Green Corrosion Inhibitory Efficiency of Isolated Flavonoid from Spermacoce hispida Leaves on Mild Steel in Acid Medium
Department of Chemistry, Holy Cross College (Autonomous), Affiliated to Bharathidasan University, Trichy-2, India.
Corresponding Author E-mail: saiganessh2014@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/390227
Article Received on : 13 Mar 2023
Article Accepted on : 22 Apr 2023
Article Published : 01 May 2023
Reviewed by: Dr. Syed Abuthahir
Second Review by: Dr. Gunawan
Final Approval by: Dr. Malinee Sriariyanun
Investigations on the effectiveness and inhibitory activity of the isolated flavonoid (Rutin) from Spermacoce hispida plants against corrosion of metal in a 1M HCl acidic solution include mass loss analysis, potentiostatic curves, and electrochemical methods. Plant extracts have been found to exhibit excellent inhibitory properties against corrosion of metals in different environments. The base metal's ability to stop corrosion improves when the green inhibitor is absorbed, and the rate of steel corrosion decreases. According to weight loss measurements, the highest corrosion inhibition efficacy is 87.99%. The electrochemical impedance study revealed that when the rutin concentration in S. hispida leaves increased, the IE value rose along with the Rct and Cdl values. As a consequence of adsorption, this pure flavonoid extract binds to the metal surface, with the adsorption kinetics being characterized by the Langmuir isotherm. According to the findings of this study, the extracted flavonoid from S. hispida leaf extract seems to be a powerful and environmentally benign basic metal inhibitor in an acid medium.
KEYWORDS:Electrochemical techniques; Green inhibitor; Rutin; Spermacoce hispida
Download this article as:| Copy the following to cite this article: Durgadevi S, Rose A. L. Green Corrosion Inhibitory Efficiency of Isolated Flavonoid from Spermacoce hispida Leaves on Mild Steel in Acid Medium. Orient J Chem 2023;39(2). |
| Copy the following to cite this URL: Durgadevi S, Rose A. L. Green Corrosion Inhibitory Efficiency of Isolated Flavonoid from Spermacoce hispida Leaves on Mild Steel in Acid Medium. Orient J Chem 2023;39(2). Available from: https://bit.ly/3NJjuIX |
Introduction
The effect of the industrial revolution on the environment has been the overuse and harm to our ecosystem, despite the many huge technologies that have just come to light as a result of the revolution. In addition, as the world of technology has progressed, corrosion has emerged as one of the most pressing problems, with yearly losses running into the millions. Corrosion, defined as the deterioration of a base metal due to electrochemical assault or redox chemical impact in an aggressive environment, is an issue faced by all home and industrial oil and petroleum facilities 1-3. Corrosion is a major problem in our industrial realm since it not only eats away at the metal surfaces of our machines but can also contaminate our food supply and our energy sources, leading to disastrous mishaps and environmental degradation. Electroplating, galvanizing, and protective coating to various rust inhibitors or corrosion inhibitors are only a few of the corrosion protection technologies available 3-5. Use of natural and inorganic corrosion inhibitors is a common technique. Polar activities involving organic matter, nitrogen or hydroxyl group in the molecule, heterocyclic compounds, and electrons contribute to the effect of natural ligands. Chromate, dichromate, and nitrite are included in the most efficient inorganic inhibitors 6-8. Nevertheless, their carcinogenic qualities limit their use. While chemically manufactured inhibitors have potent anti-corrosive effect, the vast majority are exceedingly harmful to humans and the environment, either during the production process or after being applied 7,9,11. Because of this, people are trying to find corrosion inhibitors that are safe for the planet. Compostable and free of heavy metals and other noxious byproducts, eco-friendly corrosion inhibitors are great for Mother Nature. Eco-friendly alternatives to conventional corrosion inhibitors include oils, fragrant plant extracts, spices, and medicinal herbs. These herbal inhibitors have a green origin and are thought to provide a plentiful source of normally occurring bioactive compounds that may be efficiently extracted using just mild separation procedures. Furthermore, they are non-toxic, biodegradable, and contain negligible amounts of metal ions 1-5. In order to inhibit or stop corrosion, green corrosion inhibitors use a variety of mechanisms, such as the formation of precipitates, adsorption, and/or the formation of a reactive layer on the base metal. Most naturally occurring chemicals worked by producing an almost imperceptible coating that repelled corrosion. Spermacoce hispida, a member of the family Rubiaceae, is a popular food source in northern Canada and Europe. S.hispida contains antibacterial, diuretic, antihypertensive, antimalarial, and analgesic activities 12, making it useful in a number of phytotherapeutic contexts.
The primary goal of this research is to find out the phytochemical screening of plant leaf methanol extract. The constituents were separated by fractionation and the flavonoid isolated from the S.hispida leaf by the method of chromatographic techniques. To elucidate the flavonoid effects on the corrosion behaviour of base metal in a 1 M HCl, which might be measured a usual green inhibitor, non-toxic, less-expensive, and effective natural corrosion inhibitors derived from renewable sources. This extract’s inhibitory effect was investigated using a variety of analytical tools, including mass loss measurement and electrochemical techniques.
Experimental
Specimen preparation
In industry, base metal is used. This sample was used for the mass loss method and electrochemical measurements. It was polished with a polisher and then with abrasive paper of decreasing grain size 50-400 before being cleaned with distilled water and a fresh cloth.
Preparation of Inhibitor
1mL isolated flavonoid (Rutin) is concentrated to 1000 mL solutions (ppm). The study’s content ranges from 100 to 800 ppm.
Weight reduction method
The weight of the base metal was reduced by submerging it in 100 mL of electrolyte containing 1 M HCl with and without the addition of the separated flavonoid at varying concentrations. Before to and after immersion, the sample is weighed using an electronic balance. It is cleaned with distilled water and dried before being reweighed. Optimized operating parameters (time and concentration) were applied to a second round of testing, this time with and without an inhibitor present, to establish said settings and to assess said impact of temperature.
Electrochemical Methods
Tafel polarization lines and Nyquist impedance lines were marked using an electro catalytic analyzer. Throughout the research, several cells were employed. A 1 cm2 base metal specimen, a pure platinum counter electrode (CE), and a reference electrode (RE) are used in this experiment (EIS). A flavonoid derived from the S.hispida plant is added to 1MHCl solutions of varying concentrations, and a potentiodynamic polarization curve is recorded at room temperature using a scanning rate (green inhibitor).
SEM analysis
The mild steel samples were left for three hours with and without inhibitor before being rinsed in deionized water, dried, and visually examined.
EDAX analysis
A technique for elemental composition of a sample is EDAX spectroscopy.
Result and Discussion
Phytochemical Analysis
Flavonoids, tannins, alkaloids, glycosides, triterpenoids, and phenolic compounds are only few of the numerous organic chemicals found in the methanol extract of the S.hispida plant. Inhibitory abilities are well-known to exist in the bulk of these substances. Using chromatographic methods, the major flavonoid components of S.hispida leaves were extracted and purified. Flavonoid compounds were characterized by ultraviolet (UV), far-infrared (FT-IR), nuclear magnetic resonance (NMR), and gas chromatography (GC-Mass) spectroscopy. Flavonoid extracted from S.hispida leaves has an inhibitory impact on corrosion steel, as shown by mass loss and electrochemical methods. Hence, the corrosion inhibiting action is due to the adsorption of these compounds onto the surface of the base metal, which in turn decreases the surface area accessible for corrosion 14,15.
Evaluation of Anticorrosion Activity by the Weight Reduction technique
Without separated flavonoid, the corrosion rate of base metal in 1M HCl was determined using weight reduction techniques, and the presence of inhibitor from S.hispida leaves at 300ºC and the percentage of inhibition were determined. The rate of corrosion on the steel lowers and the inhibitory efficiency improves when the inhibitor intensity increases, as shown in Table 3 and Fig. 3. The concentration of the extract at which the rate of corrosion is lowest is 700ppm. This may be attributable to the inhibition of corrosion by catalytic sites on the outside of the steel being protected 16. The significant inhibitory efficiencies17 may be explained by the existence of heteroatom-containing active molecules in the isolated flavonoid from S.hispida leaves.
Table 1: Corrosion rates of mild steel in 1M HCl by weight loss method using isolated Flavonoid.
|
S. No. |
Isolated flavonoid (ppm) (Rutin) |
Corrosion rate (mpy) |
Surface coverage(θ) |
Percentage Inhibition (%) |
|
1 |
Blank |
11.4780 |
– |
– |
|
2 |
100 |
4.3616 |
0.62 |
62.0 |
|
3 |
200 |
3.9790 |
0.65 |
65.33 |
|
4 |
300 |
3.4128 |
0.70 |
70.27 |
|
5 |
400 |
2.9231 |
0.74 |
74.53 |
|
6 |
500 |
2.3568 |
0.79 |
79.47 |
|
7 |
600 |
1.9742 |
0.82 |
82.8 |
|
8 |
700 |
1.3774 |
0.87 |
87.99 |
|
9 |
800 |
1.8212 |
0.84 |
84.13 |
 |
Figure 1: Inhibition efficiency of isolated flavonoid extract at different concentration |
Temperature Effect
To investigate the impact of temperature on IE and activation energy, experiments were carried out in the 303 – 333K temperature range, both in the absence and presence of optimum absorption of the inhibitor in 1 M Hydrochloric acid solution for 3 hours of mild steel spattering. Figure 2 shows the change in inhibitory efficiency as a function of temperature increase. The data demonstrate that the extract is most effective at preventing growth at 303 K, with an inhibitory effectiveness of 87.99%, and that this efficiency declines as the temperature rises.
Table 2: Effect of temperature on the CR of mild steel in 1M HCl by isolated flavonoid extract at different temperatures
|
S.No |
Temperature(K) |
Corrosion rate(Blank) |
Corrosion rate(inhibitor) |
Surface coverage(ᶿ) |
% I.E |
|
1 |
303 |
11.4780 |
1.3774 |
0.87 |
87.99 |
|
2 |
313 |
22.9713 |
7.5908 |
0.66 |
66.96 |
|
3 |
323 |
31.7405 |
16.2987 |
0.48 |
48.65 |
|
4 |
333 |
44.6876 |
31.6639 |
0.29 |
29.14 |
 |
Figure 2: Inhibition efficiency of isolated flavonoid at different temperatures. |
Kinetic and thermodynamic Criteria
This is because of an increase in the absorption of the passive layer and any byproducts created on the base metal that may impede the reaction, as well as an enhanced influence of temperature on the dissolution process of steel or inadequate desorption of the inhibitor from the base metal 15-18. Identifying the inhibitors’ inhibitive mechanism relies heavily on the activation factors. Using the linear regression of Log (CR) vs 1/T data equation, we find that the activation energy Ea ranges from 43.66 KJ mol-1 in the absence of the isolated flavonoid to 58.07 KJ mol-1 in its presence.
 |
Figure 3: Arrhenius plots for corrosion of mild steel in 1M HCl solution in the absence and presence of isolated flavonoid. |
The extract strongly inhibits the corrosion process by raising the barrier energy, as seen by the increase in Ea value occurrence 19. Table 3 displays the computed activation enthalpy H and entropy S values from the temperature investigations using equation 11. These values represent the absence and presence of 700 ppm extract, respectively. The thermodynamic parameters ∆S and ∆H are shown in Figure 5. The ideal temperature dependence of inhibitory activity (K).
Table 3: Kinetic and thermodynamic parameters for carbon steel in 1M HCl and isolated flavonoid rutin (700 ppm).
|
Ea(kJmol-1) |
ΔH*(kJmol-1) |
ΔS*(kJmol-1) |
|
|
Blank |
92.082 |
34.57 |
0.077 |
|
Inhibitor |
257.11 |
83.52 |
0.223 |
Adsorption parameter
Adsorption of organic molecules provides information on the nature of the interaction between the adsorbed molecules and the electrode surface. Evaluation of the isolated flavonoid extract from S.hispida leaves and the process of adsorption onto the surface of base metal led to the identification of a model that best explained the data obtained via the loss mass and electrochemical measurement techniques. With the extract added as an inhibitor, the value of increases. In the Langmuir adsorption isotherm equation, the weight reduction technique values are replaced.
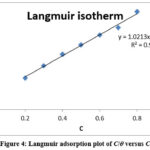 |
Figure 4: Langmuir adsorption plot of C/θ versus C |
Table 4: Langmuir adsorption of isolated flavonoid (rutin)in 1M HCl on the mild steel.
|
Inhibitor |
Intercept |
Kads |
R2 |
ΔGads0(kJmol-1) |
|
Rutin |
0.1137 |
8.7951 |
0.9935 |
-15.5985 |
The linear relationship between C/Ѳ ratio and inhibitor absorption is shown by the graph, with a regression coefficient of R2 = 0.99, very close to unity. This demonstrates that the flavonoid extract of S.hispida adheres to a layer of mild steel in 1M HCl media in accordance with the Langmuir adsorption isotherm. Hence, the isolated flavonoid from the plant S.hispida is adsorbed on the surface of the base metal by single layer adsorption, preventing electrolyte access 20. Stability of inhibitor molecules is maintained when their free energy are lowered by adsorption on the steel surface through reaction with ions or molecules near the surface 21.
Evaluation of Anticorrosion Activity by the Polarization Curves of Tafel
To illustrate how the flavonoid extract serves as a corrosion inhibitor, Figure 8 shows the superposition of Tafel lines for the extract. The inhibitory effectiveness at various concentrations is shown in Table 5, together with electrochemical data (corrosion rates, corrosion potentials Ecorr, densities Icorr corrosion, electro catalytic slopes of Tafel). The corrosion current dropped when the inhibitor was added, suggesting that the isolated flavonoid mitigated the corrosive effects of the acid medium on the mild steel. The decrease in Ecorr value 22 indicates that the inhibitor is probably an anodic inhibitor.
 |
Figure 5: Tafel polarization for base metal in 1M HCl (a) and (b) of isolated flavonoid rutin (700 ppm). |
Table 5: The potentiodynamic polarization and linear polarization of rutin
|
System |
Ecorr mV/SCE |
Icorr µA/cm2 |
βa mV/dec |
βc mV/dec
|
Corr.rate Mm/y |
|
Blank |
-553.4 |
857.7 |
90.123 |
119.159 |
392.0 |
|
Inhibitor |
-564.54 |
819.284 |
106.989 |
140.218 |
374.49 |
Electrochemical impedance parameter
Based on the measurement of a transfer function after an intentional disruption of the electrochemical system under investigation, EIS is one of the most helpful tools for studying the properties of an electrochemical system and explaining the inhibitory mechanism 23,24. In Fig. 1, shows the Nyquist diagrams for base metal in 1 M hydrochloric acid, both when the inhibitor does not present and when it does. The charge transfer process occurs at the inhibitor/steel interface, as shown by the fact that the Nyquist curves are semicircular regardless of concentration 25,26. Increasing the inhibitor concentration results in larger capacitive half-loop diameters, suggesting a higher proportion of inhibition.
 |
Figure 6a: Nyquist plot in absence of optimum concentrations of rutin. |
 |
Figure 6b: Nyquist plot in presence of optimum concentrations of Rutin. |
Table 6: The electrochemical impedance parameters of Rutin.
|
System |
Rs ῼ |
Rct ῼ m |
Cdl F m |
|
Blank |
1.67 |
0.99207 |
0.07379 |
|
Inhibitor |
4.8 |
1.8863 |
0.01449 |
SEM analysis
Surface morphology was studied using scanning electron microscopy. The images indicate that the plain samples’ surfaces have severe corrosion. With increasing temperature, the roughness of corrosive environment investigators on the hard surface in blank solution increased. These images definite the rates of steel corrosion in 1 M solution at different temperatures. The micrographs in Figure show that the metal substrates were inhibited against corrosion and that the concentration of the inhibitor increased, resulting in smoother surfaces 27,28.
 |
Figure 7: SEM micrograph of (a) plain mild steel, (b) mild steel immersed in 1 M HCl acid,(c) mild steel immersed in the presence ofisolated flavonoid in 1M HCl. |
Energy dispersive spectroscopy
The elements that can be found on the surface of mild steel were determined using EDX spectra after 3 hours contact to uninhibited and inhibited 1 M HCl. The EDX analysis of mild steel with 700 ppm isolated flavonoid is exposed in Figure. According to the EDX analysis, only Fe and oxygen were detected, implying that the reactive film contained only Fe2O3. Extra lines in the spectra indicate the presence of C.
 |
Figure 8: EDAX spectra of mild steel specimen (a) and (b) of isolated flavonoid rutin (700 ppm). |
Conclusion
In a 1M HCl solution, the corrosion inhibitory efficacy of an extract of the plant S.hispida’s isolated flavonoid rises with extract concentration. The polarization curves show that the isolated flavonoid is both an anodic and a mixed-type inhibitor. Increasing the absorption of isolated flavonoid results in a higher Rct and a lower Cdl due to inhibitor adsorption on the surface of the base metal, as measured by EIS. The adsorption of the inhibitor molecules is confirmed by the anticorrosive activity evaluation techniques, and the adsorption model follows the rules of Langmuir adsorption. SEM and EDAX spectra are used to evaluate the thin layer’s surface shape in relation to the base metal. The extracted flavonoid (Rutin) from S.hispida leaves extract is a good inhibitor, as shown by weight loss and electrochemical methods.
Acknowledgement
Thanks for Holy Cross College (Autonomous), Trichy for providing lab and library facilities.
Conflict of interest
There is no conflict of interest.
References
- Belkis G., Neman A., Journal of Advanced Research in Science and Technology, 2017,4. 433.
- Hussin M. H., Kassim M. J., Materials Chemistry and Physics, 2011, 125,461.
CrossRef - Benmessaoud Left D., Zertoubi M., Irhzo A., Azzi M., J. of Materials and Environmental Science, 2013,4, 855.
- Rahim O., Chaouch K., Touhai L., AnnalesdesSciencesetTechnologie, 2013, 5, 18
- ZaferaniS. H. and Shishesa M. R., J. of Petroleum & Environmental Biotechnology,2014, 5, 1.
- Quraishi M. A., Jamal D., J. of Applied Electrochemistry, 2002, 32, 425.
CrossRef - Raja P. B., Sethuraman M. G.,Materials letters, 2008,62, 113.
CrossRef - Nathiya R. S., Vairamuthu R., Egyptian J. of Petroleum, 2017, 26, 313
CrossRef - Casaletto M. P., Figà V., Privitera A., Bruno v, Corrosion Science, 2018,136, 91.
CrossRef - Singh A., Ahamad I., Quraishi MA.. Arabian J. of Chemistry, 2016, 9, 1584.
CrossRef - Alaneme, K. Olusegun J., Alo W., Alexandria Engineering Journal, 2016, 55, 1069.
CrossRef - Guessoum B., HadjSeyd A., Kemassi A., Rahim O., Phytothérapie, 2021,19, 69.
CrossRef - Mushira Banu, B. Arifa Farzana, and Riaz Ahamed K., Materials Today: Proceedings, 2021,47, 2036.
CrossRef - Pradeep Kumar C. B., Mohana K. N., Egyptian Journal of Petroleum, 2014, 23, 201.
CrossRef - Marsoul A., Ijjaali M., Elhajjaji F., Taleb M., Salim R., Boukir A., Materials Today: Proceedings, 2020, 27, 3193.
CrossRef - Bouknana D., Hammouti B., Jodeh S., Sbaa M., Lgaz H., Analytical & Bioanalytical Electrochemistry, 2018,10, 751.
- Oumelkheir R., Messaouda A., Manel Z., Ibtissem B., Sourya G., Samira B., Fresenius Environmental Bulletin, 2021,30, 12157.
- Jeon J. M., Yoo Y. R. and Kim Y. S.. Corrosion Science and Technology, 2021,20, 325.
- Archana P., Samatha T., Mahitha B., Chamundeswari, RamaSwamy N, International J.of Pharmaceutical and Biological Research, 2012, 3, 82.
- Khadom A. A., Ahmed N. A., South African J. of Chemical Engineering, 2018,25, 13.
CrossRef - Benarioua M., Mihi A., Bouzeghaia N., Naoun M., Egyptian J. ofPetroleum, 2019, 28, 155.
CrossRef - Lawal.D and Yunusa.I, International J. of Innovation and Applied Studies , 2013, Vol. 2 No. 4,477-483
- Alvareza E., Victoria Fiori-Bimbi M., Adriana Neske,Silvia A. Brandán, Claudio A. Gervasi, J. of Industrial and Engineering Chemistry, 2018, 58, 92.
CrossRef - Ehsani A., Mohammad Shiri H., Kowsari E., Safari R., Torabian J., Kazemi S., J. of Colloid and Interface Science, 2016 ,478, 181.
CrossRef - Coelho L., Cossement D., Olivier M.-G., Corrosion Science, 2018,130, 177.
CrossRef - Qiang Y., Guo L., Zhang S., Li W., Yu S., Tan J., Scientific Reports, 2016, 6, 33305.
CrossRef - Mitali Konwar, G D Baruah, Archives of Applied Science Research, 2011,3 (1), 214-221,.
- 28.Vidhya S , Leema Rose A and Janeeta Priya F, Asian j. of chemistry, 2019, 31,No.10 21964,.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.










