Development and Application of Newly Synthesized Tamarind 2-Hydroxy -2- Methyl Butyric Acid (THMBA) Resin for Elimination of Hazardous Metal Ions from Industrial Effluents
Anju* , Chandra Prakash
, Chandra Prakash , Ganesh Kumar Choudhary
, Ganesh Kumar Choudhary , Sarita Kumari
, Sarita Kumari , Mukesh Choudhary
, Mukesh Choudhary , and Vimla Chowdhary
, and Vimla Chowdhary
Department of Chemistry, Faculty of Science, New Campus, Jai Narain Vyas University, Jodhpur, Rajasthan, 342001, India.
Corresponding Author E-mail: anjuchoudharyjnvujodhpur@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/390233
Article Received on : 16 Feb 2023
Article Accepted on :
Article Published : 11 Apr 2023
Reviewed by: Prof. Waquar Ahmad Siddiqui
Second Review by: Dr. Nitin Agrawal
Final Approval by: Dr. B.K Sharma
The newly synthesized Tamarind 2-Hydroxy-2-Methyl Butyric Acid (THMBA) resin for elimination of hazardous waste metal ions was developed from industrial effluents. The Tamarin kernel powder (TKP) has been studied for their good metal sorption properties and found to have potential for waste management. In the laboratory, chemically produced Tamarind-2-hydroxy-2-methyl butyric acid (THMBA) resin derivative has been employed for the elimination of Zn2+, Pb2+, and Cd2+ions in aqueous solution of effluents of arid region of Rajasthan, with special emphasis in and around Pali district. These groups of ion exchanger constitute new category of newly reformed ion exchange resin for the retraction of ions of harmful metal. It was further diagnosed by computing thermal and FT-IR spectral analysis, ion exchange capacity etc. The estimation of ‘Kd’ values of these unsafe metal ions was also done at various values of pH.
KEYWORDS:Adsorption; Adsorbents; Arid region; Pollutant; Tamarind kernel powder (TKP); Textiles Effluents; Tamarind Hydroxy Methyl Butyric Acid; Waste water
Download this article as:| Copy the following to cite this article: Anju A, Prakash C, Choudhary G. K, Kumari S, Choudhary M, Chowdhary V. Development and Application of Newly Synthesized Tamarind 2-Hydroxy -2- Methyl Butyric Acid (THMBA) Resin for Elimination of Hazardous Metal Ions from Industrial Effluents. Orient J Chem 2023;39(2). |
| Copy the following to cite this URL: Anju A, Prakash C, Choudhary G. K, Kumari S, Choudhary M, Chowdhary V. Development and Application of Newly Synthesized Tamarind 2-Hydroxy -2- Methyl Butyric Acid (THMBA) Resin for Elimination of Hazardous Metal Ions from Industrial Effluents. Orient J Chem 2023;39(2). Available from: https://bit.ly/3zOIV3y |
Introduction
The water quality parameters of arid region of Pali district were studied. Ground water is the major source of drinking water and over 93% of the drinking water demand is met by groundwater in said area of Pali district. The water quality of this area is not having those standards which are recommended by the WHO. Discharge of urban, industrial and agricultural wastes have increased the quantum of various hazardous chemicals that entered in water. Without the knowledge of water chemistry, it is difficult to understand the biological phenomenon. The chemistry of water much reveals about metabolism of the ecosystem and explains the general hydro-biological interrelationship1,2 domestic wastes3,4 water quality5. Inorganic or organic ion exchange materials provide a way to polysaccharide6 natural polymers7, chitosan8 sulphate groups9,10 and physical appearance11. The relevant work was also conducted by various researchers exchange method12 oxidation processes13,14, chemical techniques15, solvent extraction16. Garg and Seth17 were also studied about characteristics of ground water samples (GWS) from the some bore wells and dug wells of different locations of Pali district in Rajasthan and found remarkable result of ground water. The discussed methods employed to develop versatile guar gum-based adsorbent18, solvent extraction19, precipitation20, hazardous metal ions21-24 Guar gum25,26.
Nature and objective of the present study
The objectives of this study are to investigate and evaluate various hazardous metal ions present in rural area of Pali district (Arid region of Western Rajasthan), and to develop newly synthesized Tamarind 2-Hydroxy -2- Methyl Butyric Acid (THMBA) resin for elimination of hazardous metal ions from industrial effluents. The purpose of using Tamarind here as the polysaccharide matrix and its easy availability from agricultural resources and effective and low-cost procedures of handling and decontamination of industrial wastewater. The study about the newly synthesized resin from tamarind kernel powder (TKP) has been sustainable application. Tamarind-2-Hydroxy-2-Methyl Butyric Acid resin (Newly developed resin, namely THMBA resin) has application to elimination of hazardous metal ions.
Material and Methods
Materials
The chemicals used in the synthesis of resin are tabulated in Table 1.
Table 1: Materials used for synthesis Tamarind 2- Hydroxy -2-Methyl Butyric Acid (THMBA) Resin
|
S.No. |
Chemical |
Specification |
|
1 |
Tamarind Kernel Powder (TKP) |
Sarabhai M, Chemicals Baroda, INDIA |
|
2 |
2- Hdroxy-2-Methyl Butyric Acid (AR) |
Sigma Aldrich Chemicals Private Ltd, Mumbai |
|
3 |
Hydrochloric Acid (AR) |
Sarabhai M, Chemicals Baroda, INDIA |
|
4 |
Epichlorohydrin (AR) |
Loba Chemie Pvt. Ltd, Mumbai |
|
5 |
Methanol (AR) |
E Merk. Bombay, India |
|
6 |
Sodium Hydroxide (AR) |
Sarabhai M, Chemicals Baroda, INDIA |
|
7 |
Dioxane (AR) |
SD Fine chem. Pvt. Ltd. Boisar |
Synthesis of Tamarind 2-Hydroxy -2- Methyl Butyric Acid (THMBA) Resin
The procedure followed to synthesize guar gum diamino benzoic acid is as follows:
Epoxy propyl ether of 2-Hydroxy 2-Methyl Butyric acid Preparation
The solution of 2-Hydroxy 2-Methyl Butyric acid, NaOH, and epichlorohydrin in double distilled water followed by 1.18 gm of 2-Hydroxy -2-Methyl Butyric acid (0.01 M) in methanol in a round bottom flask and 4.0 mL of NaOH (0.1M), and mixed all solutions, then after addition of 9.30 ml (0.10 M) of epichlorohydrin to the alkaline mixture with continuous stirring on a magnetic stirrer for about 4 hours at temperature 60-65 oC.
Tamarind 2- Hydroxy -2-Methyl Butyric Acid Resin (THMBA Resin) Preparation
After preparation of epoxy ether compound, 162 g of Tamarind kernel power (TKP) (0.5 moles) was mixed with dioxane and then it was allowed to react with the prepared epoxy propyl ether of 2-Hydroxy 2-Methyl Butyric acid. It was continuous stirring for next 4 hours at 60-65 oC temperature. This reaction mixture was kept for 24 hours. The resultant product was first filtered under vacuum then it was followed by washing with 70% methanol and HCl to neutralize 6.0 g NaOH and to eliminate inorganic contaminants and then dried. The yield of Tamarind 2- Hydroxy -2-Methyl Butyric Acid Resin (THMBA Resin) was 42.6 gram.
Stock Solutions Preparation of Metal Ions
Zinc Acetate Zn (II)
2.324g of zinc Acetate (Zn (CH3COO)2) was dissolved in a 2.5 ml of acetic acid and volume was make up by doble distilled water to 1000 volumetric flask to give 1000 ppm zinc solution.
Lead Nitrate pb (II)
In a 1000mL volumetric flask, 2.3247 g of lead nitrate was added to 1.5 ml of diluted sulphuric acid. The total volume of solution was made 1000 mL by pouring doble distilled water to the solution in volumetric flask.
Cadmium Sulphate Cd (II)
1.231g of CdSO4 H2O was dissolved in 2.0 ml concentrate sulphuric acid and the volume was raised up to mark in 1000 ml volumetric and the volume was raised up by addition of double distilled to mark in 1000 ml volumetric flask to give 1000 ppm Cadmium solution.
Determination of Distribution Coefficient by Batch Method
The value of molar distribution coefficient ‘Kd’ of various metals like, Zn(II), Cd(II), and Pb(II) which shows sound adsorption on different chelating resins were done by using batch method. Weighed amount of various chelating resins i.e., Tamarind 2- Hydroxy -2-Methyl Butyric Acid Resin (THMBA Resin) was taken in a glass stopper conical flask, which was having 1ml of 1000 ppm metal solution analogous to 1 mg metal ions. In it, suitable buffers of known volume were taken for pH adjustments. After this the contents of conical flasks were then allowed to stir on a magnetic stirrer and then equilibrated. The two phases obtained on equilibration were then separated by using Whatman 42 filter paper and a portion of filtrate obtained was examined for the concentration of metal in ions.
Calibration curves were plotted for various metals, through the examination of standard solution series of metal ions with the help of atomic absorption spectrophotometer. Air acetylene flame and different wavelength of main resonance line were used for the estimation of various metals. The values of different wavelengths taken with the help of corresponding calibration curves for different ions the concentration of metal ions in filtrates were determined. And then the respective distribution coefficients were calculated by the using the following formula
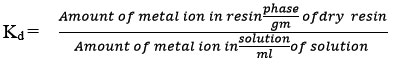
Buffers of pH 2 -8 were prepared by adding appropriate amounts of 0.2M acetic acid and 0.2M sodium acetate in different conical flasks.
Determination Of Distribution Coefficient ‘Kd’ for Zn(II), Pb(II) and Cd(II)
Table 2: Absorbance For Standard Solutions Of Zn, Pb, and Cd solution
|
S.No. |
For Zn, |
For Pb |
For Cd |
|||
|
Concentration in ppm |
Absorbance |
Concentration in ppm |
Absorbance |
Concentration in ppm |
Absorbance |
|
|
1 |
1 |
0.034 |
1 |
0.030 |
2 |
0.006 |
|
2 |
2 |
0.063 |
2 |
0.072 |
3 |
0.143 |
|
3 |
4 |
0.113 |
4 |
0.143 |
5 |
0.264 |
|
4 |
6 |
0.165 |
6 |
0.217 |
7 |
0.381 |
|
5 |
8 |
0.218 |
8 |
0.299 |
9 |
0.487 |
|
6 |
10 |
0.272 |
10 |
0.372 |
11 |
0.585 |
|
7 |
12 |
0.328 |
12 |
0.456 |
12 |
0.604 |
|
8 |
14 |
0.384 |
14 |
0.530 |
13 |
0.643 |
|
9 |
16 |
0.445 |
16 |
0.613 |
15 |
0.662 |
Calibration curves are given in Fig 4,5, and 6.
Different amounts of 0.2M acetic acid and 0.2M sodium acetate were taken in a glass stopper conical flask to obtain solutions of desired pH i.e., 2-7. Similarly, to obtain the pH of 8 suitable amount of 0.2M NH4OH and 0.2M NH4Cl were mixed. In each flask containing different pH solutions, 0.085gm of dry resin (THMBA) and 1ml of 1000 ppm Zn (II) solution were added. These contents were allowed to mix completely on a magnetic stirrer and then filtered. The resulting filtrates were examined for zinc, lead and cadmium. The results obtained were summarised in the given Table 3
Table 3: Distribution Coefficient ‘Kd’ for Zn(II), Pb(II) and Cd(II) for Thmba Resin.
|
PH |
For Zn, |
For Pb |
For Cd |
|||
|
‘Kd’ for Zn |
% Adsorption of Zn (II) by resin |
Kd’ for Pb |
% Adsorption of Pb (II) by resin |
Kd’ for Cd |
% Adsorption of Cd (II) by resin |
|
|
2 |
345.61 |
47.08 |
499.00 |
42.20 |
67.28 |
7.532 |
|
3 |
449.54 |
2.26 |
865.30 |
53.71 |
69.70 |
7.847 |
|
4 |
1045.93 |
69.14 |
1644.13 |
65.87 |
79.16 |
9.016 |
|
5 |
4759.80 |
89.89 |
3650.53 |
787.20 |
88.17 |
10.146 |
|
6 |
5950.34 |
1.58 |
1821.48 |
67.82 |
77.94 |
8.864 |
|
7 |
1977.76 |
80.25 |
408.45 |
53.31 |
69.37 |
8.795 |
|
8 |
1816.34 |
78.73 |
253.49 |
42.41 |
68.72 |
8.714 |
Inference
The value of distribution coefficient for Zn(II) on THMBA resin was maximum at pH 6 and other pH from 2 to 8, shows good absorption on THMBA. From the resin, metal ions can be eluted by HCl having pH below 2.0.
Resin Characterization
The characteristics of newly produced Tamarind resin was researched and analysed. Various analyses and determinations were used to investigate the characteristics of these resins. Diverse methods, such as I.R. spectra, pH titration, and sulphur and nitrogen content measurement, were used to validate the synthesis of various derivatives. The Shimadzu 8300 was used to do FTIR spectral investigation on all of the newly developed resins.
 |
Figure 1: Tamarind (Tamarindus indica). |
FTIR Characterisation of Tamarind 2- Hydroxy -2-Methyl Butyric Acid Resin (THMBA Resin)
FTIR spectrum studies of GDABA resin shows peculiar peaks at 3239.1 cm-1, 1010.1 cm-1, 1211.4 cm-1, 1558 cm-1 for NH2 group, C-O-C stretching, -C-O stretching and C=O stretching respectively. A peak at 2881 cm-1, shows the presence of CH and CH2 stretching for aliphatic groups, a peak for C=C conjugated group has been observed at 2322.1 cm-1, polysaccharides generally show peaks in the range of 3200 to 3600 cm-1, region. Fig 2 shows the FTIR spectrum of THMBA resin. FTIR spectrum studies of THMBA resin shows peculiar peaks at 3239.1 cm-1, 1010.1 cm-1, 1211.4 cm-1, 1558 cm-1 for NH2 group, C-O-C stretching, -C-O stretching and C=O stretching respectively. A peak at 2881 cm-1, shows the presence of CH and CH2 stretching for aliphatic groups, a peak for C=C conjugated group has been observed at 2322.1 cm-1, polysaccharides generally show peaks in the range of 3200 to 3600 cm-1, region. Fig 2 shows the FTIR spectrum of THMBA resin.
 |
Figure 2: Tamarind specifications: Tamarind Kernel and Tamarind Kernel Powder. |
 |
Figure 3: FTIR Characterization of Tamarind 2- Hydroxy -2-Methyl Butyric Acid (THMBA) Resin |
 |
Figure 4: Scanning electron micropy (SEM) Images of Tamarind-2- Hydroxy -2-Methyl Butyric Acid (THMBA) resin |
 |
Figure 6: Chelation of Pb(II) on Thmba Resin. |
 |
Figure 7: Calibration for Zn (Ii) on Thmba Resin. |
 |
Figure 8 Chelation for Cd(Ii) on Thmba Resin. |
Conclusion
Maximum adsorption of Metal ion was observed at different pH levels of maximum adsorption. So, the segregation of these metal ions from others can be done at pH 2 and 10. All observed results are reported for the development and application of newly synthesized Tamarind-2- Hydroxy -2-Methyl Butyric Acid (THMBA Resin) resin for elimination of hazardous waste metal ions. Tamarind-2- Hydroxy -2-Methyl Butyric Acid Resin (THMBA Resin) resin is profoundly selective for the elimination of hazardous metal ions from the industrial waste. On studying the results obtained, it can be inferred that with increasing pH, the ‘Kd’ values are variable for different metal ions. The Table 2,3, and Figure 3-8 shows the development and application of newly synthesized Tamarind-2- Hydroxy -2-Methyl Butyric Acid Resin (THMBA Resin) resin for elimination of hazardous waste metal ions. The trend of values of equilibrium dissociation constant (‘Kd’) at the corresponding pH values where maximum adsorption of metal ion takes place on newly synthesized Tamarind 2- Hydroxy -2-Methyl Butyric Acid Resin (THMBA Resin) resin as mentioned.
Acknowledgment
Authors are thankful to Head, Department of Chemistry, Faculty of Science, Jai Narain Vyas University, Jodhpur, Rajasthan, 342001, INDIA, for necessary laboratory facility and Anju Chaudhary (co-author) is thankful to Professor Dr. Vimla Choudhary (Research Supervisor) for scientific analysis and guidance.
Conflicts of Interest
Authors have no any conflict of interest.
References
- Majumder, S.; Dutta, T.K.; Int. J.A dv. Res., 2014, 2(3), 877-881.
- Sharma, T.K.; Ramendra, S.; Inter. J.; Cur. Microbio. App. Sci., 2016, 5(12),308-315.
CrossRef - Rao, VNR.; Valsaraj, CP.; J. Mas. Biol. Ass.,1984, 26, 58-65.
- Kudari, V.A.; Kadadevaru, G.G., India. Environ Monit Assest., 2006, 120, 387-405.
CrossRef - Koorosh, J., Sadanand. M., Ind. J. Aqua. Biol., 2009, 2(2), 26-30.
- Inamuddin, SA.; Siddiqui, WA.; Khan, A.A.; National Library of Medicine, 2007,15,814
- Li, Q.; Wang, S.; Huang, C.; Xiang, Z.; MDPI, Aug 2020, vol 12, 8, 1837
CrossRef - Liu, F.; Guan, L.; Yang, Z.; Li, Z.; De K.; J. Appl. Polym. Sci.,2001,79,1324-1335.
CrossRef - Raposo, M.F.D.J.; Morais, A.M.M.B.; Morais, R.; Drugs 2015, 13, 2967–3028.
CrossRef - Singh, R.P., Mathur, P.; Ind. J Env. Sci, 2005,9(1), 57-61.
CrossRef - Kodom, K., Onoyinka, A., Mkude, IT., Yeboah, J.O.; Int. J. Res. 2018,51,989-1007.
- Lata, G.; Georgr, BK.; Kannan, G.; Ninan, KN.; J. Appl. Polym. Sci. 2003 43(6)1159-1163.
CrossRef - Kepa, U.; Stanczyk, M E.; Stepniak, L.; Desalination 2008 233(1-3)187-193.
CrossRef - Diwani, Gel.; Rafie, SE.; Hawash, S.; J. Agri. Environ. Sci. 2009 6(1)119-128.
CrossRef - Diaz, B.; Hernan, L.; Morals, R.; Hernan, PB.; Ind. Eng. Chem. Res. 2009 48,1253-1258.
CrossRef - Kiezyk, PR.; Mackay, D.; J. Chem. Eng. 2009 49(6)747-752.
CrossRef - Garg, J., Seth, G.; Cur. World Env., 2006,1(2),139-144.
CrossRef - Sujata, M.; Sangchul H.; Sheldon QS.; Int. J Bio. Macromol., 2023, 226,368-382
CrossRef - Rios, P.; Hernan, J.; Lozano, J.; Sanchez, S.; Golinez, C; J. Chem. Eng. Data., 2010,5,605.
- Harper, TR.; Kingham, NW.; Water Environ. Res. 1992 4(3)200-203.
CrossRef - Rengaraj, S.; Yeon, KH.; Moon, SH.; J. Hazard. Mater. 2001 87(1)273-287.
CrossRef - Yurlova, L.; Kryvoruchko, A.; Kornilovich, B.; Desalination. 2002 144(1)255-260.
CrossRef - Benito, Y.; Ruiz, ML.; Desalination. 2002 142(3)229-234.
CrossRef - Singh, AV.; Kumawat, IK.; Polym Eng Sci, 2013,53: 546-554.
CrossRef - Rathore, M.; Singh AV.; Orient. J. Chem. 2023,39(1) 216-221.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









