Quantitative Analysis of Gallic Acid and Quercetin by HPTLC and In Vitro Antioxidant Activity of Averrhoa carambola Linn.
1School of Pharmaceutical Sciences, IFTM University, Lodhipur Rajput Delhi Road, Moradabad-244001 (Uttar Pradesh), India.
2Pharmacy Academy, IFTM University, Lodhipur Rajput, Delhi Road, Moradabad-244001 (Uttar Pradesh), India.
Corresponding Author E-mail: dineshpharma181@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/390324
Article Received on : 08 Apr 2023
Article Accepted on : 29 May 2023
Article Published : 14 Jun 2023
Reviewed by: Dr.Ammar A.Razzak Mahmood
Second Review by: Dr. Ahmed Awad
Final Approval by: Dr. Malinee Sriariyanun
The preliminary phytochemical screening of ethanolic extracts of Averrhoa carambola (A. carambola) leaves were done using the standard protocol. The findings of phytochemical analysis exhibited the occurrence of Carbohydrates, Alkaloids, Steroids, Tannins, Vitamin C and flavonoids. By using the Folin-Ciocalteu technique and the aluminum chloride colorimetric technique, respectively, the whole phenolic or flavonoid amounts were evaluated and were found 194.48±0.723 mg/g of dry extract as equivalent to gallic acid and 54.83±0.108 mg/g of dry extract as equivalent to quercetin respectively. In vitro, antioxidant activity was evaluated using 2, 2-diphenlyl-1-picrylhydrazyl (DPPH), Hydrogen peroxide (H2O2), and Nitric oxide (NO) method. Ethanolic extract of A. carambola leaves showed good in vitro antioxidant action. Thin layer chromatography (TLC) of the ethanolic extract was carried out with gallic acid and quercetin as the reference biomarkers. HPTLC (High-Performance Thin Layer Chromatography technique was applied to detect spots and quantification for gallic acid and quercetin. The Rf values of gallic acid and quercetin were found 0.25 and 0.53 respectively. The amounts of gallic acid and quercetin were found to be 502.7 µg and 458.3 µg /100 mg of the ethanolic extract of A. carambola leaves separately.
KEYWORDS:Antioxidant; A. carambola; Flavonoid; Gallic acid; HPTLC; Phenolic; Quercetin; TLC
Download this article as:| Copy the following to cite this article: Kumar D, Verma N, Raj V. Quantitative Analysis of Gallic Acid and Quercetin by HPTLC and In Vitro Antioxidant Activity of Averrhoa carambola Linn. Orient J Chem 2023;39(3). |
| Copy the following to cite this URL: Kumar D, Verma N, Raj V. Quantitative Analysis of Gallic Acid and Quercetin by HPTLC and In Vitro Antioxidant Activity of Averrhoa carambola Linn. Orient J Chem 2023;39(3). Available from: https://bit.ly/3CBEpGY |
Introduction
Traditionally, plants are considered an essential source of medicines and playing a crucial role in the health of the overall community. In addition, biological importance of secondary metabolites of plants on humans has been known for a long time 1.
A. carambola commonly referred as star fruit or carambola, is a well-known plant that belongs to the family Oxalidaceae. A. carambola is mainly grown in southern China, Southeast Asia, India, as well as Northern South America. The leaves of A. carambola are normally used to treat coughing, headaches, chicken-pox, ringworm, vomiting, fevers, angina, aphthous stomatitis, diabetes, and hangovers 2,3,4.
According to numerous phytochemical and pharmaceutical studies, the extract of A. carambola leaves is a reliable source of phenolic, flavonoids, saponins, alkaloids, and tannins, etc. The various reported bioactive compounds present in the leaves such as vitamin C, quercetin and gallic acid are responsible for specific healing properties 5,6,7.
Plants rich in phenolic and flavonoids mixtures such as gallic acid and quercetin are primarily capable for health advantages. Plants those have high amount of phenolic and flavonoids content, produce more significant antioxidant activity. Antioxidants are essential in the management of inflammation, diabetes, cancer, dementia, and Alzheimer’s disease 8, 9.
In the study of phytoconstituents analysis, Thin–layer chromatography (TLC) and High-performance thin-layer chromatography (HPTLC) techniques has been employed frequently. These techniques helps in the qualitative and quantitative analysis of plants constituents in tiny amounts. The uses of these techniques for evaluation of herbal medicines has grown, as well as many herbal pharmacopoeias, now involves these techniques for the identification and standardization of phytoconstituents and phytoproducts. These techniques tells the quality and quantity of the phytoconstituents in plant medicine10,11. Structures of Quantified compounds (Gallic acid and Quercetin) are available in Figure 8.
This study aimed to perform the extraction, extractive value determination, preliminary phytochemical screening, entire phenolic and flavonoid contents determination, in vitro antioxidant activity determination by various methods, TLC and HPTLC for measurement of the two marker phytoconstituents gallic acid and quercetin in ethanolic extract of A. carambola.
Material and Methods
Collection and Authentication of Plant Materials
The fresh leaves of A. carambola L. were gathered from the garden of the Dariyapur Bujurg, Distt Amroha Uttar Pradesh, India. Plant materials were taxonomically detected and verified by Dr. Sunita Garg, Former Chief Scientist, and Head, of Raw Materials Herbarium and Museum, Delhi (RHMD), CSIR- NIScPR as A. carambola L (Family: Oxalidaceae) with Authentication No.- NIScPR/RHMD/Consult/2021/3914-15-1. Plant samples were submitted in the herbarium of the same laboratory.
Chemicals and Drugs
Glacial acetic acid, Toluene, Ethyl acetate, Ethanol, Methanol, Hydrogen peroxide, and Petroleum ether (60-80°C) were acquired from Central Drug House (CDH) New Delhi. DPPH and Ascorbic acid were obtained from Sigma Aldrich. Gallic acid and Quercetin were procured from Yucca Enterprises, Mumbai. All the chemicals and drugs used were of laboratory grade.
Leaves Extract Preparation
The leaves of A. carambola were collected and dried in shade at room temperature until the leaves became well–dried. After drying leaves crushed it into a coarse powder. 50gm of dried coarse leaf powder was taken in Soxhlet and extracted with 250 ml of Petroleum ether (60-80°C) for defatted and it was further extracted with Ethanol. The extract was dried using the water bath and preserved in a desiccator for further use. The extractive value of Ethanolic extract was measured 12, 13.
Preliminary Phytochemicals Investigation
The preliminary phytochemical investigations of ethanolic extract of A. carambola leaves were performed to identify different types of phytoconstituents such as carbohydrates, Amino acids, Proteins, alkaloids, saponins, steroids, tannins, Vit C and Flavonoids 12, 14.
Total Phenolic Contents Determination
The Folin Ciocalteu (FC) technique was used for the estimation of whole phenolic amount of ethanolic extract of A. carambola leaves. 1 ml extract of 1 mg/ml conc. was added with 1 ml of FC chemical and after five minutes, ten ml of seven % sodium bicarbonate solution was mixed to the above mixture. After A few seconds, thirteen ml of distilled water was mixed systematically. The above solution was stored in the dark for ninety minutes at room temperature, after this the absorbance was noted at 760 nm. The entire phenolic content was estimated from standard curve prepared by gallic acid. Total Phenolic Content are described as gallic acid equivalent (mg/g of dry extract)15, 16.
Total Flavonoid Contents Determination
The whole flavonoid amount of the ethanolic extract of A. carambola leaves was determined by aluminum chloride spectrophotometer method. Firstly 1 ml extract of 1mg/ml was taken in a test tube and mixed with 2 ml CH3OH, 0.1 ml aluminum chloride (10%), 0.1 ml potassium acetate, and 2.8 ml distilled water. The above mixture mixed thoroughly, stored for thirty min in dark and the absorbance was noted at 415 nm using a UV spectrophotometer. The standard quercetin solution was tested in the same way. The total flavonoid contents in A. carambola leaves extract are described as quercetin equivalent (mg/g of dry extract)17.
In Vitro Antioxidant Activity
DPPH radical scavenging assay
The Antioxidant activity of the ethanolic leaves extract of A. carambola at different concentrations against DPPH was tested. 10, 20, 30, 40, 50, 60, 70 and 80µg/ml of the leaves extract and ascorbic acid as reference in similar Concentration. as filled in test tube and in every test tube, DPPH solution was mixed in similar volume. After these two milliliters of methanol was mixed in each and every test tube and the test tubes were stored for ninety minutes in a dark room. After ninety minutes, all the test tube absorbance was noted at a wavelength of 517 nm by the spectrometer. The inhibition percentages of the reference as well as the sample were calculated via equation 1. Inhibition Conc. 50 (IC) value was calculated from the percentage inhibition vs conc. graph 18,19,20,21.

All tests were performed in triplicate.
Hydrogen Peroxide (H2O2) Assay
Antioxidant activity by H2O2 was estimated according to the method of Ruch et al with slight modification 28. H2O2 (40 mM) solution was made in phosphate buffer (50 mM pH 7.4). 10, 20, 30, 40, 50, 60, 70, and 80µg/ml conc. of the leaves extract and Ascorbic acid as a reference in similar conc. was filled in a test tube and mixed 2 ml of H2O2 into each and every test tube and after that added 2 ml of phosphate buffer solution (50mM pH 7.4) in each test tube. Absorbance was noted at 230 nm by spectrophotometer. The inhibition percentages of the reference as well as sample were determined via above mention equation 1. IC 50 value was calculated from the percentage inhibition vs conc. graph 22, 23.
Nitric Oxide Scavenging Assay
The antioxidant activity of Ethanolic leaves extract of A. carambola at different conc was tested by the nitric oxide method. 10, 20, 30, 40, 50, 60, 70, and 80µg/ml conc. of the plant leaves extract and ascorbic acid as a reference in similar conc. was filled in test tube and in every test tube 4 ml sodium nitroprusside (10 millimole), 1 ml phosphate buffer (7.4 pH and all test tubes was stored at room temperature for 150 minutes. After storage 0.5ml mixture withdrawn from each test tubes and filled in new test tubes and added 1 ml sulphanilic acid and stand for few minutes. After that 1 ml of Napthyl Ethylene Diamine Dihydrochloride (NEDD) was mixed and again stored for thirty minutes. Absorbance of each test tubes noted at 540 nm by spectrometer. IC 50 value was determined from the percentage inhibition vs conc. graph 17, 24.
Thin–layer chromatography (TLC)
The various solvent system was optimized for TLC analysis of Ethanolic leaves extract of A. carambola but finally, the below mentioned solvent system presented in Table 1 was used. The Rf value of different spot or solutes was determined by the below mention formula 1.
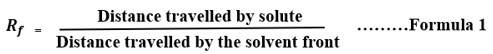
Table 1: TLC and HPTLC solvent system for Ethanolic extract of A. carambola.
|
Leaves |
Solvent Mixture |
Ratio |
Detection |
|
A. carambola |
Toluene: Ethyl Acetate: Glacial Acetic Acid |
6:3:1 |
Day Light |
HPTLC Analysis for quantitative estimation of Gallic Acid and Quercetin
Authors also wanted to find out how much amount of gallic acid and quercetin are present in ethanolic leaves extract of A carambola. for this we used HPTLC technique because HPTLC is simple and cheaper technique as compared to other techniques.
Preparation of Standard Solution
Standard amounts of gallic acid and/or quercetin, each weighing 1 mg, were each mixed with methanol on their own to produce standard solutions with a concentration of 100 µg/ml.
Preparation of test solution
100 mg ethanolic extract of A. carambola was added in 10 mL ethanol and shaken for ten minutes and filtered. Filtrate was used for HPTLC analysis.
HPTLC Method Development
One of the most effective methods for determining the levels of phytoconstituents found in various plant parts is high-performance liquid chromatography (HPTLC). An aliquot of every dilution of the standard and test solutions (ethanolic extract) has been applied to a pre-coated TLC plate (5 by 10 centimeters with a 0.2 mm aluminium base) by using Camag Linomat V. The aliquot was 10 microliters in volume. The chromatogram has been developed in a below mention solvent system mentioned in Table 1 in a saturated chamber. The plate that had been developed was first dried with a flow of hot air, then it was examined in a TLC scanner at an able to detect wavelength. The peak area, as well as the area under the curve, were plotted against the sample concentrations in order to generate a calibration curve. It was determined how much of the marker compound was present by employing an equation of regression based on the calibration curve 25, 26.
Statistical analysis
The findings of study were indicated as the mean accompanied by the ± standard deviation (SD) of three separate attempts. From the regression plots, we were able to derive the IC50 values (the concentration at which there was a 50% reduction in activity). The findings were subjected to a one-way analysis of variance (ANOVA) wherever it was relevant to do so, and the significant difference (P <0.05) between the means was significant.
Results
The Extractive value of the ethanolic extracts of A. carambola leaves is presented in Table 2.
Table 2: The result of the Extractive Value of the Ethanolic extracts of A. carambola leaves
|
S. No |
Solvent |
Extractive Value (%w/w) |
|
1 |
Ethanol |
7.308±0.46 |
Values are expressed as Mean±SD
Phytochemical Analysis
The Phytochemical analysis of the ethanolic extract of A. carambola leaves appeared that it contains carbohydrates, alkaloids, saponins, steroids, tannins, vitamin c and flavonoids. The finding of phytochemical screening is presented in table 3. Such secondary metabolites are also recognized to have several pharmacological benefits.
Table 3: Results of phytochemicals screening of ethanolic extracts of A. carambola leaves
|
S. No. |
Name of the Chemical test |
Ethanolic Extract |
|
1. |
Test for Carbohydrate |
|
|
|
Molish’s test |
+ |
|
2. |
Test for amino-acids |
|
|
|
Ninhydrin test |
– |
|
3. |
Test for Proteins |
|
|
|
Biuret test |
– |
|
|
Million’s test |
– |
|
4. |
Test for Alkaloids |
|
|
|
Dragendroff’s test |
+ |
|
|
Mayer’s test |
+ |
|
5. |
Test for Saponins |
|
|
|
Foam test |
+ |
|
6. |
Test for Steroid |
|
|
|
Salkowski reaction |
+ |
|
|
Liebermann Burchard Reaction |
+ |
|
7. |
Test for Tannins |
|
|
|
Drug + 5% Ferric Chloride |
+ |
|
|
Drug+ Lead Acetate Solution |
+ |
|
8. |
Test for vit C |
+ |
|
9. |
Test for Flavonoids |
|
|
|
Shinoda test |
+ |
|
|
Alkaline Reagent Test |
+ |
|
|
Zinc Hydrochloride Test |
+ |
(+) = Present; (-) = Absent
Total Phenolic content
Using a UV spectrophotometric technique, the overall phenolic material of the ethanolic leaves extract of A. carambola leaves was calculated. It was discovered that the total amount of phenolic content was 194.48±0.723 mg gallic acid equivalent /gweight of dry extract. The values shown are the mean±standard deviation of three distinct assessments.
Total flavonoids content
Using a UV spectrophotometric technique, the flavonoid substance of the ethanolic leaves extract of A. carambola leaves was determined. It was discovered that the total flavonoid substance was 54.83±.108 mg quercetin equivalent /g weight of dry extract. The values provided are displayed as the mean±standard deviation of three distinct assessments.
Antioxidant Activity of Ethanolic Leaves Extract
DPPH radical scavenging assay
The DPPH test method uses a stable free radical called 2,2-diphenyl-1-picrylhydrazylhydrate, which can admit an electron to transform into a stable moiety. A. carambola leaves extracts in ethanol demonstrated potent free radical scavenging abilities. The rising amount of the compounds affects the scavenging activity. The existence of antioxidants in the substances is indicated by the DPPH assay’s transition from purple to yellow. Calculating A. carambola’s % of scavenging activity and plotting it against conc. vs. % suppression Figure 1. Various conc, notably 10, 20, 30, 40, 50, or 60, 70, as well as 80 g/mL, were used for the scavenging activity. The outcomes supported the discovery of a dose-dependent inhibition activity that displayed free radical scavenging activity. Strong antioxidant activity was demonstrated by A. carambola‘s ability to scavenge DPPH free radicals, with IC50 values of 87.±.252g/mL. The percent of the total DPPH scavenging actions of distinct concentrations of ascorbic acid and A. Carambola leaves are presented in Table 4 and Figure 1 and the IC50 value of A. carambola leaves and ascorbic acid is displayed in Table 5.
 |
Figure 1: Percentage Inhibittion of A. carambola as Compared to ascorbic acid by the DPPH method. |
Table 4: Percentage of scavenging activity of Ascorbic acid and A. carambola leaves by DPPH method.
|
S. No |
Conc. (µg/ml) |
(%) Inhibition (Mean±SD |
|
|
|
|
Ascorbic Acid |
A. carambola leaves |
|
1 |
10 |
27.13±0.024 |
11.94±0.064 |
|
2 |
20 |
32.19±0.016 |
23.58±0.09 |
|
3 |
30 |
38.26±0.048 |
30.67±0.057 |
|
4 |
40 |
44.23±0.024 |
35.43±0.062 |
|
5 |
50 |
51.42±0.009 |
39.98±0.012 |
|
6 |
60 |
56.48±0.065 |
46.86±0.016 |
|
7 |
70 |
62.04±0.032 |
50.30±0.048 |
|
8 |
80 |
68.12±0.097 |
55.67±0.062 |
Table 5: IC50 for Ascorbic Acid and A. carambola during DPPH Method
|
S. No. |
Substance |
IC50 (µg/ml) |
|
1 |
Ascorbic Acid |
41.73±0.098 |
|
2 |
A. carambola |
68.11±0.252 |
H2O2 Scavenging Assay
H2O2 is quickly disintegrated into oxygen and water and this may yield hydroxyl ions. Hydroxyl ions may start lipid peroxidation as well as disruption DNA in the body 27,28. The percentage of H2O2 scavenging activities of different conc. of ascorbic acid and A. carambola are presented in Table 6 and Figure 2. IC50 value of A. carambola leaves and Ascorbic acid is displayed in Table 7.
 |
Figure 2: Percentage inhibition of A. Carambola as compared to Ascorbic Acid by H2O2 Radical Scavenging Method. |
Table 6: Percentage of scavenging activity of Ascorbic acid (vitamin C) and A. carambola during H2O2 Radical Scavenging Method
|
S. No |
Conc. (µg/ml) |
Inhibition (%) (Mean±SD) |
|
|
|
|
Ascorbic Acid |
A. carambola leaves |
|
1 |
10 |
10.76±0.056 |
10.16±0.012 |
|
2 |
20 |
22.43±0.024 |
20.93±0.046 |
|
3 |
30 |
33.40±0.064 |
29.68±0.036 |
|
4 |
40 |
44.47±0.098 |
41.65±0.076 |
|
5 |
50 |
55.03±0.042 |
50.30±0.024 |
|
6 |
60 |
67.51±0.086 |
60.06±0.092 |
|
7 |
70 |
88.63±0.012 |
68.81±0.03 |
|
8 |
80 |
90.24±0.08 |
70.93±0.018 |
Table 7: IC50 for Ascorbic Acid and A. carambola during H2O2 Radical Scavenging Method
|
S. No. |
Substance |
IC50 (µg/ml) |
|
1 |
Ascorbic Acid |
45.93±0.172 |
|
2 |
A. carambola |
62.64±0.942 |
Nitric Oxide Scavenging activity
The percentage (%) nitric oxide scavenging activities of distinct concentrations of ascorbic acid and A. carambola are presented in Table 8 and Figure 3. IC50 value of A. carambola leaves and Ascorbic acid is shown in Table 9.
 |
Figure 3: Percentage inhibition of A. Carambola as compared to Ascorbic Acid by Nitric Oxide Scavenging activity. |
Table 8: Percentage of scavenging activity of Ascorbic acid (vitamin C) and A. carambola during Nitric Oxide scavenging activity
|
S. No |
Conc (µg/ml) |
Inhibition (%) (Mean ± SD) |
|
|
|
|
Ascorbic Acid |
A. carambola leaves |
|
1 |
10 |
16.73±0.068 |
15.11±0.028 |
|
2 |
20 |
20.18±0.05 |
21.50±0.052 |
|
3 |
30 |
33.77±0.042 |
35.70±0.068 |
|
4 |
40 |
46.96±0.052 |
39.35±0.043 |
|
5 |
50 |
60.85±0.078 |
47.26±0.03 |
|
6 |
60 |
72.92±0.065 |
53.85±0.062 |
|
7 |
70 |
79.82±0.096 |
60.55±0.017 |
|
8 |
80 |
84.38±0.054 |
73.94±0.074 |
Table 9: IC50 for Ascorbic Acid and A. carambola leaves during Nitric Oxide scavenging activity
|
S. No. |
Substance |
IC50 (µg/ml) |
|
1 |
Ascorbic Acid |
42.41±0.098 |
|
2 |
A. carambola |
66.84±0.782 |
Thin layer chromatography for qualitative analysis of Gallic acid and quercetin
TLC technique was mainly used for qualitative analysis of extract. Findings of TLC analysis of the ethanolic extract of A. carambola showed the presence of Gallic acid and quercetin because Rf value of Reference Gallic acid and quercetin was similar as extract Rf value. The results of TLC are presented in Table 10 and Figure 4.
 |
Figure 4: Pictogram of developed TLC plate at daylight |
Table 10: Number of spots and Rf values at wavelength UV254 nm of Ethanolic extract of A. carambola.
|
No of Spot |
Standard Rf |
A. carambola Rf |
|
1 |
0.25 (Gallic Acid) |
0.25 (Gallic Acid) |
|
2 |
0.53 (Quercetin) |
0.53 (Quercetin) |
|
3 |
|
0.61 (Unknown) |
|
4 |
|
0.65 (Unknown) |
|
5 |
|
0.83 (Unknown) |
|
6 |
|
0.91 (Unknown) |
|
7 |
|
0.96 (Unknown) |
HPTLC Analysis for Quantitative analysis of gallic acid and Quercetin
The HPTLC fingerprint and chromatogram showed the appearance of gallic acid and quercetin in the ethanolic leaves extract of A. carambola. (Table 11 and Figure, 5 Figure 6, and Figure 7). Rf value of gallic acid and quercetin of ethanolic leaves extract of A. carambola was identical to the standard at 0.25 and 0.53 when the plates were scanned at 254nm. The correlation coefficient was 0.995. The amount of gallic acid and quercetin in the ethanolic leaves extract of A. carambola was 502.7 µg and 458.3 µg /100 mg, respectively.
Table 11: Number of spots and Rf values at wavelength UV254 nm of Ethanolic extract of A. carambola leaves
|
No of Spot |
Standard Rf |
A. carambola Rf |
|
1 |
0.25 (Gallic Acid) |
0.05 (Unknown) |
|
2 |
0.53 (Quercetin) |
0.08 (Unknown) |
|
3 |
|
0.09 (Unknown) |
|
4 |
|
0.25 (Gallic Acid) |
|
5 |
|
0.29 (Unknown) |
|
6 |
|
0.30 (Unknown) |
|
7 |
|
0.53 (Quercetin) |
|
8 |
|
0.54 (Unknown) |
|
9 |
|
0.83 (Unknown) |
|
10 |
|
0.91 (Unknown) |
|
11 |
|
0.96 (Unknown) |
 |
Figure 5: HPTLC Fingerprint of ethanolic extract of A. caramboia scanned at 254nm. |
 |
Figure 6: Chromatogram of ethanolic extract of A. carambola scanned at 254 nm. |
 |
Figure 7: Chrqmatogram of Standrad Gallic Acid and Quercetin scanned at 254 nm. |
 |
Figure 8: Straucture of Gallic acid and Quercetin found in the ethanolic leaves extract of A. caramboia |
Discussion
Extractive values play a very important role in the determination of quality and quantity of herbal drugs, also useful to evaluate the nature of phytoconstituents available in the plant drug and also useful in the assessment of particular phytoconstituents soluble in specific solvents 29. Phytochemical analysis was done and the results showed the attendence of carbohydrates, alkaloids, saponins, steroids, tannins, vitamin C, and flavonoids in ethanolic extracts of leaves. The findings of phytochemical analysis suggest that the ethanolic extracts of A. carambola leaves probably contain active phytoconstituents providing the basis for their use as a management for numerous illness 30, 31.
Phenols and flavonoids are present in plants produce antioxidants activity. Hence, we could conclude that the phenols and flavonoids are accountable for the detected antioxidant activity in this research work. Phenols and Flavonoids are the greatest significant and miscellaneous group of phytoconstituents which are mainly distributed in higher plants with extraordinary therapeutic potential. Phenols and flavonoids have a beneficial action in the management and prevention of neurological disorders such as memory loss and can delay the process of neurological disorders. Phenol and Flavonoids compounds have crucial role in delaying the progression of Alzheimer’s Disease [AD]. Many Studies recommended that phelonic and flavonoids have the ability to pass blood-brain barrier (BBB), which is important for management and prevention of neurological disorder such as memory loss. however, various flavonoid subcategories differ in ability to pass the BBB. In the management and treatment of memory loss and AD, flavonoids efficiency is attributed to the reduction of amyloid beta (Aβ) toxicity and decreasing oxidative stress. Numerous phenolic and flavonoids such as gallic acid, rutin, catechins, quercetin, kaempferol, myricetin, and apigenin are useful in prevention and management of neurodegenerative diseases, cancer, inflammation etc. have been reported. 32
The antioxidant activity of ethanolic leaves extract of A. carambola was measured by DPPH, H2O2, and NO methods. The explanation of each method are given below one by one. DPPH is extensively used to find out antioxidant activity of plant extract. It is simple and cheaper technique. DPPH dark in colour and crystalline in nature. It is prepared by free- radical elements that are stable. It is well known and a popular antioxidant method. 33. DPPH radicals were scavenged by A. carambola leaves in a conc. dependent manner. Using DPPH, we found in this study A. carambola leaves have antioxidant activity. Similarly, the ethanolic leaf extract exhibited remarkable scavenging activity when compared to standard ascorbic acid by H2O2 and NO scavenging assay method. The ethanolic leaf extracts strongly scavenge in dose-dependent manner shown in figure 2 and 3.
Because of the free radical scavenging activity of A. carambola leaves, it can be useful in the management and prevention of numerous health problems causes by free radicals.
Free radicals such as nitric oxide, and hydrogen peroxide are well-recognized inducers of cell and tissue pathogenesis which may cause numerous human diseases such as neurodegenerative diseases, aging diseases, and as well as inflammatory diseases25. Antioxidants are extremely found in phytoconstituents having the Potential to secure the human body from injury caused by free radical caused oxidative stress. The antioxidant capacity of A. carambola leaves extract examined and significant results were found20.
The HPTLC technique is a simple, specific, precise, sensitive, and accurate technique for the quantification of phytoconstituents from plant extract. We used this technique for the quantification of gallic acid and quercetin. This technique can be efficiently used for routine analysis of phytoconstituents as well as formulations containing any compounds 34. The HPTLC analysis provided the amount of gallic acid and quercetin as 502.7 µg/100 mg and 458.3 µg/100 mg of ethanolic extract of A. carambola leaves respectively. Many Studies suggested that gallic acid and Quercetin are beneficial in the management and prevention of various diseases such as neurodegenerative diseases, cancer disease, inflammatory diseases, etc. Data collected from a variety of sources suggested that Gallic acid and Quercetin have the ability to reverse amnesia in rodents induced by scopolamine. Because it inhibits oxidative stress and also decreases acetylcholinesterase levels in the rodent brain. Gallic acid and Quercetin are commonly found in edible plants 32,35,36,37.
Conclusion
These unreported parameters may be helpful in establishing the diagnostic characteristics for the recognition of the A. carambola plant also the creation of a monograph on it.
Acknowledgement
The authors are thankful to Dr. M. P. Pandey Honorable Vice-chancellor, and Prof. Sushil Kumar Director of the School of Pharmaceutical Sciences, IFTM University Moradabad, India, for providing the necessary support, facilities, and suggestions for this research. The authors are also thankful to Dr. Sunita Garg, Department of Pharmacognosy & Phytochemistry, NISCAIR, New Delhi, India, for the authentication of plant material.
References
- Gul, R.; Jan, S.U.; Faridullah, S.; Sherani, S.; Jahan, N. The Sci World J.2017,5873648.
CrossRef - Luan, F.; Peng, L.; Lei, Z.; Jia, X.; Zou, J.; Yang, Y. A Review. Front Pharmacol. 2021,12, 699899.
CrossRef - Carolino, R.O.; Beleboni, R.O.; Pizzo, A.B.; Vecchio, F.D.; Garcia, C.N.; Moyses, N M. Neurochemistry international. 2005, 46(7), 523-31.
CrossRef - Ferreira, E.B.; Fernandes, L.C.; Galende, S.B.; Cortez, D.A.G.; Bazotte, R.B. Revista Brasileira de Farmacognosia. 2008, 18(3), 655-660.
CrossRef - Aladaileh, S.H.; Saghir, S.A.M.; Murugesu, K.; Sadikun, A.; Ahmad, A.; Kaur, G. Biomedicines. 2019,7(3), 231-236.
CrossRef - Shui, G.; Leong, L.P. J of chromatography A. 2004,1022(1-2),67-75.
CrossRef - Annegowda, H.V.; Bhat, R.; Min, T. L.; Karim, A.A.; Mansor, S.M. J Food Sci Technol. 2012,49(4),510-4.
- Vermerris, W.; Nicholson, R. Dordrecht: Springer Netherlands; 2006, 235-55.
CrossRef - Kumar, S.; Pandey, A.K.; The Sci. World J. 2013,162750.
CrossRef - Kaushik, R.; Jain, J.; Mazumder, A. J. Appl. Pharm. Sci. 2018, 8(4), 90–98.
- Khan, A.D.; Singh, M.K.; Lavhale, P.M.; Kaushik. R. J. of Herbs, Spices & Medi. Plants. 2022
- Mukherjee, P.K. Q Cont and Eval. of Herb. Drugs Elsevier.2019,79-149.
- Mukherjee, P.K. Q Cont. and Eval. of Herb. Drugs: Elsevier. 2019,53-77.
- Khandelwal, K.R. Pract. Pharmaco. 2007, 227-228.
- Wairata, J.; Fadlan, A.; Setyo, P.A.; Taher, M.; Ersam, T. Arab. J of Chem. 2022,15(2),103541.
- Saeed, N.; Khan, M.R.; Shabbir, M. BMC Compl. and Altern. Medi. 2012,12(1), 221.
CrossRef - Phong, H.X.; Viet, N.T.; Quyen, N.T.N.; Van, T.P.; Trung, N.M.; Ngan, T.T.K. Materials Today: Proceedings. 2022,56, A1-A5.
CrossRef - Sachithanandam, V.; Parthiban, A.; Lalitha, P.; Muthukumaran, J.; Jain, M.; Elumalai, D. J. of biomol. Str. & dyn. 2022, 40(4), 1490-502.
- Eswaraiah, G.; Peele, K.A.; Krupanidhi, S.; Kumar, R.B.; Venkateswarulu, T.C.; J. King Saud Uni – Sci. 2020, 32(1), 842-7.
CrossRef - Al, T. B.; A Al, Q. M.; Al, Z. M.; Al, M. A.; Muhaidat, R.; Qar, J. Biomed. and Pharm J. 2018, 11(3),1239-45.
CrossRef - Wong, C.C.; Li, H.B.; Cheng, K.W.; Chen, F. Food Chem. 2006, 97(4),705- 11.
CrossRef - Amri, F.S.A.; Hossain, M.A. Egypt. J of Basic and Applied Sci. 2018, 5(4), 245-51.
CrossRef - Tong, H.; Wang, X.; Dong, Y.; Hu, Q.; Zhao, Z.; Zhu, Y. J. of Biol. Chem.. 2019, 294(12), 4583-95.
CrossRef - Sihag, S.; Pal, A.; Ravikant, S. V. Biocat. and Agri. Biot. 2022, 42,102368.
CrossRef - Bhardwaj, P.; Banarjee, A.; Jindal, D.; Kaur, C.; Singh, G.; Kumar, P. Pharm. Chem. J. 2020, 54,184.
CrossRef - Sharma, V.; Janmeda, P. Ara. J. of Chem.. 2017, 10(4), 509-14.
CrossRef - Halliwell, B.; Gutteridge, J. Free Radicals in Biology and Medicine: Oxford University Press. 2015.
CrossRef - Ruch, R.J.; Cheng, S.J.; Klaunig, J.E. Carcinogenesis. 1989, 10(6), 1003-8.
CrossRef - Chandel, H.S.; Pathak, A.K. Pharmaco. Res. 2011, 3(1), 49-56.
CrossRef - Auwal, M.S.; Saka, S.; Mairiga, I.A.; Sanda, K.A.; Shuaibu, A. A. Vet. Res. forum : an inter quar. j. 2014, 5(2), 95-100.
CrossRef - Gandagule, U.B.; Duraiswamy, B.; Zalke, A.S.; Qureshi, M.A. Anci. Sci. of life. 2013, 32(4), 245-9.
CrossRef - Khan, H.; Ullah, H.; Aschner, M.; Cheang, W.S.; Akkol, E.K. Biomolecules. 2020, 10(1), 112-118.
CrossRef - Baliyan, S.; Mukherjee, R.; Priyadarshini, A.; Vibhuti, A.; Gupta, A.; Pandey, R.P. Molecules (Basel, Switzerland). 2022, 27(4).
CrossRef - Khan, A.D.; Singh, M.K.; Lavhale, P.M.; Kaushik, R. Journal of Herbs, Spices & Medicinal Plants. 2022,1-12.
- Kahkeshani, N.; Farzaei, F.; Fotouhi, M.; Alavi, S.S.; Bahramsoltani, R.; Naseri, R. Iran. j basic medi. sci. 2019, 22(3), 225-37.
CrossRef - Nagpal, K.; Singh, S.K.; Mishra, D.N. Drug delivery. 2013, 20(3-4), 112-9.
CrossRef - Mahdi, P.B.; Jothy, S.L.; Latha, L.Y.; Chen, Y.; Sasidharan, S. Asian Pacific journal of tropical biomedicine. 2012, 2(12), 960-5.
CrossRef - Brüll, V.; Burak, C.; Stoffel, W.B, Wolffram, S.; Nickenig, G.; Müller, C. Brit j of nutri. 2015, 114(8),1263-77
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.










