Biological Importance of Phytoconstitents Isolated from the Genus Randia and Gc-Ms Analysis of Petroleum-Ether Fruit Extract of Randia Dumetorum
Manisha* and M. C. Sharma
Department of Chemistry, University of Rajasthan, Jaipur, Rajasthan India.
Corresponding Author E-mail: manialaria1989@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/390335
Article Received on : 04 May 2023
Article Accepted on : 05 June 2023
Article Published : 19 Jun 2023
Reviewed by: Dr. T. F. Abbs Fen Reji
Second Review by: Dr. Kuldeep Gupta
Final Approval by: Dr. S.A. Iqbal
Randia Genus (Indigo berry) belongs to family Rubiaceae, native to America, allocated in tropical regions. Most of the Species associated with this Genus used as ornamental, folk medicine to treat the disease of renal, malaria, cancer, dysentery, snake-bite etc. All plant parts are used by tribes for various ailment treatments. The phytochemicals generally associated with this genus are terpenoids, saponins, lignans, coumarin, iso-coumarin, flavonoids, tannins, essential oil and acid resin. This review highlights the phytochemicals and pharmacological activity reports. Phytochemical investigation of Randia dumetorum fruit extract using petroleum ether solvent, reveals the presence of 2,4-di-tert-butylphenol, octadecadienoic acid, 23(Phenylsulfanyl) lanosta-8,24-dien-3-ol, hexadecenoic acid, gamma-sitosterol, 4-tert-butylcalix[4]arene, tetracontane, tetratetracontane and octacosanol etc. Compound 1-Octacosanol (A), 9-Octadecenoic acid (B) and gamma-Sitosterol (C) were isolated with the help of column chromatographic techniques and characterized by spectral studies i.e. 1H NMR, 13C NMR, IR and mass spectroscopy.
KEYWORDS:Folk medicine; GC-MS; Phytochemistry; Pharmacology; Randia, Rubiaceae
Download this article as:| Copy the following to cite this article: Manisha M, Sharma M. C. Biological Importance of Phytoconstitents Isolated from the Genus Randia and Gc-Ms Analysis of Petroleum-Ether Fruit Extract of Randia Dumetorum. Orient J Chem 2023;39(3). |
| Copy the following to cite this URL: Manisha M, Sharma M. C. Biological Importance of Phytoconstitents Isolated from the Genus Randia and Gc-Ms Analysis of Petroleum-Ether Fruit Extract of Randia Dumetorum. Orient J Chem 2023;39(3). Available from: https://bit.ly/468kYmD |
Introduction
Randia genus commonly known as Indigo berry, belongs to Rubiaceae family, sub-family Ixoroideae, tribe- Gardeniae, native to the america, mainly distributed in tropical and subtropical regions. This is a large and well-defined family, with more than 600 genera and about 13,500 species found worldwide 1-3. This genus associated with more than 100 species. Out of them more than 10 species are ethnopharmacologically important. Randia echinocarpa, Randia matude, Randia aculeata, Randia hebecarpa, Randia ferox, Randia momantha are the most studied species 4. Excessive ethnobotanical and ethnomedicinal study of root, bark, leaves, fruit and seeds of this genus are reported in literature.
Randia dumetorum (Cautonaregam spinosa) also known as ‘Mainphal’ (hindi & Bengali) ‘Vamanaphala’(Sanskrit), highly reputed medicinal plant. It is deciduous shrub with thorns or small tree (height up to 5m.), which is distributed up to 4000 ft altitude throughout the india4. Leaves are oval, wrinkled, shiny & pubescent. Flowers are white, wrinkled solitary and possess honey like fragrance 5. Randia fruit approx. 1.8 – 4.5cm length, spherical, have persistent calyx-limb, yellowish brown coloured. Fruit contains large no. of seeds with the length of 0.4 – 0.6 cm, hard, compressed & brown 6.
Roots are used as insect repellent and insecticide. Bark is sedative, nervine and used to relieve the pain of bruises and febrile bone ache. Fruits have antiasthmatic, antitumor, analgesic, antispasmodic, antiallergic, antifertility, abortifacient & nauseantand also used as fish poison and detergent 5,7-8. The dry fruit powder with fresh milk treats gastric troubles. During emesis ‘Mainphal’ doesn’t show any side effect so it is considered as best emetic drug (vamaka dravya)5. Seeds show the antidiarrheal, antidysenteric activity. Seeds used to induce appetite and contain 14.2% protein, 1.5% fat, 1.4% organic acid, resin, mucilage 6,8.
Genus Randia exhibits a wide array of biological activities like anticancer, antioxidant, anti mutagenic, anti pyretic, anti venom, anti-inflammatory, hepatoprotective, abortifacient, haemolytic, molluscicidal, anthelmintic activities 4,8-16. The phytoconstituents associated with this genus are flavonoids, lignans, sterols, coumarins, tannis, triterpenoids, saponins mainly.
Only Randia aculeata shows the antivenom effect 4. Pseudogenosides and tyramine isolated from Randia siamensis are responsible for hypotensive and hypertensive activity respectively. Fruits of Randia echinocarpa (native to the maxico) consumed by rural areas as food/medicine 4.
Seeds of randia monantha are fiber rich. Seed oil contain palmitic acid (21.78%), oleic acid (26.12%) & linoleic acid (46.56%) 17.
Materials and Methods
Collection & pre-treatment of fruit sample
The fruit samples of Randia dumetorum fruit were collected from local market of Jaipur city Rajasthan (India). The fruit sample were washed, cleaned and dried. The plant’s identification was confirmed by Dr. Mahesh C. Sharma (Retd. Professor), Department of Chemistry, University of Rajasthan, Jaipur.
Preparation of extracts and Column Chromatography:
The air dried Randia dumetorum fruits were ground to fine powder using a grinder. Then the powdered fruits (approximately 1 kg) of Randia dumetorum were subjected to Soxhlet extraction procedure using Petroleum ether solvent (3ltr), for 12×3 days. “ Rotary Vacuum Evaporator (N.N. Series) with a digital water bath SB-651 and an aspirator; Eyela, tokyo, Japan’’ was used to remove excess solvent and further sodium sulphate remove moisture from extract. Above extract was stored in air tight container at low temperature. It was filtered by Whatman No.1 filter paper to remove grainy matter and concentrated sample of extract was obtained. The extract was dissolved in petroleum ether (20-30 mg/ml) for GC-MS analysis. Fat free extract (after acetonitrile treatment) was subjected to column chromatographic separation over a silica gel column. Column elution was done by different solvent system according to increasing polarity.
GC-MS Analysis
GC-MS analysis of Randia dumetorum fruit extract (Petroleum ether) was done at the Advanced Instrumentation Research Facility (AIRF) Lab, JNU, New Delhi using standard GCMS model as explained below.
Instrument details
Shimadzu GCMS-QP2010 Plus with thermal desorption system TD 20, AOC-20S auto-sampler & 20i auto-injector, was used to performed Gas chromatography- mass spectroscopy (mass range 1.5-1090 Daltons). The total run time of GC-MS was 29 mins. Different compounds were detected by mass spectrometer according to their different retention time. A plot of intensity v/s retention time was recorded (Chromatogram). The compounds are identified by comparing the data with the WILEY8, NIST14 & NIST14s libraries with name, molecular formula, molecular weight and structures.
The GC spectrum of the petroleum ether extract shows total nineteen compounds, were determined by the chromatographic method with the help of NIST and WILEY library as shown in Table 2. Compound Octadecadienoic acid was found to be in the highest concentration (13.36%) followed by hexadecenoic acid(11.63%), Octadecanoic acid(8.46%), 4tertbutylcalix[4]arene(5.16%), tetracontane (4.17%), tetratetracontane (3.40%), octacosanol (3.79%), other compounds were found in trace amount.
Table 1: Phytochemicals Identified in the Petroleum ether fruit extract of Randia dumeorum using GC-MS
 |
Table 1: Phytochemicals Identified in the Petroleum ether fruit extract of Randia dumeorum using GC-MS. |
 |
Figure 1: GC-MS Spectrum of Pet ether fruit extract of Randia dumetorum |
Isolation of compound A as 1-Octacosanol
Compound A was obtained by using hexane and dichloromethane with 1:4 as eluting solvents in column. After the solvents were eliminated the resultant material was crystallised with methanol to form colourless needle, m.p. 83-86oC. IR (KBr, cm-1) 3300, 2899-2840, 1460, 1064, 734, 724. 1H NMR (CDCl3, δppm) 0.88 (3H, triplet), 1.25 (-CH2, broad singlet), 3.57 (2H, triplet, -OCH2). 13C NMR (CDCl3, δppm) 14.12(-CH3), 22.69, 25.78, 29.36, 29.45, 29.61, 29.69, 31.92, 32.86 (each -CH2), 63.14 (-CH2OH). MS (m/z): 410 (M+), 390, 364, 350, 308, 292 etc. Molecular formula calculated as C28H58O.
Isolation of compound B as 9-Octadecenoic acid
Cream-coloured oil obtained when column was eluted with pet-ether and benzene in ratio 3:1. m.p. 10-120C. IR (KBr, cm-1) 2950, 1712, 1620. 1H NMR (δ ppm, CDCl3) 10.48 (s, 1H), 2.23 (t, 2H, C-2), 1.58-1.62 (m, 2H, C-3), 1.28-1.30 (20H, s), 5.24(2H, m), 0.93 (t, 3H). 13C NMR spectrum (δ ppm, CDCl3) 176.90 (C-1), 128.50 (C-9 and C-10), 32.3 (C-2, C-8, C-11), 25.10 (C-3), 28.30 (C-4), 28.65 (C-5, C-15), 29.15 (C-6, C-13, C-14), 29.75 (C-7, C-12), 30.66 (C-16), 21.82 (C-17), 13.89 (C-18). MS (m/z): 296 (M+), 264, 222, 180 etc. Molecular formula calculated as C19H36O2.
Isolation of compound C as γ-sitosterol
On eluting the column with chloroform white needle like crystals obtained. It showed m.p. 135-1370C, gave positive Liebermann burchard test. IR (KBr, cm-1) 3445-3550, 1620, 1050. 1H NMR (CDCl3, δppm) 0.88 (3H, triplet), 1.25 (-CH2, broad singlet), 3.57 (2H, triplet, -OCH2). 5.32 (t, 1H, C-6), 3.50 (m, 1H, C-3), 0.66-2.42 (m, for remaining 26 protons). 13C NMR spectrum (δ ppm, CDCl3) 31.50 (C-1), 32.31(C-2), 42.23 (C-4), 32.51 (C-7), 46.33 (C-8), 49.73(C-9), 36.25 (C-10), 21.33 (C-11), 28.70 (C-12), 43.10 (C-13), 57.31 (C-14), 24.83 (C-15), 41.51 (C-16), 56.30 (C-17), 36.25 (C-20), 36.21 (C-22), 24.57 (C-23), 40.11 (C-24), 37.51 (C-25), 32.81 (C-28), 12.50 (C-18), 19.51 (C-19), 19.52 (C-21), 23.41 (C-26), 23.42(C-27) and 30.00 (C-29). MS (m/z): 414 (M+), 397, 383, 369, 255 etc. Molecular formula calculated as C29H50O.
Results and Discussion
Isolation of compound A as 1-Octacosanol
Compound 1 was obtained by using hexane and dichloromethane with 1:4 as eluting solvents in column. After the solvents were eliminated the resultant material was crystallised with methanol to form colourless needle, m.p. 83-86oC. IR (KBr, cm-1) 3300(-OH, stretching), 2899-2840 (C-H, stretching), 1460 (-CH2-, bending), 1064 (C-O, stretching) and 734, 724 [-(CH2)n-deformation, n>4]. 1H NMR (CDCl3, δppm) 0.88 (3H, triplet), 1.25 (-CH2, broad singlet), 3.57 (2H, triplet, -OCH2). 13C NMR (CDCl3, δppm) 14.12(-CH3), 22.69, 25.78, 29.36, 29.45, 29.61, 29.69, 31.92, 32.86 (each -CH2), 63.14 (-CH2OH). MS (m/z): 410 (M+), 390, 364, 350, 308, 292 etc. Molecular formula calculated as C28H58O.
 |
Scheme 1: 1-Octacosanol. |
Characterization of compound B as 9-Octadecenoic acid
Cream-coloured oil obtained when column was eluted with pet-ether and benzene in ratio 3:1. m.p. 10-120C. Its IR spectrum (KBr, cm-1) appeared at 2950 confirmed the presence of carboxylic group. The absorption at 1712 due to carbony group whereas the absorption at 1620 confirmed the presence of olefinic (C=C stretching) group.
The 1H NMR spectrums (δ ppm, CDCl3) showed a sharp singlet for one proton of carboxylic group at 10.48 (s, 1H, -COOH). A triplet at 2.23 (t, 2H, C-2) was assigned for C-2 protons and a multiplet was observed at 1.58-1.62 (m, 2H, C-3) due to protons of C-3 position. A broad singlet at 1.28-1.30 integrated for 20 protons of ten methylene group at C-4 to C-7 and C-12 to C-17 respectively. A multiplet observed at 1.89-1.95 was assigned to the protons of methylene present at C-8 and C-11 positions. Protons attached to olefinic carbon at position C-9 and C-10 showed a multiplet at 5.24. A triplet at 0.93 (t, 3H, C-18) for three protons of methyl group at C-18 position.
The 13C NMR spectrum (δ ppm, CDCl3) showed absorption at 176.90 was confirmed the presence of -COOH group at C-1 position. The olefinic group was confirmed by the absorption at 128.50 which were assigned to C-9 and C-10 carbon positions. Other peaks were obtained at 32.3 (C-2, C-8, C-11), 25.10 (C-3), 28.30 (C-4), 28.65 (C-5, C-15), 29.15 (C-6, C-13, C-14), 29.75 (C-7, C-12), 30.66 (C-16), 21.82 (C-17), 13.89 (C-18).
From the above spectral data, it was identified as compound “2” 9-Octadecenoic acid.
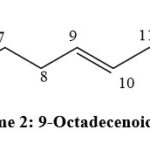 |
Scheme 2: 9-Octadecenoic acid. |
Characterization of Compound C as gamma-Sitosterol
The compound 3 was isolated as white powder, gave positive Liebermann-Burchard and Salkowski tests characterstic for sterols. The mass spectrum exhibited the molecular ion peak at m/z 414 [M]+ corresponding to its molecular composition as C29H50O. Other prominent peaks were observed at m/z 397, 383, 369, 255, etc.
In the IR spectrum (KBr, cm–1), strong absorptions at νmax3445-3550 showed the presence of the hydroxyl group. Other absorptions at 1620 and 1050 were characterized for olefinic group (C=C stretching) and for C-O stretching respectively.
The 1H NMR spectrum (δppm, CDCl3), exhibited abroad triplet at 5.32 corresponding to H-6 olefinic proton. A multiplet appeared at 3.50 for H-3 α-proton. The rest of the protons of compound appeared in high field region 0.66-2.42.
In 13C NMR spectrum (δ ppm, CDCl3), absorption at 72.21 demonstrated the presence of a hydroxyl group at C-3 position and two absorptions at 141.31(C-5) and 123.52 (C-6) can be assigned to olifinic carbons respectively. The assignments of other carbon atoms and their position were established as 31.50 (C-1), 32.31(C-2), 42.23 (C-4), 32.51 (C-7), 46.33 (C-8), 49.73(C-9), 36.25 (C-10), 21.33 (C-11), 28.70 (C-12), 43.10 (C-13), 57.31 (C-14), 24.83 (C-15), 41.51 (C-16), 56.30 (C-17), 36.25 (C-20), 36.21 (C-22), 24.57 (C-23), 40.11 (C-24), 37.51 (C-25), 32.81 (C-28), 12.50 (C-18), 19.51 (C-19), 19.52 (C-21), 23.41 (C-26), 23.42(C-27) and 30.00 (C-29).
 |
Scheme 3: γ-sitosterol. |
Conclusion
Defatted petroleum-ether extract produced 1-octacosanol (A), Octadecanoic acid (B) and γ-sitosterol (C).
In view of the immense biological, nutraceutical and pharmacological importance of the genus randia, we have systematically reviewed this genus as it may be helpful to food industry and pharmaceutical as well as phytochemists, biologists and pharmacologists.
Acknowledgement
The authors are thankful to Head, Department of Chemistry, University of Rajasthan, Jaipur, Rajasthan, India for the financial support.
Conflicts of interest
Present study does not contain any conflict of interest.
References
- Marina, D.J.; Ana, M.G.; and Roberto, S., A New Species of Randia (Rubiaceae) and the Taxonomic Significance of Foliar Anatomy in the Species of Randia of the Southern Cone of America. Systematic Botany 2020, 45 (3), 607–619.
CrossRef - Daiane, M.; and Cecilia, V.N., Secondary Metabolites from Rubiaceae Species. Molecules 2015, 20, 13422-13495.
CrossRef - Jaswant, S.S.; and Sood, S.K., Ethnomedicinal Plants Used to Treat Skin Ailments in Fringe Villages of Col. Sher Jung National Park, Simbalbara, Sirmour, Himachal Pradesh, India. Journal of Bioresearch 2023, 2(1), 1-10.
- Ayala, M.O.; Camacho, S.G.; and Vargus, F.D., Phytochemical composition and biological activities of the plants of the genus Randia. Botanical Sciences 2022, 100 (4), 779-796.
CrossRef - Timalsina, D.; Devkota, H.P.; Bhusal, D.; and Sharma, K.R., Catunaregam spinosa (Thunb.) Tirveng: A Review of Traditional Uses, Phytochemistry, Pharmacological Activities, and Toxicological Aspects. Evidence-Based Complementary and Alternative Medicine 2021, 3257732.
CrossRef - Garg, A.K.; and Chouhan, P., Madanaphala (randia dumetorum): a pharmacological and pharmacognostical review. International Journal of Recent Scientific Research 2019, 10 (4), 32061–64.
- Mazahir, R.; Anshul, K.S.; Poonam, G.; Tatheer, F., Isolation and chromatographic finger printing of randialic acid b an abortifacient agent isolated from bark of ayurvedic medicinal plant randia spinosa (poir.) rubiaceae. Asian Journal of Pharmaceutical Analysis and Medicinal Chemistry 2016, 4 (2), 107-111.
- Deepadarshan, U.; Narayanappa, M.; Sophiya, P.; Krishnaram, H.; Pramod, S.N.; and Dharmappa, K.K., Medicinal and nutritional aspects of wild edible fruits from Western Ghat of India. International Journal of Green Pharmacy 2022, 16 (4), 334-354.
- Hassan, M. M.; Sharmila, D.; Nandini, M.S.; Prabhu, K.; and Rao, M.R.K., The GCMS analysis of ethylacetate extract of one herbal plant Catunaregam spinosa. Natural Volatiles & Essential Oils. 2021, 8 (4), 6783-90.
- Anand, S.P.; Deborah, S.; and Velmurugan, G., Antimicrobial activity, nutritional profile and phytochemical screening of wild edible fruit of Catunaregamspinosa (Thunb.) triveng. The Pharma Innovation 2017, 6 (10), 106–109.
- Damle, S.; and Sharon, K., Antioxidant activity, TLC and HPLC-ESI-Q-TOF-MS fingerprinting of Catunaregamspinosa (Thunb.) triveng. Journal of Pharmacognosy and Phytochemistry 2018, 4, 2119-2124.
- Madhavan, V.; Vedavathi, B.; Raju, A.; and Murali, A.; and Yoganarasimhan, S., Sedative activity studies on the aqueous and alcohol extracts of the stem bark of Madanaphala-an ayurvedic drug Catunaregam spinosa(Thunberg) Tiruvengadam. Asian Journal of Traditional Medicines 2011, 6, 203–210.
- Saini, H.; Dwivedi, J.; Paliwal, H.; Kataria, U.; Chauhan, P.; Garg, R., Anti-inflammatory, analgesic and antipyretic activity of Catunaregamspinosa (Thumb.) Tirveng extracts. Journal of Drug Delivery and Therapeutics 2019, 5 (5), 89–94.
CrossRef - Abdullah-Al-Ragib, M.T.; Javed, H.; Mohammad, J., Antioxidant potential and cytotoxicity of Randiadumetorum Lam. leaf extract. Journal of Pharmacognosy and Phytotherapy 2017, 9 (9), 138–145.
CrossRef - Ram, C.B.; Sabir, H.; Siril ,S.; Himanika ,B.; Satish, K.; Chongtham, N.; and Anand, N.S., Ethno-ecological survey on medicinal plants for the treatment of Cancer in Hamirpur district, Himachal Pradesh, India. International Journal of Food and Nutritional Sciences 2022, 11 (11), 337-348.
- Subbu, T.; Pavithra, S.K.; Kavipriya, M.R.; and Azhagiyamanavalan, L.P., Synthesis of silver nanoparticles using Catunaregam spinosa fruit extract for their biological activities. Biomass Conversion and Biorefnery 2022, 1-14.
- Juárez-Trujillo, N.; Monribot-Villanueva, J.L.; Alvarado-Olivarez, M.; Luna-Solano, G.; Guerrero-Analco, J.A.; and Jiménez-Fernández, M., Phenolic profile and antioxidative properties of pulp and seeds of Randia monantha Benth. Industrial Crops and Products 2018, 124, 53-58.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









