The Effect of Chitosan and Glycerol Mixture on Improving Biodegradable Plastic Properties of Young Coconut Husk (Cocos Nucifera L.)
Z. Muchtar* , S. A. Sari
, S. A. Sari , S. Rahmah
, S. Rahmah , M. Zubir
, M. Zubir and G. E. Sarumaha
and G. E. Sarumaha
Chemistry Department, Faculty of Mathematics and Natural Science, Universitas Negeri Medan, Jl. Willem Iskandar, Pasar V, Medan Estate, 2022 - North Sumatera, Indonesia.
Corresponding Author E-mail: zmuchtar@unimed.ac.id
DOI : http://dx.doi.org/10.13005/ojc/390111
Article Received on : 12 Nov 2022
Article Accepted on :
Article Published : 23 Jan 2023
Reviewed by: Dr. Kansiri Pakkethati
Second Review by: Dr. N. K. Geetha
Final Approval by: Dr. Ioana Stanciu
Biodegradable plastics were synthesized from young coconut husk which taken from Medan Area district, Medan city, North Sumatera, Indonesia, and modified by increasing the mixtures of chitosan (C) and glycerol (G) with ratios of C:G and G:C as 1:1, 1:3, and 1:5. The increase of glycerol produces a thin plastic of 1.04 mm with a water resistance of 24.48%. It also produces a flexible plastic with an elongation of 96.89% and a lower tensile strength of 0.48 Pa. Meanwhile, the increase of chitosan results in a thicker plastic of 8.2 mm with a water resistance of 74.26% and tensile strength of 3.85 Pa, albeit with a lower elongation of 12.21%. The process of plastic degradation was observed for 15 days; the plastic with high glycerol content characterised an increased degradation percentage of 96.07%, while those with high chitosan content had a degradation percentage of 52.45%. The increasing amount of glycerol in the mixture results in a thinner and more flexible biodegradable plastic that decomposes easily in the soil.
KEYWORDS:Biodegradable; Chitosan; Glycerol; Plastic; Young coconut husk
Download this article as:| Copy the following to cite this article: Muchtar Z, Sari S. A, Rahmah S, Zubir M, Sarumaha G. E. The Effect of Chitosan and Glycerol Mixture on Improving Biodegradable Plastic Properties of Young Coconut Husk (Cocos Nucifera L.). Orient J Chem 2023;39(1). |
| Copy the following to cite this URL: Muchtar Z, Sari S. A, Rahmah S, Zubir M, Sarumaha G. E. The Effect of Chitosan and Glycerol Mixture on Improving Biodegradable Plastic Properties of Young Coconut Husk (Cocos Nucifera L.). Orient J Chem 2023;39(1). Available from: https://bit.ly/3H2hFli |
Introduction
Plastic is one of the most used polymer products in daily life while polypropylene is widely used for its heat and moisture resistance and excellent dimensional stability. However, as a synthetic polymer, it is difficult to decompose when discharged into the environment. Thus, decomposition takes place within tens or hundreds of years, also producing carbon emissions when burned. Burning plastic also produces methane gas, which contributes to an increase in the greenhouse effect.1-4
Biodegradable plastics characterize a solution to reduce plastic waste in the environment. They contain organic monomers (such as starch, cellulose, protein and others) that are more easily and quickly decomposed by soil microorganisms than synthetic polymers. Thus, biodegradable plastic is more environmentally friendly and does not harm living things.5-7
Cellulose is one of the organic polymers that are biocompatible, easily decomposed, non-toxic, and renewable. One of the derivative products of cellulose is carboxymethyl cellulose (CMC), which is used as a binder, gelling agent, and stabilizer in various products such as detergents, food, paper, textiles, pharmaceuticals, and paints.8,9 Based on its properties, CMC has been used as a source of monomers to produce environmentally friendly and non-toxic biodegradable plastics.10,11
Young coconuts (Cocos nucifera. L) that contain 28% cellulose can contribute to the manufacture of biodegradable plastics.12-15 Many young coconuts are obtained in Indonesia, an agricultural country that is known for producing coconuts. Generally, only the meat and the water of the coconut are used commercially, with the other parts, including the husk, being reduced to waste. The composition of the coconut husk is 35% of the fruit’s total weight, and most of the coconut waste is encompassed by the husk.16-19
Plasticizers are necessary for producing biodegradable plastics that are waterproof, elastic and flexible.20,21 It improves the physical and mechanical properties of biodegradable plastics and protects against microorganisms that may damage them.22-25 In this study, glycerol and chitosan are added to increase the mechanical strength of biodegradable plastics created from the young coconut husk fibre, CMC. It is modified by increasing the mixtures of chitosan (C) and glycerol (G) with variations of C:G and G:C being 1:1, 1:3, and 1:5.
Materials and Methods
Materials
In this study, the material used as a source of cellulose was young coconut fiber taken from the Medan Area district, Medan City, North Sumatera, Indonesia. Young coconut husks are washed and then dried in the sun and then the half-dried coir is separated and dried again until dry. The dry young coconut husk is then ground and sifted sieve 60 mesh.
Synthesis of Alfa Cellulose
A mixture of 1L of 3.5% HNO3 solution and 10 mg NaNO2 was added to 75 g of coconut husk powder. The mixture was heated at 90°C for 2 hours before filtering out and washing the residue until the pH of the filtrate was neutral (pH). Subsequently, it was digested using 750 mL of a solution containing 2% NaOH and 2% Na2SO3, heated at 50°C for 1 hour before filtering out and washing the reside until the pH of the filtrate was neutral. Then, it was bleached with 250 mL of 1.75% NaOCl solution at 70°C for 30 minutes. The residue is filtered and washed until its pH was neutral. Purified α-cellulose was formed with 500 mL of 17.5% NaOH solution at 80°C for 0.5 hours before the residue was filtered and washed until its pH was neutral. This is followed by bleaching with 10% H2O2 at 60°C before filtering and washing the α-cellulose obtained, which is then dried in an oven at 60°C before being characterized by FT-IR.
Synthesis of Carboxymethyl Cellulose (CMC)
A total of 5 g of α-cellulose powder was mixed with 150 mL of isopropanol solution and 15 mL of 25% NaOH solution and stirred for 1 hour. After this, 6 g of sodium mono-chloroacetate was added to the mixture, which is stirred for 1.5 hours and heated at 55˚C for 3.5 hours. After heating, the solution was soaked with 100 mL of methanol for 24 hours. Then, the solution was neutralized with 90% (v/v) acetic acid. The residue was filtered and washed thrice using 70% (v/v) ethanol (200 mL) for 10 minutes to remove contaminants. Then, the final product obtained was washed with methanol (100 mL), dried at 55˚C for 18 hours and characterized as the CMC powder by FT-IR.
Synthesis of Biodegradable Plastic by Increasing the Glycerol Ratio
Initially, 3 g of CMC were dissolved in 100 mL of distilled water and heated at 80°C for 10 minutes. In another glass, 3 g of chitosan were dissolved in 1% of acetic acid, heated and stirred at 80°C for 10 minutes. The CMC-chitosan solution was mixed with 3 g of glycerol. The mixture was homogenized for 15 minutes, heated again for 7 minutes at 80ºC, and then cooled at 20-25°C. Then 30 ml of the mixture was poured into a 15 cm x 5 cm glass mould and maintained at 25°C. The same treatment was carried out for the increasing ratio of glycerol (C:G), which are 1:3 and 1:5. Finally, the plastic sheet was characterized by tensile strength, elongation, thickness, water resistance, and the biodegradation process was observed in the soil for 15 days.
Synthesis of Biodegradable Plastic by Increasing the Chitosan Ratio
Initially, 3 g of CMC were dissolved in 100 mL of distilled water and heated at 80°C for 10 minutes. In another glass, 3 g of glycerol were dissolved in 1% acetic acid, heated and stirred at 80°C for 10 minutes. The solutions are mixed and homogenized for 15 minutes and heated again for 7 minutes at 80ºC. The solution was cooled at 20-25°C before pouring 30 ml of the mixture into a 15 cm x 5 cm glass mold and maintained at 25°C. The same treatment was carried out for the increasing ratios of chitosan (G:C), which are 1:3, and 1:5. Finally, the tensile strength, elongation, thickness, and water resistance of the plastic sheet were recorded. The biodegradation process was observed in the soil for 15 days.
Results and Discussion
FTIR Characterization
The purpose of adding HNO3 and NaNO2 is to remove the nitro-lignin from the young coconut husk. A brown pulp is produced in a process termed delignification before the swelling process, which occurred with the addition of NaOH and Na2SO3, though this still resulted in a brown pulp. In this process, hemicellulose, mineral salts, and ash are lost. Because the colour of the pulp is still brown, the bleaching process is carried out by adding NaOCl solution. Brownish-yellow pulp is produced through this process. Through the process of delignification, swelling process and bleaching, the product that is produced is α, β, and γ cellulose.12,26 Therefore, to separate α-cellulose, NaOH was added to the pulp. Then, α-cellulose was precipitated while β and γ celluloses were dissolved. The α-cellulose product obtained is brownish yellow. Hence, it was bleached with 10% H2O2 solution and dried in the oven for 24 h at a temperature of 60°C. The α-cellulose product obtained is 12.1 g, derived from 75 g of young coconut husk powder or 16.14% of the initial weight.
 |
Figure 1: FTIR Spectrum of Young Coconut Husk α-cellulose and Commercial α-cellulose. |
To determine whether the alpha-cellulose was successfully synthesized, FTIR was carried out by comparing samples of young coconut husk alpha-cellulose with commercial alpha-cellulose (Figure 1). No significant differences were found between the two. The synthesis of carboxymethyl cellulose (CMC) from the young coconut husk started with the alkalization process of young coconut husk α-cellulose by adding NaOH and isopropyl alcohol. The addition of NaOH solution activates the –OH groups on the α-cellulose so that its structure expands and facilitates the process for the next step, while isopropyl alcohol is a medium that homogenizes the carboxymethylation reaction. The next step is carboxymethylation with sodium monochloroacetate; herein, the carboxylate group attaches to the cellulose. This process was neutralized with 90% acetic acid solution as the previous reaction occurred in an alkaline environment and was washed with ethanol and methanol to remove various impurities. The CMC product obtained is 3.069 g, derived from 5 g of young coconut husk α-cellulose or 61.38% of the initial weight.
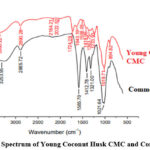 |
Figure 2: FTIR Spectrum of Young Coconut Husk CMC and Commercial CMC |
The FTIR spectra of Young Coconut Husk CMC and Commercial CMC demonstrate several wave numbers, indicating the identification of functional groups in both CMCs (Figure 2). In general, the characteristic feature of the CMC spectra is at 1500 cm-1, which indicates the carbonyl group (-C=O), and at 1400 cm-1, indicating the –CH2 bond of the carboxymethyl constituent. The two spectra contain a number pattern, which indicates the presence of the CMC functional group.
XRD Characterization
The biodegradable plastic products have a brownish-yellow colour and a sharp, sour smell that comes from the acetic acid solution. XRD analysis was carried out to observe changes in the CMC structure due to the addition of glycerol and chitosan in the synthesis of biodegradable plastics (Figure 3). The bioplastic was successfully synthesized from a mixture of CMC waste from young coconut shells with various ratios of chitosan and glycerol. Bioplastic made from the young coconut CMC with the addition of a mixture of chitosan and glycerol (1:1), showed an increase in the intensity of 2 thetas at around 43.9o and 64.8o when compared to alpha-cellulose and CMC. Increasing the concentration of chitosan and glycerol decreased the intensity of the 2 thetas as well as their crystallinity properties at both G:C = 1:5 (Figure 3a) and C : G = 1:5 (Figure 3b). This significant change in intensity indicates the inclusion of chitosan and glycerol in the CMC structure.
EXPO 2014 showed that there was no change in the crystal system, which is triclinic, although there was a slight change in the bond length, bond angle, and unit cell volume. Analysis using EXPO showed no difference in the crystal system of alpha-cellulose, CMC, and bioplastics. However, the changes that occur are not significant and do not affect the crystal system.
 |
Figure 3: XRD patterns of alpha-cellulose, CMC and a mixture of Glycerol and Chitosan, where (A) represents the increasing concentration of chitosan and (B) represents the increase in glycerol concentration. |
SEM Characterization
SEM images were also observed to consider changes in the surface and morphology of the bioplastic when the amount of chitosan and glycerol are increased in the mixture (Figure 4).
 |
Figure 4: SEM image of a mixture of Glycerol and Chitosan bioplastic. |
Increasing the amount of glycerol makes the surface of the bioplastic more slippery and homogeneous since glycerol is more easily dissolved in CMC than chitosan.26,27 Meanwhile, the increasing amount of chitosan in the mixture with glycerol causes a rougher and low homogeneous surface due to the poor solubility of chitosan in CMC.
 |
Figure 5: Thickness of Biodegradable Plastic in Various Compositions of Chitosan and Glycerol |
The thickness of each plastic was measured. Although it showed an uneven and non-homogeneous surface, the bioplastic made with the addition of chitosan to a mixture of chitosan and glycerol resulted in a thicker plastic with the greatest thickness in the mixture G:C = 1:5, which is 8.2 mm. The difficulty of dissolving chitosan in CMC makes the bioplastic clump together, resulting in increased thickness. Meanwhile, bioplastics made by increasing the amount of glycerol to a ratio of 1:5 reduce its thickness to only 1.04 mm thick, compared to the ratio of the same amount of chitosan and 1.61 mm thick glycerol.
The thickness of the biodegradable plastic was measured using a screw micrometers. Figure 5 shows that an increase in the amount of glycerol will reduce the thickness of the bioplastic. The molecular bonds are reduced so that when heating biodegradable plastics, water will be lost and biodegradable plastics with a small thickness are produced. Meanwhile, the addition of chitosan increases the thickness of biodegradable plastics significantly due to increased molecular bonding. 30,31
The interactions that occur in the manufacture of this biodegradable plastics are made possible by the hydrogen bonds formed between the -OH groups of cellulose and the -NH2 groups of chitosan. The large number of -OH groups in cellulose also allows hydrogen bonds to form with -OH groups in glycerol. This bioplastic structure is also strengthened by the presence of -OH hydrogen bonds in glycerol with -OH groups in chitosan, as shown in Figure 6. 28-30
 |
Figure 6: Interaction scheme between cellulose, glycerol and chitosan. |
Biodegradable Plastic properties
The properties of biodegradable plastics were measured using a tensile test to evaluate the maximum force they could resist until it breaks. Tensile strength performance (Figure 7) shows that increasing the amount of chitosan can enhance the tensile strength in biodegradable plastics with a variation of G:C = 1:5, which is 3.85 Pa when compared with the same ratio of chitosan and glycerol (1:1), which is 1.53 Pa. The increasing chitosan increases the number of hydrogen bonds, thereby strengthening the intermolecular bonds of biodegradable plastics.22,26 Meanwhile, the biodegradable plastic made by increasing the amount of glycerol, although producing a more homogenous yet thin bioplastic, demonstrated a reduction in tensile strength (C:G = 1:5) to 0.48 Pa.
 |
Figure 7: Tensile Strength (left) and Percentage of Elongation (right) of Biodegradable Plastic in Various Compositions of Chitosan and Glycerol |
Further, Figure 7 also shows the elongation properties of all bioplastics. Elongation determines how far the biodegradable plastic stretches before it breaks. The greatest elongation was shown by bioplastics made with a ratio of C:G = 1:5, which was 96.89%, while the increase of chitosan, G:C = 1:5, only produced a bioplastic with an elongation of 12.21%, which is lower than the ratio of chitosan and glycerol (1:1), which is 33.03%. The percentage of elongation is enhanced by the decrease in chitosan, (the trend is opposite with tensile strength) because glycerol reduces hydrogen interactions between chitosan and CMC, causing hydrogen interactions between chitosan-glycerol and glycerol-CMC to produce the more elastic biodegradable plastics.
The biodegradable plastic has good water resistance if it absorbs a little water. Increasing water absorption will enhance the solubility of the biodegradable plastics, which would make them easy to break. The addition of more glycerol causes biodegradable plastics to absorb a significant amount of water, as glycerol is hydrophilic and has a large number of –OH groups, such that it binds to water through hydrogen bonding. As chitosan is hydrophobic and does not dissolve in water, the water resistance increases with a water resistance value of 74.27% and swelling of 25.73% (Figure 8).
 |
Figure 8: Swelling (left, solid line) and Water Resistance (right, dashed line) of Biodegradable Plastic in Various Compositions of Chitosan and Glycerol |
Biodegradable plastics with a size of 1 cm x 1 cm are planted in the soil for 15 days. Every 5 days, the biodegradable plastics were weighed by cleaning the specimen first using alcohol to remove bacteria attached to the sample. The plastic degradation process was observed for 15 days. Plastics with high glycerol have an increased degradation percentage of 96.07%, while plastics with high amounts of chitosan have low biodegradation, which is represented by 52.45% (Figure 9). This indicates the hydrophilic nature of glycerol, which binds to the water contained in the land.
 |
Figure 9: Degradation Profile of Biodegradable Plastic with Various Compositions of Glycerol (A) and Chitosan (B) over 15 days |
Conclusion
Glycerol and chitosan affect the physical characteristics of the biodegradable plastics produced in terms of tensile strength, elongation percentage, thickness, water resistance, swelling and the process of decomposition in the soil. The increasing amount of glycerol in the mixtures of chitosan and glycerol produces a thinner plastic with lower water resistance, although they are flexible and decompose easily in the soil. Meanwhile, plastics with a high amount of chitosan have a greater thickness, higher water resistance, lower flexibility and longer durations for decomposition. Meanwhile, plastics with a high amount of chitosan are thicker, and highly resistant to water, but have low flexibility and soil degradation capabilities.
Acknowledgment
The authors acknowledge to Chemistry and Physics Laboratory of Universitas Negeri Medan and LPPM Universitas Negeri medan which financially fully supported by the Universitas Negeri Medan Non-Tax Revenue Fund (PNBP) funding with a reference number is 0201/UN33.8/PL-PNBP/2021.
Conflict of Interest
The authors declare that we do not have any conflicting interest.
References
- Thompson, R.C.; Moore, C.J.; Saal, F.S.; Swan, S.H., Philos. Trans. Roy. Soc. B., 2009, 364, 1973-1976. doi : 10.1098/rstb.2009.0054
CrossRef - Evode, N.; Qamar, S.A.; Bilal, M.; Hafiz, D.B.; Iqbal M.N., Case Stud. in Chem. and Environ. Engin., 2021, 4, 100142, 1-8. doi : 10.1016/j.cscee.2021.100142
CrossRef - Li, W.; Qamar, S.A.; Qamar, M.; Basharat, A.; Bilal M.; Iqbal H.M., Int. J. Biol. Macromol., 2021, 1077, 1-9. doi ; 10.1016/j.nexus.2022.100077
- Benson, N.U.; Fred-Ahmadu, O.H.; Bassey, D.E.; Atayero, A.A., J. of Environ. Chem. Engin., 2021, 9(3), . Doi : 10.1016%2Fj.jece.2021.105222
CrossRef - Vollmer, M.J.; Jenks, M.C.; Roelands, R.J.; White, T.; van Harmelen, P; de Wild, B. M.; Weckhuysen, Angew. Chem. Internat. Educ., 2020, 59(36), 15402. doi : 10.1002/anie.201915651
CrossRef - Sonawane, Y.B.; Shindikar, M.R.; Khaladkar, M.Y.; Internat. J. of Innov. Res. in Sci., Engin. and Tech., 2014, 3(9), 15903-15908. doi : 10.15680/IJIRSET.2014.0309016
CrossRef - Pannetier, P.; Morin, B.; Cl ́erandeau, C.; Laurent, J.; Chapelle, C.; Cachot, J. Environ. Pollut., 2019, 248, 1098-1107. doi : https://doi.org/10.1016/j.envpol.2018.10.129
CrossRef - Adam, I.; Walker, T.R.; Bezerra, J.C.; Clayton A., Mar. Pol. 2020, 116, 103928, https://doi.org/10.1016/j.marpol.2020.103928
CrossRef - Andrady, A.L.; Neal, M.A., Phil. Trans. of the Roy. Soc. B: Biol. Sci., 2009, 364(1526), 1977. doi : 10.1098/rstb.2008.0304
CrossRef - Barboza, L.G.A.; Vethaak, A.D.; Lavorante, B.R.B.O.; Lundebye, A.K.; Guilhermino, L., Mar. Pollut. Bull., 2018, 133, 336-348. doi : 10.1016/j.marpolbul.2018.05.047
CrossRef - Andersson, J.F.; Haarr, M.L.; Havas, V., Sci. of The Tot. Environ., 2020, 745, 141117. doi : 10.1016/j.scitotenv.2020.141117
CrossRef - Brennecke, D.; Duarte, B.; Paiva, F.; Caçador, I.; Clode, J.C.; Estuarine Coastal Shelf Sci, 2016, 178, 189-195. doi : 10.1016/j.ecss.2015.12.003
CrossRef - Qamar, S.A.; Qamar, M.; Bilal, M.; Bharagava, R.N.; Ferreira, L.F.R.; Sher, F.; Iqbal, H. M., Int. J. Biol. Macromol., 2021, 185, 1-19. doi : 10.1016/j.ijbiomac.2021.06.079
CrossRef - Bharath, K. N.; Madhu, P.; Sanjay, M.R.; Basavarajappa, S.; Suchart, S.; Alexey, K.; Gorbatyuk, S., Polym. Compos., 2022, 43(4), 1985-1995. doi : 10.1002/pc.26513
CrossRef - Qamar, S.A.; Asgher, M.; Bilal, M., Wste and BioM. Valor., 2021, 12, 6487-6459. doi : 10.1007/s12649-021-01479-x
CrossRef - Sonawane, Y.B.; Shindikar, M.R.; Khaladkar, M.Y., Int. J. of Innov. Res. in Sci., Engin. and Tech., 2014, 3(9), 15903-15908. doi : 10.15680/IJIRSET.2014.0309016
CrossRef - Gallo, F.; Fossi, C.; Weber, R.; Santillo, D.; Sousa, J.; Ingram, I.; Nadal, A.; Romano, D.; Environ. Sci. Eur. , 2018, 30 (1), 13. doi : 10.1186%2Fs12302-018-0139-z
CrossRef - Lebreton, L.C.M.; Van der Zwet, J.; Damsteeg, J.W.; Slat B.; Andrady A.; Reisser J.; Nat. Commun., 2017, 8(1), 1-10. doi : 10.1038/ncomms15611
CrossRef - Thushari, G.G.N.; Senevirathna, J.D.M.; Heliyon., 2020, 6(8), 1-16. doi : 10.1016/j.heliyon.2020.e04709
CrossRef - Gu, J.D., Environ. Sci. Pollut. Ctrl. Ser. 2021, 28(2), 1278-1282. doi : 10.1007/s11356-020-11501-9
CrossRef - Block, C.; Van Caneghem J.; Van Brecht A.; Wauters G.; Vandecasteele C., Wste and BioM. Valor., 2015, 6(2), 137-145. doi : 10.1007/s12649-014-9334-3
CrossRef - Luo, H.; Zhao, Y.; Li, Y.; Xiang, Y.; He ,D., Pan, X., Sci. of The Tot. Environ., 2020, 714, 136862. doi : 10.1016/j.scitotenv.2020.136862
CrossRef - Vanapalli, K.R.; Sharma, H.B.; Ranjan, V.P.; Samal, B.; Bhattacharya, J.; Dubey, B.K.; Goel, S., Sci. Tot. Environ., 2021, 750, 1-20. doi : 10.1016%2Fj.scitotenv.2020.141514
CrossRef - Thiounn, T., Smith, R.C., J. Polym. Sci., 2020, 58(10), 1347-1364. doi : 10.1002/pol.20190261
CrossRef - Miandad, R., Barakat, M.A., Aburiazaiza, A.S., Rehan, M., Nizami, A.S., Proc. Saf. Environ. Prot., 2016, 102, 822-838. doi : 10.1016/j.psep.2016.06.022
CrossRef - Asgher, M., Nasir, I., Khalid, N., Qamar, S.A., J. Polym. Res., 2020, 27 (11), 347. doi :10.1007/s10965-020-02314-y
CrossRef - Omoniyi, A; Babalola; Abel, O. Olorunnisola, Mater. Res. Proceed. 2019 11, 195-200. doi : https://doi.org/10.21741/9781644900178-14
CrossRef - Tavaria, F. K.; Soares, J. C.; Reis, I. L.; Paulo, M. H.; Malcata, F. X.; Pintado, M. E. J. Appl. Microbiol., 2012, 112(5), 1034–1041. doi : 10.1016/j.fm.2008.05.003
CrossRef - Tikhonov, V. E.; Stepnova, E. A.; Babak, V. G. Carbohydrat. Polym., 2006, 64(1), 66–72. doi:10.1016/j.carbpol.2005.10.021
CrossRef - Kim, K. W.; Min, B. J.; Kim, Y. T.; Kimmel, R. M.; Cooksey, K.; Park, S. I. Food Sci. Technol., 2011, 44(2), 565–569. doi :10.1016/j.lwt.2010.08.001
CrossRef - Singh, B. R. Medicine., 2013, 1, 1-3. Saegeman, V. S. M.; Ectors, N. L.; Lismont, D.; Verduyckt, B.; Verhaegen, J. Burns., 2008, 34, 205-211
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









