Thermal and Optical Properties of NiO Nano Doped in PVDF-HFP: Mg(ClO4)2 Nano Composite Solid Polymer Electrolytes
1Department of Physics, Jawaharlal Nehru Technological University, Hyderabad, Telangana.
2*Department of Physics, Osmania University, Hyderabad, Telangana.
Corresponding Author E-mail: j_siva_k@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/390327
Article Received on : 23 Apr 2023
Article Accepted on : 25 Jun 2023
Article Published : 27 Jun 2023
Reviewed by: Dr. N. Okamoto
Second Review by: Dr. Subhasis Roy
Final Approval by: Dr. Aditya Bhattacharyya
NiO doped nano composite Solid polymer electrolytes (SPEs) composed of PVDF-HFP (poly (vinylidene Fluoride hexafluoropropylene)): Mg(ClO4)2 with different weight concentration of NiO nanofillers synthesized by solution cast technique. NiO incorporated nano composite polymer electrolytes are characterized by UV-visible spectroscopy to find direct and indirect band gaps. The thermal stability and structural changes of the nano composite polymer electrolytes is studied by DSC and noticed that PSN12 sample having optimum change. The changes in band gap values maybe due to greater number of Ni and Mg atoms from NiO and Mg(ClO4)2 salt are coordinating by donating electrons to F atom of the PVDF-HFP polymer. From the optical absorption measurements, the found values direct and indirect band gap was low, and these values are 3.8252 eV and 1.6885 eV respectively for the polymer electrolyte sample PSN12 where weight ratio of NiO is12% nanofiller incorporated PVDF-HFP: Mg (ClO4)2 polymer electrolyte.
KEYWORDS:DSC; Mg(ClO4)2; Nano NiO; PVDF-HFP polymer; Polymer electrolyte; UV- vis
Download this article as:| Copy the following to cite this article: Mallikarjun A, Kumar J. S. Thermal and Optical Properties of NiO Nano Doped in PVDF-HFP: Mg(ClO4)2 Nano Composite Solid Polymer Electrolytes. Orient J Chem 2023;39(3). |
| Copy the following to cite this URL: Mallikarjun A, Kumar J. S. Thermal and Optical Properties of NiO Nano Doped in PVDF-HFP: Mg(ClO4)2 Nano Composite Solid Polymer Electrolytes. Orient J Chem 2023;39(3). Available from: https://bit.ly/44m1MQw |
Introduction
SPEs are in high demand for cutting-edge technological applications including fuel cells, energy storage devices like batteries, solar cells, sensors, etc., 1-3. The polymer electrolyte (PE) is in high demand due to its many benefits, including its no leakage issues, non-explosiveness, good thermal stability, wide range of optical absorption capacity, electrical sensitivity, and greater flexibility for compatibility with present and future technological advancements. Lithium salt-based polymer electrolytes are currently in high demand despite some disadvantages such explosiveness, higher corrosivity, and scarcity of the earth’s crust. In search of alternatives to lithium-based system research on other materials like Na and Mg-based polymer electrolytes are required. Mg-based and Na-based polymer electrolytes are essential for the growth of competitive and parallel production because they have excellent conductivity, are extremely thermally stable, and are highly compatible 4. The properties like stability and conductivity are low in pure solid polymer electrolytes and this can be enhanced by adding the right amount of nanofillers, such as NiO, ZrO2, and Al2O3, etc. 5-8. Optical characteristics improved polymer electrolytes are extremely useful for solar cell applications. The semiconducting nature of nano composite polymer electrolytes are most useful for solar cell applications 9-10. The dominance of sufficient concentration of nanofillers may result in a high quantity of disassociation of Mg (ClO4)2 salt in PVDF-HFP polymer electrolyte, resulting causes a narrowing of optical band gap of nano composite polymer electrolyte. It may also lead to reducing optical band gap 11. The conductivity of NiO nanofiller-included PVDF-HFP: Mg(ClO4)2 polymer electrolytes have shown the higher ionic conductivity 12. The present study is concerned with the optical and thermal properties of the NiO nanofiller effect in PVDF-HFP: Mg (ClO4)2Polymer electrolyte.
Materials and Methods
PVDF-HFP polymer (Mw~400,000),
Mg (ClO4)2 salt (Mw~223.21 and purity ≥98.0%), and NiO nanofillers (particle size
<50nm and 99.8 trace metal base) were bought from
Sigma-Aldrich, solvent THF and Acetone cleaner were bought from Merck
Millipore. In the solution cast method, 1000mg of
PVDF-HFP and 400mg of Mg(ClO4)2 salt and various
weight concentrations of NiO nanofillers are dissolved in THF (tetrahydrofuran)
solvent, and they are agitated for several hours at room temperature to
dissolve the total polymer, magnesium salts and dispersion of nanofillers into
the solvent and make the solvent into a homogenous nano composite polymer
electrolyte solution. The nano composition solutions are gathered as per the
weight concentrations of NiO nanofiller in Petri dishes and dried at room
temperature under vacuumed conditions. Table 1 shows polymer electrolyte
complexation samples with varied component weights. The prepared nano composite
SPEs are characterized by UV-visible spectroscopy (SHIMADZU UV 1800
spectrometer) and DSC (SHIMADZU DSC 60) measurements.
Table 1: Polymer electrolyte complexation samples with varied component weights.
|
S.No. |
Sample Code |
PVDF-HFP |
Mg (ClO4)2 |
NiO nanofiller |
|
1 |
PSN5 |
1000 |
400 |
50 |
|
2 |
PSN7 |
1000 |
400 |
70 |
|
3 |
PSN9 |
1000 |
400 |
90 |
|
4 |
PSN12 |
1000 |
400 |
120 |
|
5 |
PSN15 |
1000 |
400 |
150 |
Results and Discussion
Thermal studies – DSC
Figure 1 demonstrates variations in heat flow vs. temperature of PVDF-HFP: Mg(ClO4)2 polymer electrolytes (PEs) with different amounts of NiO nanofiller. The variation of endothermic and exothermic reactions clearly demonstrates the influence of NiO nano filler interacts with PVDF-HFP: Mg(ClO4)2 polymer electrolytes. It also shows the impact of temperature on interactions between nanofiller and polymer electrolytes. The exothermic peak of PVDF-HFP is decreasing and shifting to a higher temperature as a result of NiO nanofiller interaction with polymer electrolytes. Moreover, the exothermic peak of polymer melting temperatures shifting to higher temperatures is due to the raising of the polymer electrolyte’s most amorphous nature and stability. For optimum concentration of NiO nanofiller concentration the polymer attains maximum segmental motion due to relative dynamics with the interaction of nanofillers. Hence, the maximum amorphous nature can be seen for the sample PSN12. For higher concentrations of NiO nanofiller dispersion may cause aggregation, which will change its amorphous nature and cause it to crystallize. It may also lead to improve crystalline nature up to certain extent and it may cause phase transition of polymer leading to attain less melting temperature for PSN12 sample. The aggregation of nano filler may lead to the raising of crystallinity in polymers to a small extent due to high concentration of NiO nanofiller in polymer electrolyte of PSN15. The optimum concentration of NiO nanofiller provides a good interaction may cause for polymer chain segmental motion and the interaction will also help for disassociation of Mg (ClO4)2 as Mg2+ ion and (ClO)– ion. The smooth curve of PSN12 sample indicates good thermal stability and showed higher conductivity 12. The smooth curve also shows the higher segmental motion of the polymer chains with interaction of the NiO nanofiller 13-14.
 |
Figure 1: DSC curve of NiO incorporated a) PSN5, b) PSN7, c) PSN9, d) PSN12, and e) PSN15. |
Optical studies – UV-visible Spectroscopy
UV visible absorption spectra of the PVDF-HFP: Mg(ClO4)2 with various weight concentrations of NiO nanofillers nano composite polymer electrolyte system is show in Figure 2. Incorporating NiO nanoparticles into PVDF-HFP: Mg (ClO4)2 broadens its UV-visible spectrum absorption peak. NiO nanofiller incorporation in PVDF-HFP: Mg (ClO4)2 increases UV-visible absorption to higher wavelength range, which could lead to a greater number of Ni and Mg atoms from NiO and Mg(ClO4)2 salt are coordinating by donating/ sharing of electrons to F atom of the PVDF-HFP polymer where the electrons occupy the holes in polymers. And found higher conductive nature due more number Mg2+ ions are available to mobile 12. There is a perfect balance for nanofiller concentration in polymer electrolyte dispersion, where the absorption peak is broad found in UV-visible radiation spectrum. It may promote excellent conductivity in polymer electrolytes by providing a pathway for free ion migration 15-16. The impact of NiO nanofiller obviously can be seen in direct and indirect band gap decreasing while increasing the NiO nanofiller content in the polymer electrolyte. Relation between absorption coefficient as well as the incident photon energy is given by the flowing relation 17-18.
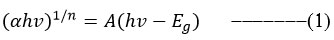
Where A is constant and denotes the material’s band gap energyand the exponent n depends on type of transition. For direct allowed transition n=1/2, and for indirect allowed transition n=2. Direct band gap of the sample is estimated by drawing graph between and then extending the straight portion of the curve on axis at and showed in Figure 3. Indirect band gap of the sample is estimated by plotting between and then extending the straight portion of the curve on axis at and showed in Figure 4. The variation direct bandgap and indirect band gap for various concentrations of NiO nanofiller incorporated polymer electrolytes are shown in Figure 5. As a result, as the concentration of NiO nanofiller in the PVDF-HFP: Mg(ClO4)2 polymer electrolyte increases, the indirect band gap and the direct band gap decreases as shown in Figure 3 and 4. The optimum nanocomposite polymer electrolyte of sample PSN12 of weights concentration 12% of NiO in PVDF-HFP: Mg(ClO4)2 polymer electrolyte shows low direct and indirect band gap, hence it is more compatible. It can be seen that increasing furthermore the dispersion of NiO nanofiller in polymer electrolytes may reduce absorption and resulting in polymer electrolytes with high direct and indirect band gaps for the higher nanofiller weight concentrations dispersion i.e.,15% NiO i.e., PSN15 sample where the possibility of formation aggregation of nanofillers in polymer electrolyte network.
 |
Figure 2: UV-visible absorbance: depict the UV absorbance Vs wavelength (nm) of all Concentration of NiO samples a) PSN5, b) PSN7, c) PSN9, d) PSN12, and e) PSN15. |
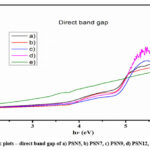 |
Figure 3: Tauc plots – direct band gap of a) PSN5, b) PSN7, c) PSN9, d) PSN12, and e) PSN15. |
 |
Figure 4: Tauc plots – indirect band gap of a) PSN5, b) PSN7, c) PSN9, d) PSN12, and e) PSN15. |
 |
Figure 5: Direct band gap and Indirect band gap variation for various nano composite PEs. |
Conclusion
Solution casting process is used to create NiO nanofiller integrated PVDF-HFP: Mg(ClO4)2 polymer electrolytes with varying weight concentrations of NiO nano fillers. DSC is used to characterize the nano composite polymer electrolytes in order to determine thermal nature and structural changes with temperature. The PSN12, NiO composite polymer electrolyte sample has a smooth curve, indicating an excellent amorphous nature. The greater number of Ni and Mg atoms from NiO and Mg(ClO4)2 salt are coordinating by donating/ sharing of electrons to F atom of the PVDF-HFP polymer. It is being noted that PSN12 is sample of good optical absorption and lowest direct and indirect band gaps in this nano composite polymer electrolyte i.e.,direct, and indirect band gap are noted as 3.8252 eV and 1.6885 eV respectively and further increase of NiO nanofiller both band gap values increases due to aggregation phase.
Acknowledgement
The authors thank HoD and BoS, Department of Physics, JNTUH Hyderabad for their encouragement and carrying out this research work.
Conflict of Interest
Authors declares no conflict of interest.
References
- Zhou D.; Shanmukaraj D.; Tkacheva A.; Armand M.; and Wang G, Chem, 2019,5(9), 2326-2352.
CrossRef - Mindemark J.; Lacey MJ.; Bowden T.; and Brandell D, Progress in Polymer Science, 2018, 81, 114-143.
CrossRef - Bocharova V and Alexei PS, Macromolecules,2020, 53(11), 4141-4157.
CrossRef - Kumar Y.; Hashmi SA and Pandey GP, Electrochimica acta, 2011, 56(11), 3864-3873.
CrossRef - Mishra T.; Mandal P.; Rout A K and Sahoo D, Composites Part C: Open Access, 2022, 100298.
CrossRef - Pleşa I.; Noţingher PV.; Schlögl S.; Sumereder C and Muhr, M, Polymers, 2016, 8(5), 173.
CrossRef - Tanaka T.; Montanari GC and Mulhaupt R, IEEE transactions on Dielectrics and Electrical Insulation,2004, 11(5), 763-784.
CrossRef - Han J and Garrett R, NSTI-Nanotech, 2008, Vol. 2,727-732.
- Hou W.; Xiao Y.; Han G and Lin JY, Polymers,2019, 11(1), 143.
CrossRef - Goyal RK.; Tiwari AN.; Mulik UP and Negi YS, Composites science and technology, 2007, 67(9),1802-1812.
CrossRef - Lin QB.; Wang LW and Huang SH, Surface Engineering and Applied Electrochemistry,2015, vol. 51, 394-400.
CrossRef - Mallikarjun A.; Sangeetha M.; Mettu MR.; Reddy MJ.; Kumar JS.; Sreekanth T and Rao SV, Materials Today: Proceedings, 2022, 62, 5204-5208.
CrossRef - Arya A and Achchhe LS, Applied Physics, 2018, 51(4), 045504.
CrossRef - da Silva Neiro SM.; Dragunski DC.; Rubira AF and Muniz EC, European polymer journal, 2000, 36(3), 583-589.
CrossRef - Rondán-Gómez V.; Montoya De Los Santos I.; Seuret-Jiménez D.; Ayala-Mató F.; Zamudio-Lara A.; Robles-Bonilla T and Courel M, Applied Physics A, 2019, 125, 1-24.
CrossRef - Liao Q.; Jin X and Fu H, Advanced Optical Materials, 2019, 7(17), 1900099.
CrossRef - Pathak CS.; Mishra DD.; Agarwala V and Mandal MK, Ceramics International, 2012, 38(7), 5497-5500.
CrossRef - Peng G.; Zhao X.; Zhan Z.; Ci S.; Wang Q.; Liang Y and Zhao M, RSC Advances, 2014, 4(32), 16849-16854.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.










