.3919 |
|
2C |
0.10877 |
2.34183 |
0.9901 |
1.40728 |
 |
Figure 5: Cytotoxicity dose response curve of compounds 1C & 2C against the four cell lines tested. |
*Using SRB 96-well format, Absorbance was measured at 540 nm using a microplate reader from BMG LABTECH®
The results of determining the pharmacokinetic characteristics and drug-likeness prediction of newly synthesized derivatives using the online version of SwissADME 19-20 are shown in Table 4. On the basis of Lipinski’s rule of five (RO5), all substances exhibited a high degree of pharmacological similarity. All of the compounds demonstrated significant bioavailability when used as medicines, indicating that they could be absorbed and transported throughout the body. All molecules were subsequently examined for their ability to predict ADMET, and it was determined that they were all appropriate drug-like molecules.
Table 4: Pharmacokinetics and drug-likeness prediction for newly synthesized derivatives by SwissADME
|
Phytochemical constituents |
Pharmacokinetics |
Drug-likeness |
||
|
GI absorption |
BBB permeability |
Log Kp (skin permeation) cm/s |
||
|
1C |
High |
NO |
-7.44 |
Yes |
|
2C |
High |
Yes |
-5.57 |
Yes |
|
3C |
High |
NO |
-5.91 |
Yes |
|
1E |
High |
Yes |
-4.83 |
Yes |
|
1G |
High |
NO |
-7.81 |
Yes |
Conclusion
In conclusion, we have selected three antioxidants (Hydroquinone, diphenylamine, and ascorbic acid) and have changed their chemical structure in some important parts, in an attempt to prove that, their biological effect as an antioxidant will be lost or changed due to this transformation. In addition, they screened for their anticancer activities based on SRB assay. Compound 2C demonstrated the highest cytotoxicity against A549 lung cancer cell lines in compared to other derivatives, where 1C exhibited a good cytotoxicity against the same cells lines, comparable to the standard drug Doxorubicin. Furthermore, compound 2C showed good cytotoxicity against other three cell lines tested (HepG2, HCT116 & MCF7). All of the compounds exhibited high soluble gastro intestinal absorption and variable BBB permeability; however, as anticipated, the drug-like properties produced positive results. These results suggested that 1C and 2C could be used as potential lead compounds for the development of lung cancer drugs. Nonetheless, there are still some significant aspects to investigate, some of which are depicted below.
Our progress toward new biological effects could be expanded with a vast number of derivatives to determine the structure-activity relationship and expand medical applications.
In the future, an in-vivo pharmacokinetic profile may have been conducted on active derivatives in order to evaluate their potential for development into therapeutic agents.
Acknowledgment
Scientific Research Deanship at University of Ha’il – Saudi Arabia through project number RG-21 173, has funded this research.
Conflicts of Interest
There are no conflicts of interest declared by the authors.
References
- Alagarsamy, V. Elsevier Health Sciences, 2013, 1, 56-65.
CrossRef - Southan, C. Elsevier, 2017, 3, 464–487. https://www.research.ed.ac.uk/ en/publications/examples-of-sar-centric-patent-mining-using-open-resources.
CrossRef - Tong, W.; Welsh, W.J.; Shi, L.; Fang, H.; Perkins, R. Environ Toxicol Chem. 2003, 22, 1680-95.
CrossRef - James H. J Pharma Reports. 2022, 06, 154.
CrossRef - Hussein, W.; Sağlık, B. N.; Levent, S.; Korkut, B.; Ilgın, S.; Özkay, Y.; Kaplancıklı, Z. A. Molecules, 2018, 23, 2033. https://doi.org/10.3390/ molecules23082033.
CrossRef - Lanchhana, D.S.; Ranjit, M.; Kumar, M.S. Journal of Pharmaceutical Negative Results, 2023, 1123-1132.
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D., Boyd, M. R. JNCI: Journal of the National Cancer Institute, 1990, 82, 1107-1112. https://doi.org/10.1093/jnci/82.13.1107.
CrossRef - Allam, R. M.; Ahmed, M.; Alaa, K.; Ola, A.; Sharaf, S.M.; Amani, E. K.; Hisham, A. M.; Ashraf, B. A. N. Toxicology Letters, 2018, 291, 77-85. https://doi.org/10.1016/j.toxlet.2018.04.008.
CrossRef - Ali, A.; Badawy, M. E. I.; Shah, R. A.; Rehman, W.; Kilany, Y. E.; Ashry, E. S. H. E.; Tahir, N. Der ChemicaSinica, 2017, 8, 446-460.
- Leyva, E.; Cárdenas-Chaparro, A.; Loredo-Carrillo, S. E.; López, L. I.; Méndez-Sánchez, F.; Martínez-Richa, A. Molecular Diversity, 2018, 22, 281-290. https://doi.org/10.1007/s11030-018-9820-9.
CrossRef - Levent, S.; Acar Çevik, U.; Sağlık, B. N.; Özkay, Y.; Can, Ö. D.; Özkay, Ü. D.;Uçucu, Ü. Phosphorus, Sulfur, and Silicon and the Related Elements, 2017, 192, 469-474. https://doi.org/10.1080/10426507.2016.1259228.
CrossRef - Voisin-Chiret, A. S.; Bazin, M. A.; Lancelot, J. C.; Rault, S. Molecules, 2007, 12, 2533-2545. https://doi.org/10.3390/12112533.
CrossRef - Zengin, H.; Yenilmez, H. Y.; Burat, A. K.; BAYIR, Z. A. Turkish Journal of Chemistry, 2014, 38, 1094-1101. https://doi.org/10.3906/kim-1405-26.
CrossRef - Silverstein, Robert M.; Clayton B. Journal of Chemical Education, 1962, 39, 546.
CrossRef - Aly, A.A.; Hassan, A.A.; Mohamed, N.K.; Ramadan, M.; Abd El-Aal, A.S.; Bräse, S.; Nieger, M. Journal of Chemical Research, 2021, 45, 562-571.
CrossRef - Bhat, A.A.; Singh, I.; Tandon, N.; Tandon, R. European Journal of Medicinal Chemistry, 2023, 246, 114954.
CrossRef - Hosseinzadeh, S.; Moghadam, M. E.; Sheshmani, S.; Shahvelayati, A. S. Journal of Biomolecular Structure and Dynamics, 2019, 38, 2215-2228. https://doi.org/10.1080/07391102.2019.1627909.
CrossRef - Keter, F. K.; Guzei, I. A.; Nell, M.; Zyl, W. E. V.; Darkwa, J. Inorganic Chemistry, 2014, 53, 2058-2067. https://doi.org/10.1021/ic4025926.
CrossRef - Abdulrahman, H.L.; Uzairu, A.; Uba.S. Bulletin of the National Research Centre. 2020, 44, 1-8.
CrossRef - Ranjith, D.; Ravikumar, C. Journal of Pharmacognosy and Phytochemistry, 2019, 8, 2063-2073.

This work is licensed under a Creative Commons Attribution 4.0 International License.









