Pharmacological Potential of Coumarin-Based Derivatives: A Comprehensive Review
1Department of pharmacy, Jagannath University, Jaipur, Rajasthan, India.
2SGT College of pharmacy, SGT university, Gurugram, Haryana, India.
Corresponding Author E-mail: sumitabajia87@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/390304
Article Received on : 05 Apr 2023
Article Accepted on : 08 May 2023
Article Published : 17 May 2023
Reviewed by: Dr. Marisa Cabeza Salinas
Second Review by: Dr T Veeraiah
Final Approval by: Dr. Jatin Mehta
By combining of benzene nucleus and pyrone ring a class of heterocyclic compounds known as benzopyrone is generated. As a basic parent scaffold 1,2- benzopyrone ring system contains by coumarins. These compounds can be divided into two groups: 1. Benzo-α-pyrone 2. Benzo-γ-pyrone. Data on different coumarin derivatives are gathered in this review article as these compounds have a wide spectrum of pharmacological actions and can be further modified to make more potent and effective medications. Derivatives of coumarin play a significant role in industries and sectors of medicine. This can be linked to their variety of chemical characteristics and multiple biological activities. Coumarin based derivatives has a phenolic hydroxyl group which is generated as one of the most derivative functional groups. The focus of this systematic and comprehensive review on synthetic pathway of coumarin affiliates and their biological activities or potential. According to authors this review could help to medicinal chemists to choose appropriate functional group for development of novel therapeutic drugs.
KEYWORDS:Antimicrobial; Antineoplastic; Coumarin; Hepatotoxic; Neuroprotective; Pharmacological;
Download this article as:| Copy the following to cite this article: Kumari S, Sharma A, Yadav S. Pharmacological Potential of Coumarin-Based Derivatives: A Comprehensive Review. Orient J Chem 2023;39(3). |
| Copy the following to cite this URL: Kumari S, Sharma A, Yadav S. Pharmacological Potential of Coumarin-Based Derivatives: A Comprehensive Review. Orient J Chem 2023;39(3). Available from: https://bit.ly/3q8d7VG |
Introduction
Coumarin, pharmacologically significant chemicals come from natural sources. Coumarins are phenolic compounds generated from cinnamic acid. These are 2H-chromen-2-one, or coumarin, is a member of the benzopyrone class 1. An oxygen heterocyclic molecule is coumarin. The greatest class of 1-benzopyran derivatives is the coumarins. They are mainly present in higher plants. The majority of naturally occurring coumarins have a Carbon-7 hydroxycoumarin; (7-hydroxycoumarin) umbelliferone is seems to be the biogenetic and structural parent of the 2H-chromen-2-one with higher oxygenation levels 2. Numerous plants naturally contain coumarins, but, woodruff, licorice, strawberries, tonka bean, cinnamon, cherries, sweet clove, apricots, bison grass and lavender have particularly high concentrations. Due to its pleasant (sweet) aroma, coumarin has been utilised in perfumes since 1882, when it was first isolated from coumarone. In 1868, it was first synthesised 3,4. In pharmaceutical industry coumarin used as precursor for various synthetic procedures. 4-hydroxycoumarins are found to be as Vitamin K antagonist type 5,6. Various methods are selected for coumarin derivative synthesis like: Perkin reaction, Reformatsky reaction, Pechmann, Friedal- craft, Witting reaction, Knoevenagel reaction 7. Coumarin derivative shows different activities such as anti-influenza, anticoagulant, antioxidant, anti-tuberculosis, antiviral, antimicrobial, anti-HIV, antineoplastic, neuroprotective, antihypertensive, anti-Alzheimer, analgesic, antidiabetic. For treatment of multiple sclerosis coumarins are best choice 8,9.
Coumarins are of two categories: 1) Benzo- ϒ -pyrone 2) Benzo- α – pyrone known as flavonoids or chromones differentiating by C=O group in heterocyclic system. 4
 |
Figure 1: benzo-ϒ-pyrone |
 |
Figure 2: benzo-α-pyrone |
 |
Figure 3: Some basic natural coumarins with introduction |
In structure 1 substitution on benzene ring, in 2 furan ring replaces C6-C7 of benzene ring, pyran ring replaces C7-C8 in structure 3, structure 4 is coumarin isomer and 5 structure is coumarin dimer.
Occurrence
Coumarins consists huge array of compounds found in plant kingdom. Some essential oils like cinnamon bark oil, lavender oil, cassia leaf oil has high concentration of coumarins. Green tea and chicory are also rich source of coumarins. Occurrence of coumarin in plant parts can be influenced by seasonal changes and environmental conditions also.10
Some members of coumarins isolated from microbial sources like aflatoxins, novobiocin from Aspergillus species and Streptomyces species respectively. These are strong inhibitors of DNA gyrase and possess 3-amino-4-hydroxy coumarin nucleus that is necessary for its pharmacological activity.11
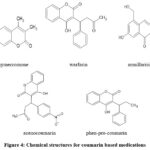 |
Figure 4: Chemical structures for coumarin based medications |
Biosynthesis
Natural biosynthetic route for coumarin generation leads to phenylpropanoids. Phenylalanine converts into cinnamic acid by (PAL) phenylalanine ammonia lyase enzyme, further leads to 4’coumaroyl-S-coA. Then variety of phenylpropanoids generated. Hydroxylation on ortho position gives coumarin, followed by side chain isomerisation and finally cyclization process.12
Metabolism of Coumarins
Two larger effective metabolic routes of coumarin compounds are: a) 7-hydroxylation b) 3,4-epoxidation in human and rats, mouse respectively. Cytochrome P450 enzyme play crucial role in biotransformation of coumarins. Important family of enzymes are CYP1, CYP2, CYP3. Enzyme responsible for 7-hydroxylation is CYP2A6. CYP3A4 enzyme leads to 3-hydroxylation followed by hydroxyphenyl acetaldehyde (o-HPA) provide hydroxyphenyl acetic acid (HPAA) on decarboxylation 13,14,15
 |
Figure 5: Schematic representation of coumarin metabolism |
Toxicity of Coumarins
Exposure of coumarin based products to humans is on high scale. Review of various studies revealed that cosmetic items and foods containing coumarin have no risk to human health 16. On the contrary, various publications observed hazardous effects i. e. hepatoxicity effects of coumarin and its affiliates 17-19. Metabolism and species dependent carcinogenic and cytotoxicity effects of coumarins was found by literature survey 20. Tolerated dose for coumarin in human found to be 0.1mg/Kg by body weight 21.
Table 1: Toxicity Effects of Different Species on Varying Doses
|
Species |
Sex |
Lethal Dose |
Route of administration |
Effect |
Reference |
|
Rat |
Male |
290-680mg/Kg |
Oral |
Carcinogenic |
22 |
|
Rat |
Male |
125-500mg/Kg |
Oral |
Hepatotoxic |
23 |
|
Mice/Rat |
Female |
250mg/Kg |
Oral |
Hepatotoxicity |
23 |
|
Rat |
Male |
0.5% |
Oral |
Carcinogenic |
23 |
|
Rat |
Male |
36mg/Kg |
Gavage |
Carcinogenic |
23 |
|
Mouse |
Male |
280mg/Kg |
Gavage |
carcinogenic (renal tubule carcinoma) |
23 |
|
Dog |
— |
100mg/Kg |
Oral |
Increased liver weight |
23 |
Coumarinyl Derivative Synthesis
Various strategies followed for coumarinyl derivatives. In polymeric materials, medications and agrochemicals coumarin skeleton is used in wide range. For development of different synthetic routes ongoing efforts were applied, leads to cyclization heterocyclic rings 26,27.
Recently, usage of new technologies like microwave irradiation, solvent free reactions, ultrasound methods, green solvents increased access to coumarin derivatives 28,29.
For coumarin synthesis important classical methods are: Pechmann condensation 30,31 and Knoevenagel reaction 32,33
Pechmann condensation
Pakdel et al. reported coumarin affiliates in magnetic-core with Fe3O4– -shell by Pechmann reaction 30.
|
|
Scheme 1 |
Yield obtained as 90% in solvent free condition. In presence of FeCl3.6H2O coumarin also obtained by Pechmann reaction. In this method 92% yield was obtained. 30
Coumarins synthesis via Pechmann condensation performed by Porsatitworakal et al. by using resorcinol and ethylacetoacetate. 34
 |
Scheme 2 Click here to View Scheme |
7-hydroxy-4-methyl coumarin synthesis with different catalysts in microwave irradiation without solvent developed by Bouasla et al. 35
 |
Scheme 3 Click here to View Scheme |
Mirosanloo et al developed coumarin derivatives by using solvent free Pechmann condensation in cellulose nanocrystals presence. 36
 |
Scheme Click here to View Scheme |
Knoevenagel Condensation
For coumarin and its derivatives most frequently used synthetic route is Knoevenagel reaction. Synthesis of coumarin affiliates by using Knoevengel reaction through PIDA mediated reaction reported by Khan et al. 37
 |
Scheme 5 |
In DES by Knoevenagel condensation green synthesis of coumarin affiliates showed by Keshavarzipour and Tavaked in scheme 38
 |
Scheme 6 Click here to View Scheme |
Different coumarin affiliates synthesized from active methylene compounds and salicylaldehyde by using Knoevenagel condensation performed by Silveira Pinto and Souza described in scheme 39
 |
Scheme 7 Click here to View Scheme |
Ghomi & Akbarzadeh performed Knoevenagel condensation for 3-substituted coumarins without solvent, yield is 63-73%.40
 |
Scheme 8 Click here to View Scheme |
Pharmacological Effects of Coumarins
Antimicrobial Effects
New coumarin derivatives synthesised by M. Nibin Joy etr.al screened against P. aeruginosa, S. aureus atrains for antimicrobial effect. Compounds 1,2 and 3 shows better antimicrobial effect via using ciprofloxacin as reference with MIC 0.2-0.6µg/ml. Antibacterial activity enhances due to fluoro group associated with pyrimidine ring and chloro group 41. 2-OH and fluoro-derivatives of coumarin reveals antimicrobial action for gram positive and negative microbes using ampicillin and ciprofloxacin as standard. Amine and pyridine derivative of coumarin showed antimicrobial activity in range of 88% against gram-negative and gram-positive bacteria E. coli using ampicillin, cotrimoxazole as standard 42,43.
 |
Scheme 9 Click here to View Scheme |
Anti-viral Effects
Various researches are performed or focussing on coumarin containing compounds synthesis to minimize or overcome shortcomings related to various viral drugs like anti-HIV, anti-hepatitis, anti-influenza, anti-chikungunya etc. 44 Pyranocoumarins shows active against hepatitis virus by binding antigens presents at cell surfaces and viral replication. 3-phenylcoumarins, 4-OH coumarins, pyranocoumarins, furanocoumarins showed pharmacological effects against infection of anti-HIV by inhibiting viral DNA replication, HIV protease. 4-thiazolidinone coumarin derivatives inhibit anti-dengue virus by inhibiting binding sites of protease. 45 Against chikungunya virus uracil-coumarin-arenes conjugates effective order of inhibiting action against chikungunya virus is uracil<thymine< benzouracil. Methoxy moiety having conjugates are more effective than another moiety. 46
 |
Figure 6: Anti-viral effects of coumarin affiliates against different viruses |
Anticancer Effects
Coumarin and its affiliates having anticancer activity for laryngeal, colon, breast, renal, leukaemia and lung cancer. Coumarin-chalcone, coumarin-metal complexes, coumarin-triazole have been found more effective than coumarin. Coumarins are capable of reducing toxic and side effects produced by radiotherapy, not only effective towards carcinogenicity47. For development of hybrid molecules; these anticancer benefits of coumarins are favourable. Cytotoxic effects or activity of coumarin uracil hybrids reported against human pancreatic cancer cell line PANE1 and breast cancer also. Coumarin scaffolds containing OCH3 and OH groups showed greatest anticancer activity against human HepG2 & MCF7. 48,49 Structure of coumarin scaffolds:
 |
Figure 7: Azocoumarin derivatives with cytotoxic activity |
Geiparvarin; natural coumarin derivatives shows anticancer activity by killing cancer cells. 50
 |
Scheme 10 Click here to View Scheme |
Antioxidant Effects
Coumarin, a naturally occurring drug having hydrophobic characteristic showed good antioxidant capability.51
 |
Scheme 11: Structures with antioxidant activities. Click here to View Scheme |
Antifungal Effects
Generally, in nails, skin and hairs fungal infection found. Certain coumarins; shows antifungal activity against P.Capsici, R. Solani, S. Sclerotiorum species described with structure. 52
 |
Scheme 12 |
Furan coumarin derivatives shows antifungal activity at large extent.53
Antitubercular Effects
TB is a major health problem caused by mycobacterium tuberculosis 54. Effective antitubercular activity by some synthetics showed against strain M. tuberculosis due to presence of different substituents and parent ring 55 described in table 2.
 |
Table 2: Compounds Shown Antitubercular Activity |
Anti-inflammatory Effects
In patients, inflammation is major threat 59. Rakesh et. Al observed heat-induced denaturation of protein inhibition against Aceclofenac reference drug by following coumarin derivatives shown in structure due to methoxy and hydroxyl group 60
 |
Scheme 13: Structure with anti-inflammatory activity. |
As Fluorescent Probes
For detection of biomaterials, metals and enzymes; emphasized on coumarin scaffold as fluorescent probes. For diagnosis of many illnesses, these probes have excellent potential 61. In drinking water, copper metal detection carried out by using coumarins (Structure 4). Various reviews focussed on iron detection in water (structure5) 62,63,64. In release of drugs (controlled) coumarins employed as photocleavable linkers. In cancer treatment, target the mitochondria with iron complex of 7- hydroxy methyl substituted aminocoumarins 61,65. Different reviews observed cytochrome P450 enzymes activity via use of 7- hydroxy coumarin and its affiliates 66.
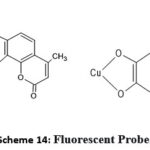 |
Scheme 14 Click here to View Scheme |
Perspectives
For biological purpose preferred structures are coumarins. Various affiliates can be found by structural modification in its ring system. Thus, most emerging topic could be structure activity studies. In year 2022, various reviews and research papers on coumarin could be found. So coumarin’s potential in medicinal chemistry is main purpose of our review. The studies that were most relevant are included. In our opinion for next few years, as fluorescent probes coumarin potential appears to be most promising topic of research.
Conclusion
Due to extensive biological and pharmacological activities coumarin derivatives gaining more attention. We discussed the antioxidant, antiviral, antifungal, antimicrobial, antitubercular, anticancer activities of synthetic as well as natural coumarins. The most significant impact is anticancer, due to this property coumarins may be a new target for cancer treatment. This review is important for design and development of coumarin derivatives as innovative lead molecules.
Acknowledgment
This research has not been supported by any external funding.
Conflict of Interest
The authors declare no conflict of interest.
References
- Patel, G.; Banerjee, S.; Coc 2020, doi:10.2174/ 1385272824999200709125717, 24, 2566–2587.
- Pereira, T. M.; Franco, D. P.; Vitorio, F.; Kummerle, A. E.; Ctmc, 2018, doi:10.2174/1568026618666180329115523,18, 124–148.
- Kulkarni, M.V.; Kulkarni, G.M.; Lin, C.H.; Sun, C.M.; Current Medicinal Chemistry, 2006, 13(23), 2795-818.
- Al-Warhi, T.; Sabt, A.; Elkaeed, E. B.; Eldehna, W. M.; Bioorg. Chem. (2020), doi: 10.1016/j.bioorg.2020.103, 104-163.
- Lipeeva,A. V.; Khvostov, M. V.; Baev, D. S.; Shakirov, M. M.; Med. Chem., 2016, 12, 674.
- Singh, H.; Singh,J. V.; Bhagat,K.; Gulati, H. K.; Sanduja, M.; Kumar, N.; Kinarivala,N.; Sharma, S.; Bioorg. Med. Chem. 2019, 27, 3477.
- Potdar, M.K.; Mohile, S.S.; Salunkhe, M.M.; Tetrahedron Lett., 2021, 42, 9285-9287.
- Mustafa, YF.; Mohammed, ET.; Khalil, RR.; Egyptain journal of chemistry, 2021, 64, 4461-4468.
- Mustafa, YF.; Applied Nanoscience, 2021, Doi:10,1007/s 13204-021-01872-x.
- Cooke D.;Dublin City University, Dublin, Ireland, 2016.
- Singh, H.; IJPSR, 2016; 7(2), 482-50.
- Vogt, T.; Molecular Plant, 2020, 3, 2–20.
- Mustafa, YF.; Khalil, RR.; Mohammed, ET.; Systematic Reviews in Pharmacy, 2020, 11, 382–387
- Mustafa, YF.; Bashir, MK.; Oglah, MK.; Systematic Reviews in Pharmacy 2020, 11, 598–612.
- Oglah, MK.; Bashir, MK.; Mustafa, YF.; Systematic Reviews in Pharmacy 2020, 11, 717–725.
- Mustafa, YF.; Abdulaziz, NT.; Systematic Reviews in Pharmacy, 2020, 11, 438–452.
- Mustafa, YF.; Abdulaziza, NT.; Jasima, MH.; Egyptian Journal of Chemistry 2021, 64, 1807–1816.
- Mustafa, YF.; Kasim, SM.; AlDabbagh, BM.; Applied Nanoscience, 2021.
- Tanaka, Y.; Fujii, W.; Hori, H.; Food and Chemical Toxicology, 2016, 90, 1–9.
- Ratanasavanh, D.; Lamiable, D.; Biour, M.; Fundamental and Clinical Pharmacology, 2016, 10, 504–510.
- Abraham, K.; Wöhrlin, F.; Lindtner, O.; Molecular Nutrition and Food Research, 2014, 54, 228–239.
- Cohen, A; Ehrlich, A; Cohen, M; Science Translational medicine, 2021, 13, 582.
- Lake, B.G; Food chem toxicology, 2001, 37(4), 423-453.
- Caltron, A; Manvati, S.; Heliyon, 2010, 6.
- Murer, H.K.; Zeitin, B.R.; The journal of Pharmacology & Experimental Therapeutics, 2014, 118(3), 348-358.
- Vekariya, RH.; Patel, HD.; Tetrahedron., 2014, 70, 3949– 3961.
- Barot, KP.; Jain, S V.; Kremer, L.; Medicinal Chemistry Research, 2015, 24, 2771–2798.
- Detsi, A.; Kontogiorgis, C.; Hadjipavlou-Litina, D.; Expert Opinion on Therapeutic Patents, 2017, 27, 1201–1226.
- Calcio Gaudino, E.; Tagliapietra, S.; Martina, K.; RSC Advances 2016, 6, 46394–46405.
- Sharma, RK.; Katiyar, D.; Synthesis (Germany), 2016, 48, 2303–2322.
- Lončarić, M.; Gašo-Sokač, D.; Jokić, S.; Molnar, M.; Biomolecules., doi: 10.3390/biom10010151, 2020, 10; 151.
- Calcio Gaudino, E.; Tagliapietra, S.; Martina, K.; RSC Advances, 2016, 6, 46394–46405.
- Molnar, M.; Lončarić, M.; Kovač, M.; Curr. Org. Chem., doi: 10.2174/1385272824666200120144305, 2020, 24, 4–43.
- Pakdel, S.; Akhlaghinia, B.; Mohammadinezhad, A.; Chem. Afr., 2019, doi: 10.1007/s42250-019-00042-5, 2, 367–376.
- Pornsatitworakul, S.; Boekfa, B.; Maihom, T.; Treesukol, P.; Mon. Chem. Chem. Mon.,2017, doi: 10.1007/s00706-017-1962-4. 148, 1245 –1250.
- Bouasla, S.; Amaro-Gahete, J.; Esquivel, D.; López, M.I.; Jiménez-Sanchidrián, C.; Teguiche, M; Romero-Salguero, F.J.; Molecules, 2017, 22, doi: 10.3390/molecules22122072 2072.
- Mirosanloo, A.; Zareyee, D.; Khalilzadeh, M.A.; Appl. Organomet. Chem,2018, doi: 10.1002/aoc. 32, 4546.
- Khan, D.; Mukhtar, S.; Alsharif, M.A.; Alahmdi, M.I.; Ahmed, N.; TetrahedronLett.,2017, doi: 10.1016/j.tetlet.2017.07.018,58,3183–3187.
- Keshavarzipour, F.; Tavakol, H.; J. Iran. Chem. Soc.,2016, doi: 10.1007/s13738-015-0722-913, 149–153.
- Pinto, L.D.S.; De Souza, M.V.N.; Synthesis, 2017, 49, 2677–2682.
- Nibin joy, M.; Bakulev, V., Yadav, B.D.; Telkar, S.; Pharm.chem.J; 2020, 54,604-621.
- Ghomi, J.S.; Akbarzadeh, Z.; Ultrason. Sonochem, 2018, doi: 10.1016/j.ultsonch.2017.06.022,40, 78–8..
- Tandel, T.; Kishor, H.; Patel, K S.; Indian journal of chemistry, 2019, 58, 594-602.
- Bowersox, J.; NIH News, 1999. Archived from the original on May 5, 2007.
- Kapoor, R. J.; Tsay, M.; Lin, S.; Chu, C. K.; Antivir. Res., 2015, 1-6.
- Kannan, S.; Kolandaivel, P.; Comput. Biol. Chem., 2017.
- Yang, Hu.; Weichao, C.; Yufeng, S.; Bin, Z.; Gao-Xue, W.; Bioorganic & medicinal chemistry Letters, 2019.
- Akkol, E.K.; Genç, Y.; Karpuz, B.; Sobarzo-Sánchez, E.; Capasso, R.; Cancers., 2020, doi: 10.3390/cancers12071959, 12, 1959.
- Yasueda, A.; Urushima, H.; Ito, T.; Integr. Cancer Ther., 2016, doi: 10.1177/1534735415610427,15,17–39.
- Sanduja, M.; Gupta, J.; Singh, H.; Pagare, P.P.; Rana, A.; J. Saudi Chem. Soc. 2020, doi: 10.1016/j.jscs.2019.12.001, 24, 251–256.
- Yasameen, K.; Al-Majedy, D.; Al-Duhaidahawi, K.; Al-Azawi, A.; Kadhum, A.; Mohamad, AB.; Molecules., doi: 10.3390/molecules21020135, 2016, 21, 135-146.
- Liu, C.; Guan, A.; yang, J.; Chai, B.; Li, M.; Li, H.; Yang, J.; J. Agri. Food chem, 2016, 64, 4-51.
- Ahmed, A.; Al-Ameiry.; Kadham, A. A. H.; Mohamad, A. B.; molecules, 2012, 17, 5713-5723.
- Pires, C.T.A.; Vieria, L.; Scodro, R.B.L.; Cortez, A.G.D.; Siqueira, V.; Furute Med. Chem; 2020, 2018-0281.
- Patil, S.B.; Results in chemistry; 2022, 4.
- Godge, R.; Kunkulol, R.; Journal of Drug Delivery and Therapeutics; 2018.
- Khan, Y.S.; Osman, H.; Khan, M.S.; Mohamad, S.; Sulaiman, O.; Johansah, N.; Medicinal Chemistry Research, 2017, 26,1139-1148.
- Kumbar, S.S.; Hosamani, K.M.; Gouripur, G.C.; Joshi, S.D.; R. Soc. Open Sci,2018, 5.
- Alshibl, H.M.; Al-Abdullah, E.S.; Haiber, M.E.; Alkahtani, H.M.; Bari, A.; Villinger, A.; Molecules, 2020, 25(14), 3251.
- Chavan, R.R.; Hosamani, K.M.; R.Soc. Open Sci, 2018, 5; 175435; doi: org/10.1098/rsos.172435.
- Ying, W.; Xiaohui, H.; Lixun, L.; Luyao, G.; Xumin, R.; Yonggang, W.; Rsc Advance, doi: 10.1039/c9ra10632d, 2020, 10, 6109-6113.
- Arslan, F.N.; Geyik, G.A.; Koran, K.; Ozen, F.; Aydin, D.; Elmas, S.N.K.; Gorgulu, A.O.; Yilmaz, I.; J. Fluoresc., doi: 10.1007/s10895-020-02503-4., 2020, 30, 317–327.
- Hien, N.K.; Bay, M.V.; Bao, N.C.; Vo, Q.V.; Cuong, N.D.; Thien, T.V.; Nhung, N.T.A.; Van, D.U.; Nam, P.C.; Quang, D.T.; ACS Omega.,doi: 10.1021/acsomega.0c03097, 2020, 5, 21241–21249.
- Liu, J.; Guo, Y.; Dong, B.; Sun, J.; Lyu, J.; Sun, L.; Hu, S.; Xu, L.; Bai, X.; Xu, W.; Sens. Actuator B-Chem, doi:10.1016/j.snb.2020.128361,2020, 320:128361.
- Sarkar, T.; Bhattacharyya, A.; Banerjee, S.; Hussain, A.; Chem. Commun., doi: 10.1039/D0CC03240A, 2020, 56, 7981–7984.
- Xia, Z.; Chen, D.; Song, S.; van der Vlag, R.; van der Wouden, P.E.; van der Merkerk, R.; Cool, R.H.; Hirsch, A.K.H.; Melgert, B.N.; Quax, W.J.; J. Med. Chem., doi: 10.1021/acs.jmedchem.0c01160, 2020, 63, 11920–11933.

This work is licensed under a Creative Commons Attribution 4.0 International License.











