Comparative Study of Solvent Effect for Mandelic Acid Oxidation by Different Oxidants
Bhawana Arora and Pallavi Mishra*
Department of Chemistry, J N V University, Jodhpur 342 005, India.
Corresponding Author E-mail: pallavianuk@gmail.com>
DOI : http://dx.doi.org/10.13005/ojc/390234
Article Received on : 17 Mar 2023
Article Accepted on : 17 Apr 2023
Article Published : 21 Apr 2023
Reviewed by: Dr. Subhojit Ghosh
Second Review by: Dr. D. Ilangeswaran
Final Approval by: Dr. S. A. Iqbal
The mandelic acid oxidation has been carried out by benzimidazolium fluorochromate (BIFC), morpholinium fluorochromate (MFC), benzimidazolium dichromate (BIDC), morpholinium chlorochromate (MCC) and tetraethylammonium chlorochromate (TEACC) using different solvents as dimethyl sulphoxide (DMSO), dimethyl formamide (DMF), chloroform (CF), acetone, dichloromethane (DCM) and 1,2-dichloroethane (DCE) at 308K, which results in the corresponding oxoacids formation. By using the TOPSIS method, we analysed the solvent effect and found the order by the solvent’s performance score for the reaction as DMSO>DMF>DCE>ACETONE>DCM>CF. The oxidation with all the oxidants resulted in the formation of corresponding oxo acid. The mechanism involved the construction of a cyclic chromate ester in a fast pre-equilibrium and then the decomposition of the ester in a subsequent slow step via a cyclic concerted symmetrical transition state leading to the formation of corresponding oxo acid in all the cases.
KEYWORDS:mandelic acid; Oxidants; TOPSIS method; solvents; solvent effect
Download this article as:| Copy the following to cite this article: Arora B, Mishra P. Comparative Study of Solvent Effect for Mandelic Acid Oxidation by Different Oxidants. Orient J Chem 2023;39(2). |
| Copy the following to cite this URL: Arora B, Mishra P. Comparative Study of Solvent Effect for Mandelic Acid Oxidation by Different Oxidants. Orient J Chem 2023;39(2). Available from: https://bit.ly/3HqeSDB |
Introduction
α- Hydroxy acids are chemical molecules that include hydroxyl and carboxylic groups. Some important members of α-hydroxy acids like glycolic acid, mandelic acid, lactic acid, malic acid, citric acid and tartaric acid are obtained from sugarcane, milk, apple, citrus fruits, grape wine and bitter almonds, respectively. These α-hydroxy acids and their derivatives have a large application area like biological, vital, cosmetic and organic synthesis. We have used mandelic acid for our oxidation study. Studying various organic compounds’ oxidation in non-aqueous environments is crucial in synthetic organic chemistry. For this study, many Cr (VI) derivatives have been reported1-5. The oxidation study of α-hydroxy acids by PBC6, PDC7, PCC8, IDC9, QCC10, PzCC11, MCC12, TEACC13,IFC14, PipCC15, MFC16, BIDC17 etc. has been reported in DMSO. We have studied oxidation reactions of mandelic acid by BIFC, MFC, BIDC, MCC and TEACC in 6 different solvents as dimethyl sulphoxide (DMSO), dimethyl formamide (DMF), chloroform (CF), acetone, dichloromethane (DCM) and 1,2-dichloroethane (DCE) at temperature 308K. α-Hydroxy acids either oxidised as alcohols, which results in the formation of corresponding oxoacids or undergo oxidative decarboxylation, which gives ketone. We have identified the best solvent under the different alternatives using the TOPSIS method18 and proposed a suitable mechanism.
Hwang and Yoon19 invented the TOPSIS approach, which Yoon20 subsequently enhanced. Hwang et al. 21 also published a new approach to multi-objective decision-making.
Material and Method
Material
We have used A. R. (analytical reagent) grade mandelic acid. The reported method was used to prepare BIFC22, MFC23, BIDC24, MCC25 and TEACC26 and their purity was determined using an iodometric approach. Solvents were purified using standard procedures27.
Kinetic Experiments
Pseudo, first-order constant, was obtained using a significant excess (*15 times or more) of the substrate over oxidants. For our research, we used six different solvents. The study of reactions was done at a constant temperature of 308K. The reaction mixtures were prepared by mixing the appropriate amount of solvent, substrate etc. and keeping them at a constant temperature in a thermostatic water bath. The reaction was started by introducing an oxidant solution previously stored in the water bath. The decrease in the concentration of oxidants was monitored spectrophotometrically. For carrying out our experiments, we have used the UV-Vis spectrophotometer model (AIMIL India model MK2) in which we have selected λmax 364 nm, 356 nm, 365 nm, 350 nm and 352 nm, respectively, for BIFC, MFC, BIDC, MCC and TEACC as these oxidants showed (absorbance) λmax at 364 nm, 356 nm, 365 nm, 350 nm and 352 nm.
Results and Discussion
We have done the oxidation of mandelic acid by different oxidants using six solvents, which results in the formation of corresponding oxoacids. The product is also confirmed by spectral analysis. FTIR spectra of mandelic acid oxidation by BIFC in DMSO solvent have been given, and similar results have been obtained with other oxidants and solvents. A suitable mechanism has been proposed which involves the formation of a chromate ester in a fast pre-equilibrium and then the decomposition of the ester in a subsequent slow step via a cyclic concerted symmetrical transition state leading to the formation of corresponding oxo acid in all the cases. A suitable mechanism for all the oxidants has been given. We have used the TOPSIS approach to compare the rate constants k2 in six solvents for specific oxidants to find out which solvent is best suited for the oxidation of mandelic acid.
 |
Graph 1: FTIR Spectrum of mandelic acid |
 |
Figure 2: FTIR Spectrum of corresponding oxyacids formed by oxidation of mandelic acid by BIFC in DMSO. |
Mechanism
 |
Scheme 1: Acid Dependent Path Mechanism 1 |
 |
Scheme 2: Acid Dependent Path Mechanism 2 |
Where B = BI-Benzimidazolium, M-Morpholinium, TEA-Tetraethylammonium
TOPSIS Study
Table 1: At 308 K decision matrix of rate constants (104 k2 s-1) for the mandelic acid oxidation by given oxidants
|
Oxidant/ Solvent |
BIFC |
MFC |
BIDC |
MCC |
TEACC |
|
DMSO |
62.9 |
72.4 |
52.4 |
74.6 |
68.2 |
|
DMF |
31.7 |
46.6 |
28.9 |
40.4 |
39.2 |
|
CF |
20.5 |
23.4 |
15.0 |
19.9 |
19.2 |
|
ACETONE |
22.8 |
25.1 |
16.3 |
24.1 |
25.8 |
|
DCM |
21.6 |
30.3 |
17.2 |
22.8 |
23.6 |
|
DCE |
23.8 |
27.8 |
19.1 |
26.5 |
28.9 |
 |
Figure 3: Decision matrix of the rate constants for mandelic acid oxidation by given oxidants |
Calculations of the Normalized Matrix
Calculations of the Normalized matrix Xij done by this formula:
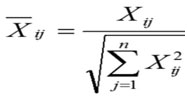
i = 1, 2,…, m
j = 1, 2,…, n
Table 2: Normalized matrix calculated using rate constants (104 k2 s-1) for the mandelic acid oxidation by given oxidants at 308 K
|
Oxidant/ |
BIFC |
MFC |
BIDC |
MCC |
TEACC |
|
DMSO |
0.0091 |
0.0070 |
0.0111 |
0.0079 |
0.0079 |
|
DMF |
0.0045 |
0.0045 |
0.0061 |
0.0043 |
0.0046 |
|
CF |
0.0029 |
0.0023 |
0.0032 |
0.0021 |
0.0022 |
|
ACETONE |
0.0033 |
0.0024 |
0.0034 |
0.0026 |
0.0029 |
|
DCM |
0.0031 |
0.0029 |
0.0036 |
0.0024 |
0.0027 |
|
DCE |
0.0034 |
0.0027 |
0.0040 |
0.0028 |
0.0034 |
Calculations of the weighted normalized matrix
Calculations of the weighted normalized matrix Vij done through this formula:
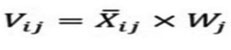
i = 1, 2,…, m
j = 1, 2,…, n
Table 3: Weighted normalized matrix calculated using normalized matrix for the mandelic acid oxidation by given oxidants at 308 K
|
Oxidant/ Solvent |
BIFC |
MFC |
BIDC |
MCC |
TEACC |
|
DMSO |
0.0018 |
0.0014 |
0.0022 |
0.0016 |
0.0016 |
|
DMF |
0.0009 |
0.0009 |
0.0012 |
0.0009 |
0.0009 |
|
CF |
0.0006 |
0.0005 |
0.0006 |
0.0004 |
0.0004 |
|
ACETONE |
0.0007 |
0.0005 |
0.0007 |
0.0005 |
0.0006 |
|
DCM |
0.0006 |
0.0006 |
0.0007 |
0.0005 |
0.0005 |
|
DCE |
0.0007 |
0.0005 |
0.0008 |
0.0006 |
0.0007 |
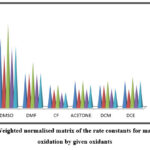 |
Figure 4: Weighted normalised matrix of the rate constants for mandelic acid oxidation by given oxidants |
oxidation by given oxidants
Calculations of the positive ideal and negative ideal values
Max Vij is V+ (positive ideal value), and Min Vij is V – (negative ideal value).
Positive Ideal Values V+:
|
0.0018 |
0.0014 |
0.0022 |
0.0016 |
0.0016 |
Negative Ideal Values V–:
|
0.0006 |
0.0005 |
0.0006 |
0.0004 |
0.0004 |
Calculations of the Euclidean distance from positive ideal values and negative ideal values
Calculations of the Euclidean distance from positive ideal values (Si+) done by this formula
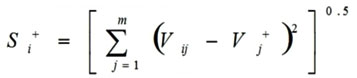
|
DMSO |
DMF |
CF |
ACETONE |
DCM |
DCE |
|
0 |
0.0017 |
0.0028 |
0.0025 |
0.0026 |
0.0024 |
Calculations of the Euclidean distance from negative ideal values (Si–) done by this formula:
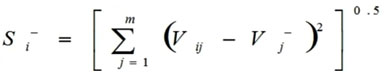
|
DMSO |
DMF |
CF |
ACETONE |
DCM |
DCE |
|
0.0028 |
0.0011 |
0 |
0.0003 |
0.0002 |
0.0004 |
Calculate the performance score
Calculations of Performance score Pi done as follows:
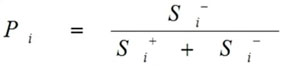
|
DMSO |
DMF |
CF |
ACETONE |
DCM |
DCE |
|
1 |
0.393 |
0 |
0.11 |
0.07 |
0.14 |
Table 4: Rank by the performance score of the solvents
|
Solvent |
Pi |
Rank |
|
DMSO |
1 |
1 |
|
DMF |
0.393 |
2 |
|
CF |
0 |
6 |
|
ACETONE |
0.11 |
4 |
|
DCM |
0.07 |
5 |
|
DCE |
0.14 |
3 |
 |
Figure 5: Rank by the performance score of the solvents |
Conclusion
In this study, we have done the oxidation of mandelic acid by different oxidants. The resultant product was corresponding oxoacids. The mechanism involved the formation of a cyclic chromate ester in a fast pre-equilibrium and then the decomposition of the ester in a subsequent slow step via a cyclic concerted symmetrical transition state leading to the formation of corresponding oxo acid in all the cases. The comparative study of the solvent effect is an essential concept of physical organic chemistry. The TOPSIS method is helpful for this study. This is the multiple criteria decision-making method. In this study, we have done the oxidation of mandelic acid by different oxidants and solvents and based on the TOPSIS method, the following order by the performance score of the solvents for the reaction- DMSO>DMF>DCE>ACETONE>DCM>CFwas obtained. Thus it was concluded that the oxidation of mandelic acid by different oxidants gives the best results when DMSO is used as a solvent.
Acknowledgement
We are thankful to HOD, Chemistry, Jai Narain Vyas University, Jodhpur, India, for providing us with valuable lab facilities to conduct our research work.
Conflict of Interest
The authors declare no conflict of interest in the present work.
References
- Corey, E.J.; Suggs W.J., Tetrahedron Lett, 1975, 16(31),2647-2650.
- Bhattacharya, M.N.; Chaudhuri, M.K.; Dasgupta, H.S.; Roy, N.; Kathing, D.T., Synthesis, 1982, 1982(07), 588-590.
- Balasubramanian, K.; Prathiba, V., Indian J. Chem., 1986, 25B, 326-327.
- Li, M.; Johnson, M.E., Synth Commun, 1995, 25, 533-537.
- Pandurangan, A.; Murugesan, V.; Palamichamy, P., J. Indian Chem. Soc., 1995, 72, 479.
- Aparna, P.; Kothari, S.; Banerji, K.K., J. Chem. Res., V, 1994, 367-367.
- Sundaram, S.M.; Amutha, M.; Sockalingam, R.M., Chem. & Env. Res., 1996, 5,229-234.
- Sethumathavan, D.; Sankaran, K.R., Oxid. Commun, 1997, 20, 587-591.
- Meenakshisundaram, S.; Sathiyendiran, V., Oxid. Commun., 1998, 21, 71-76.
- Gurumurthy, R.; Karthikeyan, B., Orient. J. Chem., 1998, 14, 435-437.
- Sekar, K.G.; Prabakaran, A., Oxid. Commun., 2008, 31, 879-884.
- Malani, N.; Pohani, S.; Baghmar, M.; Sharma, P.K., Ind. J. Chem., 2008, 47A, 1373-1376.
- Swami, P.; Yajurvedi, D.; Mishra P.; Sharma, P.K., Int. J. Chem. Kinet., 2009, 1-6.
- Sekar, K.G.; Anbarasu, K., Oxid. Commun., 2013, 36, 979-983.
- Anbarasu, K.; Selvi, P., Rasayan J. Chem., 2014, 7, 365-369.
- Purohit, V.; Mishra, P., Int. J. Innov. Res. Rev., 2018, 6(1),27-37.
- Pandey, D.; Kachawa, T.; Kothari, S., Prog. React. Kinet. Mech., 2018, 43(3-4), 300-314.
- Rao, A.; Kumar, G., J. Appl. Chem., 2019, 8(4), 1798-1804.
- Hwang, C.L.; Yoon, K., Multiple Attribute Decision Making: Methods and Applications, New York, Springer-Verlag, 1981.
- Yoon, K., Journal of Operational Research Society, 1987, 38(3), 277-286.
- Hwang, C.L.; Lai, Y.J.; Liu, T.Y., Computer and Operational research, 1993, 20, 889-899.
- Sivamurugan, V.; Rajkumar, G.A.; Arabindoo, B.; Murugesan, V., Ind. J. Chem., 2005, 44B, 144-147.
- Zahra, S.; Alangi, S.; Ghotbabadi, H.S.; Baei, M.T.; Naderi, S., E-J. Chem, 2011, 8(2), 815-818.
- Meng, Q.H.; Feng, J.C.; Bian, N.S.; Liu, B.; Li, C.C., Syn. Commun., 1998, 28(6), 1097-1102.
- Sheikh, H.N.; Sharma, M.; Hussain, A.; Kalsotra, B.L., Oxid. Commun., 2005, 28(4), 887-893.
- Pandurangan, A.; Murugesan, V., J. Indian Chem. Soc., 1996, 73, 484-486.
- Perrin, D.D.; Armarego, W.L.; Perrin, D.R.; Pergamon Press, Oxford, 1966.

This work is licensed under a Creative Commons Attribution 4.0 International License.









