Syngonium podophyllum-based ZnO/Ag Nanocomposites: Biogenic Synthesis and Antimicrobial Activity against Bacterial Isolates and Saccharomyces cerevisiae
1Department of Applied Sciences and Humanities, Faculty of Engineering and Technology, Jamia Millia Islamia (A Central University), New Delhi-110025, India.
2Department of Biosciences, Jamia Millia Islamia, (A Central University), New Delhi-110025, India.
Corresponding Author E-mail: wsiddiqui@jmi.ac.in
DOI : http://dx.doi.org/10.13005/ojc/390109
Article Received on : 18 Jan 2023
Article Accepted on : 18 Feb 2023
Article Published : 21 Feb 2023
Reviewed by: Dr. Anil Gambhire
Second Review by: Dr. E. Kumar
Final Approval by: Dr. S.A. Iqbal
There is a growing need for engineers to develop effective, powerful, and economical nanocomposites with effective antimicrobial effects. The co-precipitation method was used to fabricate Zinc oxide/Silver nanocomposites (ZnO/Ag NCs). FTIR analysis confirms the presence of a functional group responsible for the stabilization and reduction of fabricated NCs. TEM and SEM analysis showed the internal morphology of the ZnO/Ag NCs as nearly spherical and the average crystallite size of NCs 16.64nm. The NCs are polycrystalline, as demonstrated by the selected area electron diffraction (SAED) pattern. The NCs show good antimicrobial action against Prokaryotic and Eukaryotic organisms. The Minimum inhibitory concentration (MIC) of the NCs are 12.50 and 50µg/mL for E.coli and S. aureus respectively. The finding reveals, ZnO/Ag is found to be an efficient combat agent against prokaryotic and eukaryotic organisms and may be employed as an antimicrobial in the future.
KEYWORDS:Antibacterial activity; Nanocomposites; Minimum Inhibitory concentration; ZnO/Ag
Download this article as:| Copy the following to cite this article: Naaz R, Siddiqi W. A, Mohsin M.Syngonium podophyllum-based ZnO/Ag Nanocomposites: Biogenic Synthesis and Antimicrobial Activity against Bacterial Isolates and Saccharomyces cerevisiae. Orient J Chem 2023;39(1). |
| Copy the following to cite this URL: Naaz R, Siddiqi W. A, Mohsin M.Syngonium podophyllum-based ZnO/Ag Nanocomposites: Biogenic Synthesis and Antimicrobial Activity against Bacterial Isolates and Saccharomyces cerevisiae. Orient J Chem 2023;39(1). Available from: https://bit.ly/3IOF0ZX |
Introduction
NCs that comprise two or more various components have been fabricated and have become increasingly popular in recent decades. Due to the fact that their special multifunctional nano-assembled system concurrently displays fresh and improved features, nanoscale materials have gained tremendous popularity as advanced nanomaterials for various applications, including biomedicine, catalysis, electronics, and photonics. 1 There has been a long history of using metals as antimicrobial agents in ancient times. To treat bacterial diseases, a vast range of medications have been developed, including silver, copper, zinc, and arsenic. Oxidative stress kills pathogens in eukaryotic cells generated by zinc, silver, and copper, which are associated with the process.2 There has been a recent increase in microbial resistance to conventional anti-bacterial drugs due to their widespread use. Globally, the majority of population deaths are going to be primary cause by this situation and it is approaching a perilous level. besides, designing and creating new antibiotics have been slowed by financial constraints, existing regulatory obstacles and scientific impediments. 3 Under its stability and antibacterial activity, nanotechnology-based antibacterial approaches have gained much attention as novel strategies for eradicating bacteria. They supply antibacterial advantages through the unique shape, size, and structure of their material. Some nanomaterials are inherently incapable of killing germs. Although, Due to their form, size, and surface charge, silver nanoparticles are able to affect the integrity of bacterial cell membranes, respiration, and ATP production.4 A nanoscale particle size comparable to that of the microorganisms that live inside the body and environment has rendered nano-ZnO an extraordinary material with marvelous antibacterial activity. It can therefore enter the cell wall of bacteria and damage multiple parts of their structure, including their DNA and proteins. in addition, Larger surface area, surface charge density, shape, crystal flaws, and optical characteristics are also thought to contribute to ZnO antibacterial properties. 5
The antibacterial properties of both Ag and ZnO particles appear satisfactory, but they agglomerate when used individually, reducing their effectiveness. By using noble metals like silver, ZnO chemical stability and antibacterial capabilities can be improved (Ag). Various nano ZnO/Ag preparation techniques have been reported in recent years, including photocatalysis 6, photothermal/ photodynamic therapy 4, and antimicrobial activity. 7–6
This study presents a method for preparing zinc oxide/silver nanocomposites (ZnO/Ag NCs) by co-precipitation. Nanocomposites were characterized by understanding their morphology and structure. The investigation was conducted using a well diffusion approach for antimicrobial properties of the synthesized samples. Antibacterial activity of ZnO is improved by surface modification with Ag nanoparticles.
Experimental Details
Reagents
zinc sulphate heptahydrate (ZnSO4.7H2O) (CAS 7447-20-0) were brought from Merck chemicals. silver nitrate (AgNO3) (CAS 7761-88-8) was used as a silver source, sodium hydroxide (NaOH) (CAS 1310-73-2) was used to keep the basic pH, all chemicals used were high purity grade and bought from Sigma Aldrich. Muller Hinton Agar, Yeast extract peptone dextrose, and Luria broth media were obtained from Hi Media. Double-deionized water was used in all experiments, along with autoclaved borosilicate glassware. Whatman paper grade 1 from GE Healthcare Wipro Pvt. Ltd. filtered the sample.
Method of synthesispreparation of Syngonium podophyllum aqueous extract
Using distilled water and tap water, Syngonium podophyllum leaves were rinsed four times for complete removal of dirt or dust particles and semi-air dried to remove the external moisture of the leaves before chopping the leaves into small pieces. The leaves weighing 25gm were heated on a Soxhlet apparatus at 50°C for 30 minutes after being suspended in 250 ml of double-distilled water in a flask. Mixture was set aside to cool down at room temperature before filtration by Whatman filter paper no.1 to eliminate the solid residues of the extract. After that, the mixture was used, and the remaining was stayed at 4°C for later work.
Formulation of ZnO NPs and other nanocomposites
For nanoparticle synthesis: 100 mL of double-distilled water were used to prepare 1mM of ZnSO4.7H2O and AgNO3. The prepared sample was reduced with arrowhead leaf extract to form Zinc Oxide and silver nanoparticles in the ratio of 1:2 respectively.
Fabrication of ZnO/Ag binary nanocomposite: These reduced Ag metal nanoparticles were further poured into the matrix of synthesized ZnO while stirring at 10000rpm at 80°C for 1h. After that, the pH was raised to 8 by adding dropwise, 5 M NaOH solution. Room temperature was used to rest the solutions for 18 hours. To confiscate impurities, ethanol followed by distilled water was added to the solution and was centrifugated at 7000 rpm for 8 min, After every wash, the supernatant was removed until pH comes to neutral. The pellet obtained was dried at 50°C. The ultimate greyish-black powder was mashed in mortar and pestle and subjected to characterization for confirmation of the formed sample.
Characterization of nanocomposites
The morphologies and the crystal composition of the nanocomposites were characterized with a Transmission Electron Microscope (TEM) Model No. JEM 2100 Make JEOL, Japan to obtain the internal morphology size SEM analysis was carried out by model number JSM 6510LV, made by JEOL in Japan. A Rigaku X-ray diffractometer determine the X-ray diffraction patterns (XRD) (Japan).8 Organic compounds were classified using FTIR (Bruker Tensor37).9
The Antimicrobial potential of ZnO/Ag nanocomposites
Antibacterial properties of the synthesized ZnO/Ag nanocomposites were studied method by Romana et.al with few modifications. 10 A standard well diffusion process was used to assess the antimicrobic sensitivity of the Escherichia coli and Staphylococcus aureus isolates including Saccharomyces cerevisiae yeast. All of the materials and apparatus used in these studies were autoclave and sterilized for 20 minutes at 121°C. In 180 mm diameter Petri plates with a diameter of 4 mm, sterilized Muller Hinton agar (Hi-Media, Mumbai) and yeast extract peptone dextrose for yeast were added, and the plates were left to set. The isolates of S. aureus and E. coli were grown in 20 ml of Luria Broth and kept at 37°C overnight prior to use. After pouring 2µl of bacteria on Mueller Hinton Agar (MHA) plates and yeast suspension on YEPD Petri plates respectively. A sterile glass rod spreader was rotated several times to ensure the consistent spreading of the inoculant on top of the side of the petri plate. A 6 mm-diameter well was created on each plate using a cork borer. The different concentrations of 20, 40, and 60 μg/mL ZnO/Ag nanocomposites were placed in each well and incubation for 18 h for bacterial culture at 37 ºC and 48h for yeast culture.11 For the bacterial culture, the antibiotic ampicillin served as a positive control.12 Scales were used to measure the clear zone of inhibition surrounding the wells to determine the sensitivity. The studies were repeated twice to calculate the zone of inhibition and drop handling errors.
Minimum inhibitory concentration (MIC)
MIC of fabricated nanocomposites were evaluated by the broth dilution technique in 96 well plates. In a nutshell, Luria broth (LB) was made with consecutive two-fold concentrations of the manufactured ZnO/Ag nanocomposites ranging from 200.0 to.75 µg/ mL. A suspension of young colonies of the tested bacteria was used to create the inoculum, and the turbidness was altered to meet the 0.5 McFarland standard, resulting in a bacterial concentration of 1.5×108 CFU/mL. Each tube had 1 mL of LB supplemented with varying quantities of nanocomposites, and 100 mL of the diluted solution was added to each tube. This suspension was diluted to 106 CFU/mL in LB medium. In a shaker incubator, at 37°C, each test tube was incubated overnight. The term “MIC” refers to the lowest amount at which no bacterial growth was seen.13
Results and discussion
An in-depth study of fabrication of ZnO/Ag nanocomposites through Co- precipitation technique using Syngonium podophyllum as reductant and stabilizing agent.
Structural analyses (XRD)
On the synthesized nanocomposites, an XRD examination was done to determine their phase characteristics. The ZnO/Ag NCs XRD spectrum is shown in Fig 1 (b) 31.76°, 34.44°, 36.24°, 47.56°, 56.66°, 62.90°, 66.42°, 68.04°, 72.64°, and 76.98° equivalent to the crystal lattice of (100), (002), (101), (102), (110), (103), (200), (112) has been listed to the structure of wurtzite ZnO(*) (JCPDS No. 89-1397).14 Besides, doping with Ag salt results in four additional peaks in the ZnO/Ag nanocomposite, characteristics peaks at 38°, 44.3°, 64.3° and 77.74° Ag (#) were listed to cubic form in (JCPDS No. 89-3722)15 for ZnO/Ag NCs relating to the crystal planes of (111), (200), (220) and (311) respectively as shown in Fig 1(b). With no peaks connected to other phases or contaminants, the XRD patterns show the samples excellent purity. ZnO/Ag NCs were found to consist solely of ZnO and Ag phases. These pure peaks prove the synthesis of ZnO decorated Ag nanocomposites. The average size of ZnO/Ag NCs was determined by applying equation Debye–Scherrer 16
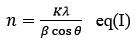
n denotes to Average Crystallite size
K = with a range of 0.9 to 1(Scherrer Constant),
λ is denoted as 1.5418 Å X-ray wavelength
β represents Full width half maxima
θ = Bragg angle
It is estimated that ZnO/Ag composites have an average size of 24.39 nm using the Scherrer equation.
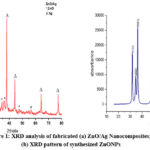 |
Figure 1: XRD analysis of fabricated (a) ZnO/Ag Nanocomposites; (b) XRD pattern of synthesized ZnONPs |
Vibrational analysis (FTIR)
From Fig. 2 it was clear that FTIR vibrational analysis was used to examine the organic composition of the fabricated ZnO/Ag nanocomposites by identifying functional groups. A C = O appeared at peak 1392 cm−1,9 and C=O stretch vibrations of carboxylic acids contributed to a peak at 1739cm-1.17 The strong peak at 2112cm-1 can be ascribed as C ≡O.18 The vibration of the NED functional groups was associated with a band that was identified at 2384 cm-1.19 A band peak at 2656 cm−1 is associated to carboxylic acid stretch in the O-H direction.20 whereas, peak around 1000 cm-1 is accredited to the presence of metal oxide.21 According to the ZnO/Ag FT-IR spectra the peaks between 3200 and 3600 cm-1, corresponds to the stretching vibration of the inter – molecular bond (O-H) that exists in between zinc oxide oxygen and the adsorbed water molecule.22 The fabrication of ZnO results in a prominent band at 650 cm-1, It is strongly consistent with the available research.
 |
Figure 2: FTIR analysis of fabricated ZnO/Ag |
Analysis of morphology, size, and pattern (SEM, TEM, SAED pattern)
SEM morphology of fabricated AgNPs, ZnO and ZnO/Ag NCs in Fig (3a, b, c) respectively, showed that majority of nanoparticles were spherical shape. Whereas fabricated ZnONPs are pure sphere-shaped. Another interesting part of the fabrication is knowing how the size, shape, surface, and aggregation state of the Ag and ZnO nanoparticles change after blending. According to the SEM analysis, the AgNPs are spherical, but ZnO shows the structure is purely spherical. The ZnO/Ag nanocomposite shows a transitional sphere-like structure. The synthesized ZnONPs in Fig. 3(b) were completely spherical, whereas the synthesized AgNPs in Fig. 3(a) were almost spherical and diameter of AgNPs ranges from 4-58 nm.
To define possible role of supernatant biomolecules in the fabrication, reducing, and stabilizing AgNPs, ZnO, and ZnO/Ag TEM analysis was performed. The TEM analysis in Fig. 4(a) revealed that fabricated ZnO/Ag nanocomposites are transitional sphere shaped with an average size of the fabricated nanocomposite were 16.64nm depicted in fig. 4(c) . As ZnO-NPs and Ag NPS both are synthesized in an aqueous medium, they have a high surface energy. It may be possible because of combining nanoparticles as composites which densified the fine gaps between them.23
 |
Figure 3: SEM analysis (a) AgNPs;(b) ZnO;(c) and (d) ZnO/Ag synthesized from Syngonium podophyllum. |
The SAED pattern in Fig. 4 (b) shows that the particles have a substantially crystalline appearance and also display a configuration of rings including spots, which suggests that nanocomposites have a higher grain dimension, regular morphology, and polycrystalline in structure.23
 |
Figure 4: (a)TEM analysis of ZnO/Ag; (b) SAED pattern of ZnO/Ag; (c) Average size distribution curve |
Applications
Antibacterial Analysis
Antimicrobial potential was evaluated by using the well diffusion technique for E. coli, and S. aureus onto MHA plates, as well as YEPD media for saccharomyces cerevisiae. Each inhibition zone for nanocomposites and commercially available antibiotics has been visualized in terms of its antimicrobial activity. Ampicillin was used as a positive control against bacterial species because of its high antibacterial performance. According to this study, the quantity of ZnO/Ag NCs affects the antimicrobial potential of E. coli, S. aureus, and S. cerevisiae. The antibacterial action that results from the ZnO/Ag NCs distribution on the agar surface limits microbial growth in the space that is already occupied by them.24 The MIC of the biosynthesized ZnO/Ag NCs against Staphylococcus aureus and Escherichia coli was assessed at various concentrations. The MIC study was done by broth dilution technique.13 The lowest inhibitory concentrations of nanocomposites, which ranged from 12.50 to 50 µg/mL and was liable for preventing the growth of isolated strains, was determined (MIC). After 5 h of treatment, fabricated ZnO/Ag nanocomposites exhibited approximately 75% toxicity against tested isolates. As a result of this antibacterial study, fabricated ZnO/Ag nanocomposites showed potential effectiveness against isolates as an antimicrobial. Silver and ZnO nanoparticles were already widely recognized to have antibacterial properties.
Table 1: Antimicrobial potentials of ZnO/Ag NCs
|
Micro organism |
various concentration of ZnO/Ag NCs for ZOI mm |
|||
|
+ve control /Antibiotic |
20µgm/L |
40µgm/L |
60µgm/L |
|
|
E. coli S. aureus S. cerevisiae |
23 0 – |
10±0.3 8±0.8 12±0.1 |
12±0.6 10±0.5 12±0.5 |
13±0.2 11±0.5 13±0.7 |
Anti-yeast analysis
The Zone of Inhibition proved S. cerevisiae sensitivity to ZnO/Ag NCs (Table 1). Thus, the antimicrobic potential of ZnO/Ag changes with the concentration of NCs. As the concentration of NCs increases the zone around the well subsequently increases. The result revealed that the anti-yeast activity is dose-dependent (NCs).
Conclusion
The current study focused on the fabrication of ZnO/Ag nanocomposites by co-precipitation with Syngonium podophyllum aqueous leaves extract, and their antimicrobic sensitivity against E. coli, S. aureus, and yeast S. cerevisiae. ZnO/Ag NCs synthesized showed smaller sizes based on XRD, SEM, and TEM analyses. The SAED pattern showed the polycrystallinity of the nanocomposites. FTIR analysis suggested that the functional group, for example flavonoids, terpenoids, etc. exhibit in leaf extract are liable for the reducing and stabilization of the nanocomposites. A possible antiseptic resultant can be obtained from the green fabricated ZnO/Ag nanocomposites due to their promising characteristics and their favorable antimicrobial efficiency against tested pathogens.
Acknowledgment
Romana Naaz one of the authors would like to acknowledge the University Grants Commission in New Delhi for granting funds in the form of a non-Net fellowship. Romana Naaz too recognizes the university sophisticated instrument facility (USIF), Aligarh Muslim University for providing instrumentation facilities for providing experimental facilities, and prof. Shamsul Hayat Aligarh Muslim University for his consistent support throughout the study.
Conflict of Interest
There are no conflict of interest.
References
- Amarjargal, A., Tijing, L. D., Im, I. T. & Kim, C. S. Simultaneous preparation of Ag/Fe3O4 core-shell nanocomposites with enhanced magnetic moment and strong antibacterial and catalytic properties. Chemical Engineering Journal 226, 243–254 (2013).
CrossRef - Cuadra, J. G. et al. ZnO/Ag Nanocomposites with Enhanced Antimicrobial Activity. Applied Sciences 12, 5023 (2022).
CrossRef - Mohammadi-Aloucheh, R., Habibi-Yangjeh, A., Bayrami, A., Latifi-Navid, S. & Asadi, A. Enhanced anti-bacterial activities of ZnO nanoparticles and ZnO/CuO nanocomposites synthesized using Vaccinium arctostaphylos L. fruit extract. Artificial Cells, Nanomedicine and Biotechnology 46, 1200–1209 (2018).
CrossRef - Obeng, E. et al. Multifunctional phototheranostic agent ZnO@Ag for anti-infection through photothermal/photodynamic therapy. Frontiers in Chemistry 10, 1–14 (2022).
CrossRef - Ashar, A. et al. Integrated hydrothermal assisted green synthesis of ZnO nano discs and their water purification efficiency together with antimicrobial activity. Journal of Materials Research and Technology 15, 6901–6917 (2021).
CrossRef - Raj, R. B., Umadevi, M. & Parimaladevi, R. Effect of ZnO/Ag Nanocomposites Against Anionic and Cationic Dyes as Photocatalysts and Antibacterial Agents. Journal of Inorganic and Organometallic Polymers and Materials 31, 500–510 (2021).
CrossRef - Saranya, A., Murad, A., Thamer, A., Priyadharsan, A. & Maheshwaran, P. Preparation of Reduced ZnO/Ag Nanocomposites by a Green Microwave-Assisted Method and Their Applications in Photodegradation of Methylene Blue Dye, and as Antimicrobial and Anticancer Agents. ChemistrySelect 6, 3995–4004 (2021).
CrossRef - Ma, L. & Chen, W. ZnS:Cu,Co water-soluble afterglow nanoparticles: Synthesis, luminescence and potential applications. Nanotechnology 21, 385604 (2010).
CrossRef - Kumari, S. et al. A Novel Synthesis of the Graphene Oxide-Silver (GO-Ag) Nanocomposite for Unique Physiochemical Applications. ACS Omega 5, 5041–5047 (2020).
CrossRef - Naaz, R., Siddiqui, V. U., Qadir, S. U. & Siddiqi, W. A. Green synthesis of silver nanoparticles using Syngonium podophyllum leaf extract and its antibacterial activity. Materials Today: Proceedings 46, 2352–2358 (2021).
CrossRef - Zanet, V. et al. Activity evaluation of pure and doped zinc oxide nanoparticles against bacterial pathogens and Saccharomyces cerevisiae. Journal of Applied Microbiology 127, 1391–1402 (2019).
CrossRef - Jana, T. K. et al. Photocatalytic and antibacterial activity of cadmium sulphide/zinc oxide nanocomposite with varied morphology. Journal of Colloid and Interface Science 480, 9–16 (2016).
CrossRef - Bagherzade, G., Tavakoli, M. M. & Namaei, M. H. Green synthesis of silver nanoparticles using aqueous extract of saffron (Crocus sativus L.) wastages and its antibacterial activity against six bacteria. Asian Pacific Journal of Tropical Biomedicine 7, 227–233 (2017).
CrossRef - Jalal, R. et al. ZnO nanofluids: Green synthesis, characterization, and antibacterial activity. Materials Chemistry and Physics 121, 198–201 (2010).
CrossRef - Sundararajan, B., Mahendran, G., Thamaraiselvi, R. & Ranjitha kumari, B. D. Biological activities of synthesized silver nanoparticles from Cardiospermum halicacabum L. Bulletin of Materials Science 39, 423–431 (2016).
CrossRef - Vadlapudi, V., Kaladhar, D., Behara, M., Sujatha, B. & Naidu, G. Synthesis of Green Metallic Nanoparticles (NPs) and Applications. Oriental Journal of Chemistry 29, 1589–1595 (2013).
CrossRef - Hanifah, M. F. R. et al. Synthesis of graphene oxide nanosheets via modified Hummers’ method and its physicochemical properties. Jurnal Teknologi 74, 195–198 (2015).
CrossRef - Jain, S. & Mehata, M. S. Medicinal Plant Leaf Extract and Pure Flavonoid Mediated Green Synthesis of Silver Nanoparticles and their Enhanced Antibacterial Property. Scientific reports 7, 15867 (2017).
CrossRef - Djilani, C. et al. Elimination of organic micropollutants by adsorption on activated carbon prepared from agricultural waste. Chemical Engineering Journal 189–190, 203–212 (2012).
CrossRef - Özer, Ç. & İmamoğlu, M. Removal of ciprofloxacin from aqueous solutions by pumpkin peel biochar prepared using phosphoric acid. Biomass Conversion and Biorefinery (2022) doi:10.1007/s13399-022-02832-3.
CrossRef - Rasli, N. I., Basri, H. & Harun, Z. Zinc oxide from aloe vera extract: two-level factorial screening of biosynthesis parameters. Heliyon 6, e03156 (2020).
CrossRef - Geetha, A., Sakthivel, R., Mallika, J., Kannusamy, R. & Rajendran, R. Green synthesis of antibacterial zinc oxide nanoparticles using biopolymer azadirachta indica gum. Oriental Journal of Chemistry 32, 955–963 (2016).
CrossRef - V., R., G., S., R., A. & S., B. Green synthesis of zinc oxide nanoparticles using Hyptis leaf extract and Activated carbon based Zinc oxide composite of Supercapacitor Applications. Indian Journal of Science and Technology 12, 107–8 (2019).
CrossRef - Aunkor, M. T. H. et al. Antibacterial activity of graphene oxide nanosheet against multidrug resistant superbugs isolated from infected patients: Graphene Oxide Antibacterial Activity. Royal Society Open Science vol. 7 at https://doi.org/10.1098/rsos.200640rsos200640 (2020).
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.










