Synthesis, Characterization and Dye Adsorption Studiesof Silver Nanoparticles by Biowaste Plant Caesalpinia Pulcherrima
Department of chemistry, Erode arts and Science College, Erode, Tamil Nadu, India.
Corresponding Author E-mail: santhisendil@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/390334
Article Received on : 12 Apr 2023
Article Accepted on : 26 Jun 2023
Article Published : 01 Jul 2023
For their unique physical, chemical and biological characteristics, silver nanoparticles (AgNPs) are used in a variety of applications in the sectors of health and wellness as well as consumer and industrial products. We describe here the production of silver nanoparticles (AgNPs) using activated carbon from locally accessible biowaste Caesalpinia pulcherrima pod is the goal of this work, which aims to characterise the activated carbons generated by oxidation of activated carbons of comparable porosity with Sulphuric acid, nitric acid, and phosphoric acid, respectively. Using Caesalpinia pulcherrima plant extract as a reducing agent, silver particles may be made. TEM was used to determine the particle size, distribution, shape, shape heterogeneity, and aggregation. Analysis of the silver nanoparticle's composition has been done using EDAX. According to the findings, activated carbon derived from Caesalpinia pulcherrima pod is ideal for the adsorption of textile dyes such as Eriofast Blue (EB) and Erionyl Orange (EO) and might be employed as a low-cost efficient adsorbent in the treatment of industrial wastewater.
KEYWORDS:Activated Carbon; Caesalpinia pulcherrima; Dye Adsorption; Silver Nanoparticles; TEM
Download this article as:| Copy the following to cite this article: Krishnamoorthy R, Santhi M, Asaithambi M. Synthesis, Characterization and Dye Adsorption Studiesof Silver Nanoparticles by Biowaste Plant Caesalpinia Pulcherrima. Orient J Chem 2023;39(3). |
| Copy the following to cite this URL: Krishnamoorthy R, Santhi M, Asaithambi M. Synthesis, Characterization and Dye Adsorption Studiesof Silver Nanoparticles by Biowaste Plant Caesalpinia Pulcherrima. Orient J Chem 2023;39(3). Available from: https://bit.ly/3JFi7rK |
Introduction
The study of structures and materials at the nanoscale size is known as “nanoscience.” The field of nanoscience deals with the study of very tiny things. The characteristics of nanoparticles are examined in nanoscience. The term “nanoparticles” refers to particles that are smaller than 100nm in diameter. When it comes to transport and attributes, a particle in nanotechnology is described as a tiny entity that acts as a single unit. The addition of nanoparticles to materials improves their strength while also reducing their weight. Socks now include silver nanoparticles. The antimicrobial qualities of the nanoparticles prevent them from absorbing the stench of sweaty feet. Technological innovations like semiconducting nanowires are being investigated by IBM scientists to enhance transistor design that has been around for more than half a century.1
In 1981, Gerd Binnig and Heinrich Rohrer devised the scanning tunnelling microscope, which revolutionised our capacity to handle solid surfaces the size of atoms. Materials and gadgets developed by nanotechnologists have the potential to transform our daily lives. There are nanoscale pumps and switches, but the small self-replicating machines envisioned by pioneers in nanotechnology remain a long way off, at best. But we’ve already shown their effectiveness. A successful nanoscale, self-replicating molecular machine, DNA produces structures molecule by molecule in living creatures and is essential to their survival. As a result of the nanoparticle’s interaction with electromagnetic radiation, the electric field it generates induces the formation of a dipole, which confers the nanoparticle’s restorative force. As a result of this effect study ofproperties and multiple applications of nanoparticles has progressed in parallel.2
Silver has a wide range of applications, but one of the most intriguing is its usage as an antibacterial disinfectant. This research aims to discuss some of the ways for generating silver nanoparticles, characterise them, and pay particular attention to their antibacterial potential as a result of the enormous prosperity that has prevailed in nanotechnology. Silver nanoparticles have the highest surface area-to-volume ratio, making them the most useful for technical applications. The nanoparticle surface is critical and should be carefully monitored because even a small change in the particle’s surface size can cause significant changes in its physical and chemical properties. Between 1 and 100 nanometers in diameter (nm), the properties of the particles change significantly in terms of their chemical and physical properties, As a result, altering their size and form enables for control over features such as temperature, redox potential, colour and conductivity, chemical stability, electrical properties, and optics.3-4
Research shows that the characteristics of nano silver particles are impacted substantially by the experimental circumstances of their synthesis and interactions with reducing agents and the absorption processes of their stabilising agent. As a result, the synthesis process used determines the specifics of the desired silver nano particle, including its form, size, and distribution. There are several ways to synthesise metallic silver salts, but the first step is to determine the desired form of the nanoparticle, whether it is spherical or triangular or cubic or pyramidal or rod-shaped or cylindrical. Because nanoparticle shape is determined by reaction speed and stabilising agent interaction, the technique that best suits the nanoparticle shape must be chosen after the form is known.5-7 Sulfuric acid, nitric acid, and phosphoric acid extract charcoal were used to create a plant extract from the caesalpinia pulcherrima plant in order to synthesise and characterise silver nanoparticles.8 By using UV visible spectroscopy and FTIR analysis, the silver nanoparticles generated have been examined in detail. SEM and TEM have been used to examine the particle size, distribution, shape, shape heterogeneity, and aggregation. EDAX has researched the components that make up the silver nanoparticles.9-10
Materials and Methods
Collection and identification of plants
In the TamilNadu district of Krishnagiri, charcoal from Caesalpinia pulcherrima (CP) plants was harvested year-round. The plants are chosen from among those found naturally around the state of Tamil Nadu. St. Joseph’s College’s Rapinat Herbarium and Centre for Molecular Systematics validated the chosen plants.
Preparation of activated carbon
Activated Carbon is made from Caesalpinia pulcherrima pods, which are employed as a precursor in the process. To make activated carbon, the pods were rinsed in distilled water, dried for five days in the sun, then crushed into minute pieces. Powdered carbonised material was studiedutilising physico-chemical techniques and surface analysis to determine its composition andproperties.
Carbonization procedures
For carbonization, the pod material is first treated for an hour with a boiling solution of 10% H2S04, HNO3, H3PO4 and then soaked for 24 hours in the same solution. Decanting and drying the remaining solution took place at the conclusion of the 24-hour period. Subsequently, a muffle furnace at 120-130 degrees Fahrenheit carbonised the raw material The powdered material was heated to 800°C in a muffle furnace for 60 minutes before being activated. After that, a huge amount of water was used to eliminate any remaining acid, and the material was dried and pulverised.
Acid process
For 24 hours, the dry material was thoroughly soaked in an excess of a suitable acid solution. When the material is heated, charring begins instantly, followed by a release of heat and gas. The surplussulphuric acid was decanted and air dried after 24 hours. It wa.\fien placed in the muffle furnace to carbonise at 140-160 °C after the reaction had ceased. At the end of this period, the product was washed with a significant amount of water to eliminate free acid.
Characterization of the activated carbon
According to the usual testing procedures, physio-chemical features of the Caesalpinia pulcherrima pod activated carbon samples were analysed.
Methods of synthesis of silver nano particles
Beakers containing 100 ml of the activated charcoal of Caesalpinia pulcherrima plant material produced with various acids are used to dissolve the solution. Stirred for 15 minutes, that mixture was then made up of a few drops of silver nitrate. Silver nanoparticles were then generated as a result of this procedure. Separation and dryjng of silver nanoparticles were performed. Characterization of Silver nanoparticles were carried out by TEM, EDAX.11-16
Batch adsorption studies
A known amount of activated carbon sample was put in a series of flasks holding varied concentrations of Eriofast Blue (EB) and Erionyl Orange (EO) solutions. The flasks were then shaken at constant temperature until equilibrium was reached. Centrifugation was then used to remove the carbon compounds. Adsorbate concentrations in the filtrates were measured using a UV-vis Spectrophotometer at 650 nm and a calibration curve. qe (mg/g) of EB and EO uptake at equilibrium was estimated.
Results and Discussion
Transmission Electron Microscopy
TEM is a valuable, frequently used, and significant technique for the characterization of nano-materials, used to obtain quantitative measures of particle and grain size, size distribution, and morphology 17-18. TEM images of Silver nanoparticles formed by using activated charcoal prepared by using Caesalpinia pulcherrima plant in Sulphuric acid, Nitric acid and Phosphoric acid are shown in figure 1 (a, b, c, d, e & f) respectively. The magnification of TEM is mainly determined by the ratio of the distance between the objective lens and the specimen and the distance between objective lens and its image plane. TEM has some advantages than SEM, that is, it can provide better spatial resolution and the capability for additional analytical measurements. The disadvantages include a required high vacum, thin sample section, and the vital aspect of TEM is that sample preparation is time consuming. Therefore, sample preparation is extremely important in order to obtain the highest-quality images possible.
 |
Figure 1: (a – b). TEM image of silver nanoparticles formed by using activated charcoal prepared by using Caesalpinia pulcherrima plant in Sulphuric acid. |
 |
Figure 1: (c – d). TEM image of Silver nanoparticles formed by using activated charcoal prepared by using Caesalpinia pulcherrima plant in Nitric acid. |
 |
Figure 1; (e – f). TEM image of silver nanoparticles formed by using activated charcoal prepared by using Caesalpinia pulcherrima plant in Phosphoric acid. |
Energy Dispersive Analysis of X-rays (EDAX)
The Silver particle present in sample has been characterized by EDAX. The presence of Silver ion in Silver nanoparticles is shown in figure 2a, 2b, 2c respectively by using activated charcoal prepared by using Caesalpinia pulcherrima plant in Sulphuric acid, Nitric acid and Phosphoric acid. The reducing agent that is plant extract which reduce bulk particle of silver ion into nanoparticle of silver 19-20.
 |
Figure 2a: EDAX image of Silver nanoparticles formed by using activated charcoal prepared by using Caesalpinia pulcherrima plant in Sulphuric acid. |
 |
Figure 2b: EDAX image of Silver nanoparticles formed by using activated charcoal prepared by using Caesalpinia pulcherrima plant in Nitric acid. |
 |
Figure 2c: EDAX image of Silver nanoparticles formed by using activated charcoal prepared by using Caesalpinia pulcherrima plant in Phosphoric acid. |
Dye Adsorption Studies
Effect of PH:
PH is the notable majority significant parameter in the effect of dye adsorption study, as the PH controls the adsorbent adsorbate interactivity brings difference in pH values. Hence it is vital to find out the optimum PH Conditions.21 The optimum activity of pH of activated carbon on removal of dye was estimated by varying the pH of the Methyl orange and Methylene blue dye solution ranges from 2 to 10. Figure 3 (a,b,c) clearly explains the elimination efficiencies of the EO and EB dyes on the synthesized activated carbon for different PH. The graph suggests that the highest removal efficiencies of MO dye of activated carbon was found at PH 2 (93% for Sulphuric acid, 92% for Nitric acid, 90% removal capacity for phosphoric acid) and noticeably decrease efficiency with increase in pH of dye solution. At the maximum PH value 10.0 all the synthesized activated carbon dropped nearly to 30%. In Contrast, the graph of EB dye shows that removal capacity increased as the PH value increased, optimum removal capability found at PH 5.5 with 93% for sulphuric acid, 85% for Nitric acid, 88% for Phosphoric acid synthesized activated carbon respectively. This observable fact can be due to transmit and elimination of protons on the existing effective groups on the surface of the adsorbent, and also due to the anionic and cationic nature of the target dyes of different PH. Similar research result were reported by Kananijazi22 in absorption experiment studies of activated carbon derived from citrous fruits peels for absorption of EO and EB dye solution.
 |
Figure 3: Effect of PH on dye removal of Activated carbon synthesized by using a) Sulphuric acid b) Nitric acid c) Phosphoric Acid. |
Effects of adsorbent dosage and contact time:
Additional significant Parameters for dye elimination are a mass of adsorbent and its result on the efficiencies of removal of the EO and EB dyes. Zhao result suggested that there is an extreme raise in removal efficiency as the mass of adsorbent concentration is varied from 0.01-0.1g. Maximum adsorption and equilibrium efficiency were obtained between 0.05- 0.1g.23 Hence to assess the adsorbent mass of the synthesized activated carbon the adsorbent mass concentration dosage preferred from the range of 0.05-0.09g/l at a given contact time. Figure (4- a, b, c) the graph obtained for the contact time and adsorbent dosage on the adsorptive uptake of biowaste Activated Charcoal synthesized by Sulphuric Acid, Nitric Acid and Phosphoric Acid. The result of the entire activated charcoal synthesized nanoparticle exhibits the adsorption quantity at equilibrium (qe) reduced with an increase in dosage of adsorbent from 0.05 to 0.09 g/L with more or less same values for all the samples. This is due to outcome of limited conglomeration, which appears at higher amount of adsorbent ensuing in reducing the active sitesComparable result was found for the phenol adsorption onto activated carbon and fungal biomass.24 Likewise another result had suggested strong evidence for the adsorption of various adsorbates onto varies adsorbents based on the literature.25-26
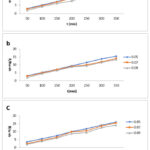 |
Figure 4: Effect of Adsorbent dosage and contact time on the adsorptive uptake of biowaste Activated Charcoal synthesized by a) Sulphuric Acid b) Nitric Acid c) Phosphoric Acid. |
Effect of the temperature
The effect of temperature of adsorption onto AC was assessed at most favorable dosage of adsorbent. The temperature dependence of dye onto activated charcoal was considered to be optimum at adsorbent dosage of 0.05 g/L. Figure5 a, b, c demonstrates dye adsorption ability onto activated charcoal samples at temperatures ranging from 30 to 50 °C. The results reveal that when the temperature of the solution climbed from 30 to 50 °C, the equilibrium adsorption capacity of the dye increased. The increase in adsorption capacity may be due to strong bonds between the dye molecules and the active sites of synthesized activated charcoal. Related phenomenon has been found in the adsorption of remazol brilliant blue and methylene blue (dyes onto orange peel adsorbent.27-28
As proof, several study results demonstrate that food dye adsorption increased as temperature increased, perhaps enhancing the pace of reaction at an ideal temperature. The most likely explanation is that higher temperatures raise the pore depth and surface area, increasing the likelihood of dye passing through the outside border layer. This backs with previous research findings.29-30
 |
Figure 5: Effect of solution temperature on the adsorption of the dye onto AC synthesized by a) Sulphuric acid b) Nitric Acid c) Phosphoric acid (conditions: W=0.05 g/L; C0=10 mg/L). |
Conclusion
Nowadays, nanotechnology necessitates the creation of delegable and ecologically acceptable ways for producing metallic nanoparticles. The biofunction approach of reducing silver nitrate solution using Caesalpinia pulcherrima aqueous extract as the reducing agent is employed here to generate stable silver nanoparticles that are activated by various acids. Analysis of the silver nanoparticles was carried out utilising the FTIR, SEM, EDX, and TEM methods. The findings showed that silver nitrate was reduced to silver nanoparticles that were stable and free of impurities. Since activated charcoal was created using three distinct acidssuch as sulfuric acid, nitric acid (Nitrate), and phosphate (Phosphoric), the same wasemployed to synthesise solver nanoparticles. FTIR, EDX, SEM, and TEM analysis revealed theexistence of all of the aforementioned silver nanoparticle types. The average size of silvernanoparticles generated by comparing experimental data was around 80 nm. The approach proved applicable to actual wastewater samples, with good EB and EO removal percentages. According to the findings of this investigation, biosorbent may be successfully used to remove textile colours.
References
- Meisel D. andLeey PC., J Phys Chem.,1986, (17), 3391‒3395.
- Wei LY.; Lu JR. and Xu HY., Drug Discovery Today.,2015, 20(5), 595‒601.
CrossRef - Baláz M.; Daneu N. andBalázˇová., Advanced Powder Technology.,2017, 28(12), 3307–3312.
CrossRef - Khodashenas B. and Ghorbani H., Arabian Journal of Chemistry.,2015, 1(1), 1‒16.
- Zielinska A.; Skwarek E. and Zaleska A., Procedia Chemistry.,2009, 1560‒1566.
CrossRef - Esvandi, Z.; Foroutan, R.; Peighambardoust, S.J.; Akbari, A. andRamavandi, B. Surf. Interfaces.,2020, 21, 100754.
CrossRef - Duman O.; Tunc¸ S. and Polat T. G., Microporous Mesoporous Mater., 2015, 210:176–84.
CrossRef - Zhao, Z.; Bai, C.; An, L.; Zhang, X.; Wang, F.; Huang, Y.; Qu, M. and Yu, Y. J. Environ. Chem. Eng. 2021, 9, 104797.
CrossRef - Hassan, A.F.; Abdel-mohsen.; A.M. and Elhadidy, H., Int. J. Biol. Macromol., 2014, 68, 125–130.
CrossRef - Jawad, A.H. and Abdulhameed, A.S., Energy Ecol. Environ., 2020. 5 (6), 456–469.
CrossRef - Ajitha B.; Ashok Kumar Reddy A. and Hwan-Jin Jeon., Advanced Powder Technology. 2018, 29(1), 86–93.
CrossRef - López-Lorente ÁI and Mizaikoff B., TrAC Trends in Analytical Chemistry., 2016, 84:97‒106.
CrossRef - Calderón-Jiménez B.; Johnson ME. and Montoro Bustos AR., Frontiers in chemistry. 2017, 5:6.
CrossRef - Agnihotri A,; Mukherji S. and Mukherji S.,RSC Advances.,2014, 4(8), 3974–3983.
CrossRef - Gurunathan, S.; Han, J.W.; Kim, E.S.; Park, J.H. and Kim, J.H., Int. J. Nanomed.,2015, 10, 2951–2969.
CrossRef - Mukherjee, P.; Ahmad, A.; Mandal, D.; Senapati, S.; Sainkar, S.R.; Khan, M.I.; Renu, P.; Ajaykumar, P.V.; Alam, M. and Kumar, R., Nano Lett. 2001, 1, 515–519.
CrossRef - Shankar, S.S.; Ahmad, A.; Sastry, M. Biotechnol. Prog. 2003, 19, 1627–1631.
CrossRef - Pyatenko, A.; Yamaguchi, M.; Suzuki, M. J. Phys. Chem. C 2007, 111, 7910–7917.
CrossRef - Das, R.; Nath, S.S.; Chakdar, D.; Gope, G.; Bhattacharjee, R. J. Nanotechnol. 2009, 5, 1–6.
CrossRef - He, R.; Qian, X.F.; Yin, J.; Zhu, Z.K. J. Mater. Chem. 2002, 12, 3783–3786.
CrossRef - Baloo,I.; Isa, M.H.;Sapari, N.Bin, Jagaba, A.H.; Wei, I.J.; Yavari, S.; Razali, R. and Vasu, R.,Ale.. Eng. J., 2021, 60 (6), 5611-5629.
CrossRef - Kanani –Jazi, M.H. and Akbari, S. J. Environ. chem. Eng. 2021. 9(3), 105214.
CrossRef - Zhao, W., Zhao,Y., Zhang, H., Hao, C. and Zhao,P. Physicochem. Eng. Asp., 2022, 633 (P1), 127680.
CrossRef - C. O. Nweke. And G. C. Okpokwasili, International Journal of Biosciences., 2013, 3 (11), 11-21.
- 25. F. Oguz Erdogan., Journal of Textiles and Engineering., 2017, 107, 181.
- Z. Huang, X. Wang, D. and Yang, Water Science and Engineering., 2015, 8, 226.
CrossRef - M. R. Mafra,; L. Igarashi-Mafra,; D. R. Zuim,; É. C.Vasques. and M. A. Ferreira. Brazilian Journal of Chemical Engineering., 2013, 30, 657.
CrossRef - P. S. Kumar.; P. S. A. Fernando.; R. T. Ahmed.; R. Srinath.; M. Priyadharshini.; A. M. Vignesh. and A. Thanjiappan., Chemical Engineering Communications., 2014, 201, 1526.
CrossRef - T. Erdogan. And F. Oguz Erdogan., Analytical Letters., 2016, 49, 917.
CrossRef - F. Oguz Erdogan, Journal of Textiles and Engineering., 2017, 107, 181.

This work is licensed under a Creative Commons Attribution 4.0 International License.










