Evaluation of Chiral Selectivity of Quinine Immobilized Chitin and Chitosan
Department of Chemistry, Jai Narain Vyas University, Jodhpur, Rajasthan, India.
Corresponding Author E-mail: garimanish.mathur@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/390333
Article Received on : 03 May 2023
Article Accepted on : 05 June 2023
Article Published : 19 Jun 2023
Reviewed by: Dr. Shruti Chopra
Second Review by: Dr. Hemant Naidu
Final Approval by: Dr. Ioana Stanciu
Quinine is a well-known chiral reagent that has been used for enantio-separation by conventional methods. With the development of chromatographic techniques various polysaccharides too have been explored for application as chiral adsorbents. However, polysaccharides shows only limited ability for real application. Quinine immobilized cellulose and starch have been reported for improved enantio-selectivity. Guaran, a seed polysaccharide with abundance of cis-1, 2-hydroxyl groups when anchored with quinine significantly improved chiral selectivity. This study reports about evaluation of chiral-selectivity of quinine derivatized chitin and chitosan. Racemic borate complexed mandelic acid and bio-active ofloxacin are efficiently resolved by chromatography using Quinine modified chitin and chitosan. All the materials used for the current study are natural, non-toxic, readily available and in-expensive. Methods employed are simple and results clearly establish a correlation between structural component and enantio-separation.
KEYWORDS:Chiral; Enantio-separation; immobilization; Stereospecific
Download this article as:| Copy the following to cite this article: Mathur G, Mathur R. Evaluation of Chiral Selectivity of Quinine Immobilized Chitin and Chitosan. Orient J Chem 2023;39(3). |
| Copy the following to cite this URL: Mathur G, Mathur R. Evaluation of Chiral Selectivity of Quinine Immobilized Chitin and Chitosan. Orient J Chem 2023;39(3). Available from: https://bit.ly/3NhTWAI |
Introduction
Asymmetric synthesis and optical resolution of racemates have drawn significant attraction in the past few decades. It is a widely accepted fact that more than 50 % drugs currently in use are chiral and most of them exist as racemates. It is also well-established that bio-activity of these chiral medicines shows a prominently different behavior in their pharmacological, toxicological activity for the two enantiomers. This difference emphasizes the need for chirally pure drugs. A systematic and economically viable method of enantio-separation is the need not only for natural bio-active racemates but also for synthetic drugs.
Chromatographic enantio-separation is one of the most versatile and widely studied approaches. Numerous chiral stationary phases (CSP), based on a large number of natural and synthetic solid adsorbents have been employed. CSPs based polysaccharides; an important class of bio-polymer being inherently chiral is comprehensively accepted. However, an exhaustive study reveals their poor ability towards enantio-separation. Hence, polysaccharides are chemically derivatized to enhance their ability for enantio-separation.
For many years, researchers have been interested in the derivatization of seed polysaccharides including starch, cellulose, guar gum, etc., with a wide range of chiral chemicals. The marine animal polysaccharides chitin and chitosan have recently attracted attention in the realm of enantio-separation. Natural biopolymers called chitin and chitosan may be found in the cell walls of fungus, insects, and mollusks as well as in the exoskeletons of insects and crustaceans. These biopolymers can be used in a wide range of industries, including the biomedical, food, and agricultural sectors [2]. Their biocompatibility, biodegradability, potent antibacterial [3] effect, and lack of toxicity are all factors in these applications. In contrast to cellulose, chitin and chitosan have greater nitrogen contents of 5-8%, making them ideal for common amine reactions. In addition to the use of modification reaction, the presence of amine groups in chitosan permits certain biological roles. A well-known alkaloid called quinine has been employed in enantio-separation. Numerous scientific publications discuss the use of quinine for polymeric adsorbents, including polysaccharides, to immobilize drugs.
Although sufficient research has been done to use chitin and chitosan as CSP, almost all of them use them along with silica gel or other common adsorbents. This study reports an exclusive use of derivatized chitin and derivatized chitosan for enantio- separation. A sample study is done with mandelic acid as well as borax complexed mandelic acid. We report here the derivatization of chitin and chitosan and study their use in enantio- separation of borax complexed mandelic acid, ofloxacin and cetirizine.
 |
Figure 1: (A). Chemical structure of chitin and chitosan. |
 |
Figure 1: (b). Chemical structure of ofloxacin. |
 |
Figure 1: (C). Chemical structure of cetirizine |
Methodology
Derivatization Reaction of Polysaccharide
Synthesis of chitin and chitosan derivatives containing immobilized quaternary quininium salt. This quaternary derivatization reaction was carried out in two steps:
In the first step, a bifunctional reagent namely epichlorohydrin is made to react with quinine. This results in formation of chlorohydrin containing quaternised quinine. Nitrogen [4] of the quinuclidine ring gets quaternised in this process. The chlorohydrin, when treated with alkali, forms oxirane. In the second step, oxirane so formed is immobilized on polysaccharide in presence of alkali as catalyst. Nucleophilic [5] center of polysaccharide attacks the oxirane ring.
Two steps of immobilization can be reversed which however, suffers from a few drawbacks. When epichlorohydrin in excess is first treated with polysaccharide, the latter gets crosslinked. Thus, availability of free oxirane functionality for carrying out further reactions becomes uncertain. Therefore, it is impossible to predict the theoretical content of quinine.
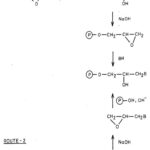 |
Figure 2: Covalent binding of a nucleophile (B, Quinine) to the polysaccharide matrix |
 |
Figure 3: Mechanism involved in epoxide ring opening. |
 |
Figure 4: Covalent binding of quinine to polysaccharide through quaternization. |
Procedure
Synthesis of Quaternary Quininium derivatized
Chitin Step-1
Synthesis of Quininium Epoxide
Treatment with 1.2 g (0.005 M) of barium chloride diluted in 50 ml of water resulted
in the conversion of 3.9 g (0.005 M) of quinine sulphate into more soluble quinine hydrochloride. The resulting quinine hydrochloride remained dissolved in water, which was then quickly filtered to remove the insoluble barium sulphate. Epi-chlorohydrin, 0.9 g (0.01M), was added to the filtrate and the reaction mixture was stirred on a magnetic stirrer at around 350 for around 4 hours. This was transformed into an insoluble product that was filtered out after being treated with 0.4 g (0.01 M) of sodium hydroxide.
Step-2: Synthesis Of Chitin Containing Quininium Derivative
A suspension of 20.3 g of chitin and 80 ml of dioxan was placed in a 500 ml round-bottom flask. An aqueous solution of 1.0 g of sodium hydroxide (50%) and 0.3 g of tetrabutylammonium bromide, a phase transfer catalyst (PTC) was added. Quininium epoxide as prepared in step-1 dissolved in dioxan and added to the chitin slurry while being continuously stirred on a magnetic stirrer cum hot plate. CSPs with different load of quinine were prepared. Three samples of derivatized chitin and chitosan with different degree of substitution are prepared by varying the amount of quininum epoxide (Table-1). The temperature is maintained at 60°C and the reaction mixture is stirred for another 6 hours. The product was filtered under vacuum on a Buchner funnel and washed, first with 2 x 50 ml of 80% aqueous methanol containing little acetic acid. It was then washed thoroughly with pure methanol to remove untreated low molecular weight compounds, and dried in an oven at 80°C. Derivatized Chitin is obtained as free flowing powder while derivatized chitosan is obtained as crumbs which are subsequently finely powdered by grinding. Product recovery was about 98%.
Result and Discussion
Derivatized chitin and chitosan are characterized by infrared spectroscopy. IR spectra of KBr pellets were scanned. All the products displayed quinine molecule-specific frequencies at 1656.67cm-1, 1563.66cm-1, 1259.02cm-1, and 1205 cm-1. Absorption frequencies at 1432.43cm-1 because of C-N linkage and at 2361.33cm-1 due to quaternary salt were also seen. Along with these absorption frequencies, the frequencies of Chitin and Chitosan were also observed as shown in Spectra1 and Spectra 2. Actual loading of quinine molecules in terms of degree of substitution was determined by nitrogen estimation.
Derivatized chitin and chitosan are used as CSP for enantio- separation studies. Both of these CSPs formed are finely powdered for their application in column chromatography as well as thin layer chromatography.
 |
Figure 5: TLC with derivatized chitin, separation of borate complexed-mandelic acid. |
 |
Figure 6: TLC with derivatized chitosan, separation of borate complexed-mandelic acid. |
 |
Figure 7: Spectra 1: FTIR spectra of derivatized chitin |
 |
Figure 8: Spectra 2: FTIR spectra of derivatized chitosan. |
Conclusion
A preliminary study on thin layer chromatographic separation, using methanol : benzene (9:1) of mandelic acid, borate complexed-mandelic acid, bio-active cetirizine and ofloxacin shows importance of multipoint interaction in effective entio-separation. Whereas borate complexed-mandelic acid and ofloxacin are better resolved on the other hand, mandelic acid and cetirizine show poor separation. This shows a clear importance of effective multi-point interaction. Borate-complexed mandelic acid has a pair of hydroxyl groups and ofloxacin possesses a β-carbonyl carboxylic acid structural set. Such combinations of interaction are lacking in mandelic acid and cetirizine. It is therefore concluded that an appropriate combination of structural groups is the primary requirement of an effective enantio-separation.
The literature review highlights the significance of derivatized chitin and derivatized chitosan as promising stationary phases for chromatographic enantio-separation. The extensive body of research discussed demonstrates the successful application of these materials in separating a wide range of chiral compounds. The review further emphasizes the importance of surface modification and the introduction of specific functional groups to enhance enantio-selectivity. Overall, derivatized chitin and derivatized chitosan offer considerable potential for advancing enantio-separation techniques and addressing the growing demand for chiral analysis in various industries.
Acknowledgment
Authors acknowledge the Department of Chemistry, Jai Narain Vyas University for instrumental analysis.
Conflict of Interest
Authors declare no conflict of interest for this work.
References
- Kumar, S.; & Dubey, R. K. Chitin and chitosan: Biopolymers for wound management. International Journal of Biological Macromolecules 2019, 121, 38-51
- Kaur, G.; Adhikari, R.; Cassady, A. I.; & Duran, R. S. Chitosan‐cellulose composite membranes: preparation, characterization, and transport properties. Journal of Applied Polymer Science 2016, 133
- J. W.; Kim, J. H.; Jeong, S. H.; & Moon, H. J. Antibacterial properties of chitin and chitosan oligomers against Vibrio vulnificus and Vibrio parahaemolyticus. International Journal of Biological Macromolecules 2015, 79, 202-207
- Saska, S.; & Teixeira, M. A. Chitin and chitosan-based biomaterials: current trends, challenges and applications. Journal of Materials Science 2017, 52
- Wei, X.; Du, Y.; & Deng, H. Advances in chitin and chitosan based porous materials. Current Organic Chemistry 2016, 192-193
- Li, R.; Li, X.; & Xiong, G. Recent advances in chitin and chitosan-based hydrogels for wound healing. International Journal of Biological Macromolecules 2021, 169
- Kozak, C.M.; Ambrose, K.; Anderson, T.S. Copolymerization of carbon dioxide and epoxides by metal coordination complexes. Coord. Chem. Rev. 2018, 376, 565–587.
CrossRef - Jhawar, R.; & Kumar, A. Chitosan-based nanoformulations for drug delivery: A review. Journal of Microencapsulation 2020, 325-342
- Mi, F. L.; & Shyu, S. S. Advances in chitosan science and technology. Journal of molecular science 2019, 965-978
- Shao, H.; Han, M.; Jiang, L.; & Liu, F. Derivatized chitosan as a stationary phase for enantioseparation. Journal of Chromatography A 2015, 124-133
- Ching, C. B.; Chong, C. W.; Yap, Y. H.; & Show, P. L. Derivatized chitin as an enantioselective stationary phase for chiral separation. 2019, 120-127
- Song, Z.; Zhu, Z.; Wang, J.; & Liu, F. Derivatized chitin stationary phase for enantioseparation of basic chiral compounds in high-performance liquid chromatography.Journal of Chromatography A 2012 , 138-144

This work is licensed under a Creative Commons Attribution 4.0 International License.










