Silver nanoparticles: Green Synthesis using Derris trifoliata Seed Extract and its Applications as a Sensor, Photocatalyst and Antibacterial Agent
Reshma Augustine1 , Navya S. Kollamparambil1
, Navya S. Kollamparambil1 , Krishnaraj M. V.2
, Krishnaraj M. V.2 , Ushamani M.1
, Ushamani M.1 , Saritha Chandran A.1*
, Saritha Chandran A.1*
1Department of Chemistry and Centre for Research, St. Teresa’s College (Autonomous), Ernakulam, Kerala, India.
2Department of Botany, Baselius College, Kottayam, Kerala, India.
Corresponding Author E-mail: sarithachandran@teresas.ac.in
DOI : http://dx.doi.org/10.13005/ojc/390106
Article Received on : 28 Nov 2022
Article Accepted on : 23 Jan 2023
Article Published : 23 Jan 2023
Reviewed by: Dr. Swaroop T R
Second Review by: Dr. Parthiban D
Final Approval by: Dr. B. K Sharma
This work focuses on the preparation of silver nanoparticles (AgNPs) by a green synthesis using the seed extract of Derris trifoliata. The optimum time and temperature for the extraction of seeds were determined using FTIR analysis. The seed extract acted as a reducing agent and silver nitrate was used as the metal precursor for the preparation of AgNPs. Optimization of reaction conditions for the preparation of AgNPs and its characterization was done by UV-Vis spectroscopy, FTIR, HRTEM and SAED images. The UV- Visible spectrum of AgNPs revealed a characteristic SPR peak at 433 nm. HRTEM and SAED images confirmed nearly spherical nature of the AgNPs with a diameter of 2–20 nm. H2O2 sensing capacity and the photocatalytic dye degradation of the AgNPs was investigated using UV-Vis spectroscopy. Antibacterial activity of silver nanoparticles on E-coli bacteria was also studied using microtitre plate method.
KEYWORDS:Derris trifoliata; Green synthesis; Hydrogen peroxide; Silver nanoparticles
Download this article as:| Copy the following to cite this article: Augustine R, Kollamparambil N. S, Krishnaraj M. V, Ushamani M, Chandran A. S. Silver nanoparticles: Green Synthesis using Derris trifoliata Seed Extract and its Applications as a Sensor, Photocatalyst and Antibacterial Agent. Orient J Chem 2023;39(1). |
| Copy the following to cite this URL: Augustine R, Kollamparambil N. S, Krishnaraj M. V, Ushamani M, Chandran A. S. Silver nanoparticles: Green Synthesis using Derris trifoliata Seed Extract and its Applications as a Sensor, Photocatalyst and Antibacterial Agent. Orient J Chem 2023;39(1). Available from: https://bit.ly/3XJJoO8 |
Introduction
Nanotechnology is one of the most prominent field of research in modern science giving emphasis to non-polluting technology with production of wide range of environment friendly and safe nanomaterials 1. Nanoparticles synthesized using metals have wide range of applications as antibacterials and therapeutics 2-3, optoelectronics 4, catalysis 5, biosensors, drug delivery, nanodevice fabrication and medicine 6.
Different nanomaterials like alginate 7, gold 8, magnesium 9, titanium 10, copper 11, zinc 12 and silver 13 are known. But AgNPs have more popularity than others when researchers found they could produce it at nanoscale. It exhibits distinctive morphologies 14,15 and characteristics 16. AgNPs are commonly used in many applications because of their relative high stability 17, unique optical properties 18-19 and strong conjugation ability with biomolecules 20. They also possess anti-inflammatory 21, antimicrobial and anticoagulant 22 antifungal 23-24, anti-bacterial 25, 26, antiviral 27,28, antitumor 3,29 and anti-platelet 22,30 properties.
For the synthesis of AgNPs, different methods like radiation assisted process 31, microwave assisted process 16, electro chemical methods 32, sonochemical process 33 and thermal decomposition 34 of Ag compounds are available, however poor conversion of materials, high energy demands, challenging and wasteful purifications, associated environment toxicity etc are the major defects. As a result, plant extract-based green synthesis of AgNPs has emerged as a quick and effective substitute for physical and chemical synthetic processes 37,38,39.
Now-a-days, synthesizing silver nanoparticles using a number of microorganisms like bacteria 40,41 and fungi 42,43, as well as by using different plant extracts like the seed extract of Avicennia marina 44, tuber of Curcuma longa 2 ,berry of Solanum xanthocarpum 26, stem bark of Callicarpa maingayi 45, leaf extract of Carica papaya 46, fruit of Citrus lemon 47, callus of Sesuvium portulacastrum 48 and seed extract of Artocarpus heterophyllus 49 are in practice. The rate of synthesis of nanoparticles by plant extracts is proportional to the chemically done process and are faster than green synthesis done by microorganisms. According to studies, seed extracts are the most efficient reducing agents for the synthesis of AgNPs that exhibit antifungal and antibacterial action 49, 50. This paved the way for the biosynthesis of silver nanoparticles using Derris trifoliata seed extract.
Derris trifoliata is liana, belonging to the flowering plant family Leguminosae. It is distributed in coastal parts of East Africa, Tropical Asia and Australia. Fruits are 3-4 cm, flat, pale yellow in color.Seeds are proven to have antibacterial, antiplasmodial properties and are larvicidal agents 51, 52. Photo induced and phytomediated synthesis of AgNPs using Derris trifoliata was also established 52. This species also have bioactive compounds like flavonoids, isoflavanoids, phenolic acids and polysaccharides 53.AgNPs prepared from Derris trifoliata is known to degrade dyes 54 and also known as a sensor of mercury ions in aqueous solutions 55.
In the present study, we prepared AgNPs using Derris trifoliata seed extract as reducing agent and silver nitrate as metal precursor. We have optimized the conditions of seed extraction and preparation of silver nanoparticles using FTIR, UV-Vis spectroscopy, HRTEM and SAED. We studied the possibility of the use of AgNPs as a sensor for Hydrogen peroxide and in the photodegradation of a hazardous dye, methylene blue. Since silver nanoparticles are known to have antibacterial properties, this property was also investigated on E-coli bacteria.
Materials and Methods
Materials
The seeds of Derris trifoliata were collected from marshy areas of Paravur, Kerala, India. Silver nitrate, Hydrogen peroxide (30% w/v), and Methylene blue were procured from Nice Chemicals Pvt. Ltd. Kochi, Kerala, India and used without further purification.
Preparation of AgNPs
Preparation of Derris trifoliata seed extract at optimized conditions
Derris trifoliata seeds were dried and ground into fine powder. 5 g of this finely powdered seed was soaked in 25 mL distilled water for 30 minutes and filtered using Whatman no.1 filter paper. The process of extraction was done under room temperature, 35°C, 40°C, 45°C, and 50°C. Optimum temperature for the extraction process was investigated using FTIR spectra of each sample. Likewise, extraction was repeated at the optimum temperature for 15, 30, 45, 50, 55 and 60 minutes to find optimum time for the extraction. Later, the extract was prepared at these optimized reaction conditions.
Preparation of AgNPs at optimized conditions
Optimum temperature for the preparation of AgNPs was determined using UV-Vis spectroscopy. For this, 250 µL of seed extract was added to 5 mL of 1 mM silver nitrate solution. It was kept under room temperature, under sunlight and at 35°C, 40°C, 45°C, and 50°C for 15 minutes. Absorption spectra of each sample of AgNPs were recorded and optimum condition for the synthesis of AgNPs was determined.
At the optimized condition, preparation of AgNPs was done by keeping the reaction mixture for 15, 30, 45 minutes and 1 hour. From the absorption spectra, optimum time of preparation was also determined. Later, AgNPs were prepared at these optimum conditions.
Characterization of synthesized AgNPs
Characterization of the AgNPs was done using FTIR, UV-Vis spectroscopy, HRTEM and SAED. The FTIR spectra of the prepared AgNPs were recorded on a Thermo Nicolet, Avatar 370 spectrophotometer using KBr pellets. UV- Visible spectra of the prepared AgNPs were recorded on Thermo Scientific Evolution 201 UV-Visible spectrophotometer with wavelength range 200 nm-1000 nm. HRTEM & SAED photographs were taken using Joel/ JEM 2100 spectrometer with resolution point: 0.23 nm, lattice: 0.14 nm.
AgNPs as Hydrogen peroxide sensor
Hydrogen peroxide sensing of AgNPs was investigated [56]. UV-Vis absorption spectrum of AgNPs was recorded. 1 mL of 20 mM hydrogen peroxide was then added to the AgNPs solution and thoroughly mixed. The absorption readings were taken at regular intervals of time and the changes in absorption was noted.
AgNPs as catalyst for photo degradation of methylene blue
The photo degradation of the dye can be followed by measuring the absorbance of the dye at its λmax 660 nm. Photo degradation of methylene blue was studied in the presence and absence of of AgNPs. Methylene blue solution was prepared by adding 10 mg of the dye to 1000 mL distilled water. To this solution, 5 mL of AgNPs was added and stirred well. This dispersion was kept under sunlight. At specific intervals of time, absorbance of the solution was measured using UV-Vis spectrophotometer. The same process was done for a blank solution of methylene blue.
AgNPs as Antibacterial agents
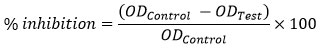
Antibacterial activity of the synthesized AgNPs was checked on E- coli bacteria using microtiter plate method. Using aseptic techniques, a single pure colony was transferred into a 10 ml of nutrient broth, capped and placed in incubator overnight at 37°C. The turbidity of suspensions was calculated and adjusted using McFarland standards as a reference. A sterile 96-well plate was labelled. A volume of 10 μL, 20 μL, 30 μL, 40 μL of test material was pipetted into the wells. 100 μL of nutrient broth was added to each well. Finally, 100 μL of microbial suspension was then added to each well. The plates were incubated at 37°C for 24 hrs and Optical Density (OD) reading was taken (OD600) after sufficient incubation. Optical density was obtained as follows.
Results and Discussion
Optimization of reaction conditions for Derris trifoliata seed extraction using FTIR
The reduction of metal salts to nanoparticles can be mediated by the biological components in the Derris trifoliata seed extract 57. The functional groups of these components may encapsulate the surface of silver ions by stabilizing the nanoparticles as capping agents 57. The FTIR spectra of the seed extracts prepared at different temperatures are depicted in fig. 1a. Only the sample prepared at room temperature showed a peak at 1061 cm-1 which arises due to –CN stretching of amines. –CN group can aid the reduction of Ag+ to metallic Ag during the formation of AgNPs. Thus we assume that room temperature is the optimum temperature condition for the preparation of seed extract. After optimizing the temperature for the preparation of theseed extract, we investigated the optimum time for the same. For this, the extraction was carried out at room temperature which is the optimum temperature condition for different time durations. Fig. 1b represents the corresponding IR spectra. The peaks in the range 3417-3469 cm-1, 2067-2079 cm-1, 1636 cm-1, 537-566 cm-1 was present in all reaction conditions. –CN stretching of amines at 1061.97 cm-1was present in sample extracted for 30 minutes and not found in any other reaction conditions. The same peak was seen for room temperature extraction during optimization of temperature for extraction. Hence the time of extraction was optimized at 30 minutes for the preparation of the seed extract.
 |
Figure 1: FTIR spectral analysis of Derris trifoliata seed extract for a) temperature optimization b) time optimization. |
Optimization of reaction conditions for the synthesis of AgNPs using UV-Vis spectroscopy
AgNPs were prepared under sunlight and also at different temperatures. The UV-Vis spectrum of these reaction mixtures is given in fig. 2. It shows an absorption peak between 400 – 450 nm. This absorption peak represents the presence of AgNPs. None of the samples prepared under different temperature conditions gave the absorption peak corresponding to the formation of AgNPs. So, sunlight irradiation is the optimum condition for the preparation of AgNPs using theseed extract.
 |
Figure 2: Absorption spectrum of AgNPs prepared under different temperature conditions |
Fig. 3 shows the absorption spectrum of the AgNPs recorded for optimization of time of reaction. Metallic nanoparticles show characteristic UV-Vis absorption spectra at 433 nm due to Surface Plasmon Resonance (SPR). The SPR bands are influenced by size, shape, morphology, composition and dielectric environment of the prepared nanoparticles 55. Intensity of absorption peak indicates the concentration of AgNPs. The appearance of the SPR increased with increase in reaction time. Irradiation for one hour shows maximum absorbance of 1.483 at 433 nm. Thus, we optimized this condition- 1 hr for the synthesis of AgNPs.
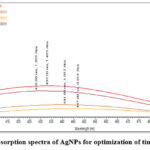 |
Figure 3: Absorption spectra of AgNPs for optimization of time of reaction. |
Optimized process for the preparation of AgNPs
5 g of finely ground seeds was soaked in 25 mL distilled water for 30 minutes at room temperature, filtered and the extract was collected. This seed extract was used as reducing agent for the synthesis of AgNPs. The seed extract was treated with 1 mM AgNO3 solution and kept under sunlight for 1 hr. Appearance of reddish- brown colour specifies the formation AgNPs.
Characterization of synthesized AgNPs
Characterization of AgNPs were done using UV- Vis spectroscopy, FTIR spectroscopy and HRTEM- SAED images.
UV-Visible spectrum
The UV-Vis absorption spectrum of the AgNPs prepared under optimum conditions is given in fig. 4.
 |
Figure 4: Absorption spectrum of AgNPs prepared at optimum conditions. |
The AgNPs showed a broad characteristic absorption peak at a wavelength of 433 nm because of SPR. According to Mie theory 58, a single SPR band shows the formation of small spherical or quasi-spherical nanocrystals. A broad peak indicates that the AgNPs are spherical and polydispersed. The spherical shape of the AgNPs was thus confirmed.
FTIR spectrum
The FTIR spectrum of the AgNPs prepared under optimum conditions are given in fig. 5. The important peaks present in FTIR spectrum of AgNPs were at 3448.20 cm-1, 2101.46 cm-1, 1637.0 cm-1, 1384.61 cm-1 and 543.50 cm-1. They correspond to the -OH stretching or -NH stretching of amines, alkyne group present in phyto constituents, carbonyl stretching in carboxyl group of proteins, germinal methyl of secondary metabolites and halide, respectively. The peak at 1061.97 cm -1 corresponding to -CN stretching of amines seen in the FTIR spectrum of the seed extract was absent here. The disappearance of this peak in the FTIR spectrum of synthesized AgNPs indicates that the amine groups present in bioactive compounds or secondary metabolites cause the reduction of Ag+ to Ag0. This reveals the role of biological molecules in the synthesis and stabilization procedures 59. The stabilization of AgNPs is due to surface bound proteins through free amine group 60.
 |
Figure 5: FTIR spectrum of AgNPs |
HRTEM
Fig. 6 gives the HRTEM and SAED images of AgNPs. The size of the synthesized AgNPs obtained were found in the range of 2-20 nm. Nearly spherical shape of the prepared AgNPs that is assumed from the absorption spectrum was also confirmed here.
 |
Figure 6: The HRTEM and SAED images of AgNPs |
AgNPs as Hydrogen peroxide sensor
The absorption spectra of the reaction mixture of the AgNPs and hydrogen peroxide at regular intervals of time are given in fig. 7. As time increased, the absorbance at 433 nm of the samples decreased. Finally, this SPR peak vanished and the brown colour of the reaction mixture became completely colourless. These results are in accordance with the results obtained by Tagad et al. 61. According to Mohan and coworkers 62, “the addition of AgNPs to H2O2 resulted in the formation of free radicals which initiated the degradation of the AgNPs. This led to the oxidation of silver to silver ions and resulted in a decrease in absorbance.” These findings suggest that the AgNPs can be successfully used to sense the concentration of H2O2 present in various samples.
 |
Figure 7: Absorption spectra of AgNPs and H2O2 reaction mixture at regular intervals of time. |
AgNPs as catalyst for photo degradation of methylene blue
Fig. 8 a and b shows the photograph of blank solution of methylene blue and methylene blue solution with AgNPs at different time intervals. The colour remained same throughout the time of study of the blank solution. For the solution with AgNPs, the deep blue colour of the methylene blue solution started to fade during irradiation of sunlight and the degradation of was complete within 12 hours of irradiation.
 |
Figure 8: Photograph of methylene blue at varying time intervals (a) blank solution |
Fig. 9 indicates the absorption spectra of the blank solution at different time intervals. Methylene blue shows an absorption band at 660 nm. It is clear from the graph that the absorbance of methylene blue in the blank solution decreases only slightly with time.
 |
Figure 9: Absorption spectrum of methylene blue (blank solution) at varying time intervals. |
Fig. 10 indicates the UV-Vis spectra of methylene blue in the presence of AgNPs at varying time intervals. With increase in time, the absorption of methylene blue decreased with simultaneous increase of absorption of AgNPs. After 12 hrs of exposure, the absorbance of methylene blue dye almost approached the base line and the absorbance of AgNPs was maximum. This may be due to the photocatalytic degradation of methylene blue dye by AgNPs. Thus the degradation of the hazardous dye methylene blue can be enhanced by using AgNPs.
 |
Figure 10: Absorption spectrum of methylene blue in the presence of AgNPs at different time intervals. |
AgNPs as antibacterial agents
Table 1 lists the percentage inhibition of the AgNPs on E.coli bacteria. An increase in percentage of inhibition with respect to concentration of AgNPs was found. The percentage of inhibition has highest value for the 40 µL mixture. The antibacterial action of synthesized AgNPs was thus proved.
Table 1: Analysis of % of inhibition of synthesized AgNPs on E.coli bacteria.
|
Concentration of AgNPs (μL) |
Optical Density |
% of inhibition |
|
Control |
1.422 |
|
|
10 |
0.371 |
73.7% |
|
20 |
0.32 |
77.5% |
|
30 |
0.253 |
82% |
|
40 |
0.24 |
83% |
Conclusions
Our study reports a cost effective, non-toxic, ecofriendly and easy method for the preparation of AgNPs, where Derris trifoliata seed extract act as a reducing and stabilizing agent. The preparation of seed extract and synthesis of AgNPs were optimized through spectroscopic methods. UV- Visible spectrum of AgNPs showed a characteristic Surface Plasmon Resonance peak at 433 nm. HRTEM and SAED images confirmed nearly spherical nature of the AgNPs with a diameter of about 2–20 nm. FTIR spectra of the seed extract and silver nanoparticles revealed that the seed extract can act as a biological agent which can perform the dual function of reduction and stabilization. Ability of AgNPs to sense hydrogen peroxide was demonstrated using UV-Visible absorption spectra which would find applications in the development of sensors to detect the presence of hydrogen peroxide in samples. The use of the prepared AgNPs in the photodegradation of one of the hazardous dyes, methylene blue was investigated using UV-Vis spectroscopy. The decrease in methylene blue absorption with increase in exposure time indicates the photocatalytic activity of AgNPs. Also, we analysed the antibacterial activity of the AgNPs on E-coli bacteria which would find applications in development of new antibacterial drugs.
Conflict of Interest
The authors have no conflict of interest.
References
- “Nanotechnology and the Environment: Report of a National Nanotechnology Initiative Workshop,” p. 66.
- Venkatpurwar, V.; Pokharkar, V. Mater. Lett. 2011, 65, 6, 999-1002 https://doi.org/10.1016/j.matlet.2010.12.057.
CrossRef - Zhang, X.-F.; Liu, Z.-G.; Shen, W.; Gurunathan, S. Int. J. Mol. Sci.2016, 17, 9, 1534 https://doi.org/10.3390/ijms17091534.
CrossRef - Muruganandam, S.; Anbalagan, G.; Murugadoss, G. Appl. Nanosci. 2015, 5, 245–253 https://doi.org/10.1007/s13204-014-0313-6.
CrossRef - Crooks, R. M.; Zhao, M.; Sun, L.; Chechik, V.; Yeung, L. K. Acc. Chem. Res. 2001, 34, 3, 181–190 https://doi.org/10.1021/ar000110a.
CrossRef - Jain, P. K.; Huang, X.; El-Sayed, I. H.; El-Sayed, M. A. Acc. Chem. Res. 2008, 41, 12, 1578–1586 https://doi.org/10.1021/ar7002804.
CrossRef - Ahmad, Z.; Pandey, R.; Sharma, S.; Khuller, G. K. The Indian Journal of Chest Diseases & Allied Sciences, 2006, 48.
- Gu, H.; Ho, P. L.; Tong, E.; Wang, L.; Xu, B. Nano Lett. 2003, 3, 9, 1261–1263 https://doi.org/10.1021/nl034396z.
CrossRef - Haas, I.; Gedanken, A. Chem. Commun. 2008, 15, 1795-1797 https://doi.org/10.1039/B717670H.
CrossRef - Schabes-Retchkiman, P. S.; Canizal, G.; Herrera-Becerra, R.; Zorrilla, C.; Liu, H. B.; Ascencio, J. A. Opt. Mater. 2006, 29, 1, 95-99. https://doi.org/10.1016/j.optmat.2006.03.014.
CrossRef - Din, M. I.; Rehan, R. Anal. Lett. 2017, 50, 1, 50-62 https://doi.org/10.1080/00032719.2016.1172081.
CrossRef - Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N. H. M.; Ann, L.C.; Bakhori, S. K. M.; Hasan, H.; Mohamad, D. Nano-Micro Lett.2015, 7, 219–242 https://doi.org/10.1007/s40820-015-0040-x.
CrossRef - Beyene, H. D.; Werkneh, A. A.; Bezabh, H. K.; Ambaye, T. G.; Sustain. Mater. Technol. 2017, 13, 18-23 https://doi.org/10.1016/j.susmat.2017.08.001.
CrossRef - Shenashen, M. A.; El-Safty, S. A.; Elshehy, E. A. Sustain. Mater. Technol. 2014, 31, 3, 293-316 https://doi.org/10.1002/ppsc.201300181.
CrossRef - Rauwel, P.; Küünal, S.; Ferdov, S.; Rauwel, E. Adv. Mater. Sc. Eng.2015 https://doi.org/10.1155/2015/682749.
CrossRef - Pastoriza-Santos, I.; Liz-Marzán, L. M.; Langmuir, 2002, 18, 7, 2888–2894 https://doi.org/10.1021/la015578g.
CrossRef - González, A. L.; Noguez, C.; Beránek, J.; Barnard, A. S. J. Phys. Chem. C, 2014, 118, 17, 9128–9136 https://doi.org/10.1021/jp5018168
CrossRef - Evanoff Jr., D. D.; Chumanov, G. Chem. Phys. Chem., 2005, 6, 7, 1221-1231https://doi.org/10.1002/cphc.200500113.
CrossRef - Wu, W.; Wu, M.; Sun, Z.; Li, G.; Ma, Y.; Liu, X.; Wang, X.; Chen, X. J. Alloys. Compd. 2013, 579, 117-123 https://doi.org/10.1016/j.jallcom.2013.05.044
CrossRef - Ravindran, A.; Chandran, P.; Khan, S. S. Colloids Surf. B: Biointerfaces 2013, 105, 342-352 https://doi.org/10.1016/j.colsurfb.2012.07.036.
CrossRef - Martínez-Gutierrez, F.; Thi, E. P.; Silverman, J. M.; de Oliveira, C. C., Svensson, S. L.; Hoek, A. V.; Sanchez, E. M.; Reiner, N.E.; Gaynor, E. C.; Pryzdial, E. L. G.;. Conway, E. M; Orrantia, E.; Ruiz, F.; Av-Gay, Y.; Bach, H. Nanomed: Nanotechnol. Biol.Med. 2012, 8, 3, 328-336 https://doi.org/10.1016/j.nano.2011.06.014.
CrossRef - Dakshayani, S. S.; Marulaisiddeshwara, M. B.; Sharath Kumar, M. N.; Ramesh, G; Kumar, P. R.; Devaraja, S.; Hosamani, R. Int. J. Biol. Macromol. 2019, 131, 787-797 https://doi.org/10.1016/j.ijbiomac.2019.01.222.
CrossRef - Monteiro, D. R.; Silva, S.; Negri, M.; Gorup, L. F.; de Camargo, E. R.; Oliveira, R.; Barbosa, D. B.; Henriques, M.; Lett. Appl. Microbiol. 2012, 54, 5, 383-391 https://doi.org/10.1111/j.1472-765X.2012.03219.x.
CrossRef - Krishnaraj, C.; Ramachandran, R.; Mohan, K.; Kalaichelvan, P. T. Spectrochim. Acta A: Mol. and Biomol. Spectrosc. 2012, 93, 95-99 https://doi.org/10.1016/j.saa.2012.03.002
CrossRef - Choudhary, M. K.; Kataria, J.; Cameotra, S. S.; Singh, J.; Appl. Nanosci. 2016, 6, 1, 105–111 https://doi.org/10.1007/s13204-015-0418-6
CrossRef - Amin, M.; Anwar, F.; Janjua, M. R. S. A.; Iqbal, M. A.; Rashid, U. Int. J. Mol. Sci.2012,13, 8, 9923-9941 https://doi.org/10.3390/ijms13089923.
CrossRef - Galdiero, S.; Falanga, A.; Vitiello, M.; Cantisani, M.; Marra, V.; Galdiero, M.; Molecules 2011, 16, 10, 8894-8918; https://doi.org/10.1177/135965350801300210.
CrossRef - Jeevanandam, J.; Krishnan, S.; Hii, Y. S.; Pan, S.; Chan, Y. S.; Acquah, C.; Danquah, M. K.; Rodrigues, J. J. Nanostruct. Chem. 2022, 12, 809–831 https://doi.org/10.1007/s40097-021-00465-y.
CrossRef - Sriram, M. I.; Kanth, S. B. M.; Kalishwaralal, K.; Gurunathan, S. Int. J. Nanomedicine. 2010, 5, 753-62. doi: https://doi.org/10.2147/IJN.S11727.
CrossRef - Shrivastava, S.; Bera, T.; Singh, S. K.; Singh, G.; Ramachandrarao, P.; Dash, D. ACS Nano 2009, 3, 6, 1357–1364 https://doi.org/10.1021/nn900277t.
CrossRef - Fierascu, R. C.; Fierascu, I.; Lungulescu, E. M.; Nicula, N.; Somoghi, R.; Ditu, L. M.; Ungureanu, C.; Sutan, A. N.; Draghiceanu, O. A.; Paunescu, A.; Soare, L. C. J. Mater. Sci. 2020, 55, 1915–1932 https://doi.org/10.1007/s10853-019-03713-3.
CrossRef - Rodríguez-Sánchez, L.; Blanco, M. C.; López-Quintela, M. A. J. Phys. Chem. B, 2000, 104, 41, 9683–9688 https://doi.org/10.1021/jp001761r.
CrossRef - Zhu, J.; Liu, S.; Palchik, O.; Koltypin, Y.; A. Gedanken, Y. Langmuir, 2000, 16, 16, 6396–6399 https://doi.org/10.1021/la991507u.
CrossRef - Navaladian, S.; Viswanathan, B.; Viswanath, R. P.; Varadarajan, T. K. Nanoscale Res. Lett. 2007, 2, 44 https://doi.org/10.1007/s11671-006-9028-2.
CrossRef - Iravani, S.; Korbekandi, H.; Mirmohammadi, S. V.; Zolfaghari, B. Res. Pharm. Sci. 2014, 9, 6, 385-406.
- Jara, N.; Milan, N. S.; Rahman, A.; Mouheb, L.; Boffito, D. C.; Jeffryes, C.; Dauhoumane, S. A. Molecules, 2021, 26, 15, 4585 https://doi.org/10.3390/molecules26154585.
CrossRef - Kotakadi, V. S.; Rao, Y. S.; Gaddam, S. A.; Prasad, T. N. V. K. V.; Reddy, A. V.; Gopal, D. V. R. S. Colloids Surf. B: Biointerfaces, 2013, 105, 194-198 https://doi.org/10.1016/j.colsurfb.2013.01.003.
CrossRef - Mubayi, A. ; Chatterji, S.; Rai, P. K.; Watal, G. Adv. Mater. Lett. 2012, 3, 6, 519-525 http://doi.org/10.5185/amlett.2012.icnano.353.
CrossRef - Jorge de Souza, T. A.; Rosa Souza, L. R.; Franchi, L. P. Ecotoxicol. Environ. Saf. 2019, 171 https://doi.org/10.1016/j.ecoenv.2018.12.095.
CrossRef - Javaid, A. ; Oloketyi, S. F.; Khan, M. M.; Khan, F. Bio Nano Sci. 2019, 8 https://doi.org/10.1007/s12668-017-0496-x
CrossRef - Singh, R.; Shedbalkar, U. U.; Wadhwani, S. A.; Chopade, B. A. Appl Microbiol Biotechnol, 2015, 99, 1 https://doi.org/10.1007/s00253-015-6622-1.
CrossRef - Guilger-Casagrande, M.; de Lima, R. Front. Bioeng. Biotechnol. 2019, 7 https://doi.org/10.3389/fbioe.2019.00287.
CrossRef - Zhao, X.; Zhaou, L ; Rajoka, M. S. R.; Yan, L.; Jiang, C.; Shao, D.; Jing, Z.; Shi, J.; Huang, Q.; Yang, H.; Jin, M. Crit. Rev. Biotechnol. 2018, 38, 6 https://doi.org/10.1080/07388551.2017.1414141.
CrossRef - Naidu, K. S. B.; Murugan, N.; Adam, J. K.; Sershen, Bio. Nano. Sci. 2019, 9, 2 https://doi.org/10.1007/s12668-019-00612-4.
CrossRef - Shameli, K.; Ahmad, M. B.; Zamanian, A.; Sangpour, P.; Shabanazadeh, P.; Abdollahi, Y.; Zargar, M. Int. J. Nanomed. 2012, 7 doi: 10.2147/IJN.S36786.
CrossRef - Banala, R. R.; Nagati, V. B.; Karnati, P. R. Saudi J. Biol.Sci. 2015, 22, 5 https://doi.org/10.1016/j.sjbs.2015.01.007.
CrossRef - Mosae Selvakumar, P.; Antonyraj, C. A.; Babu, R.; Dakhsinamurthy, A.; Manikandan, N.; Palanivel, A. Synthesis and Reactivity in Inorganic, Metal-Organic, and Nano-Metal Chemistry, 2016, 46, 2 https://doi.org/10.1080/15533174.2014.971810.
CrossRef - Nabikhan, A.; Kandasamy, K.; Raj, A.; Alikunhi, N. M. Colloids Surf. B: Biointerfaces, 2010, 79, 2 https://doi.org/10.1016/j.colsurfb.2010.05.018
CrossRef - Jagtap, U. B.; Bapat, V. A. Ind. Crops Prod. 2013, 46 https://doi.org/10.1016/j.indcrop.2013.01.019.
CrossRef - Otari, S. V.; Patil, R. M.; Ghosh, S. J.; Pawar, S. H. Mater.Lett. 2014, 116 https://doi.org/10.1016/j.matlet.2013.11.066.
CrossRef - Yenesew, A.; Twinomuhwezi, H.; Kabaru, J. M.; Akala, H. M.; Kiremire, B.T.; Heydenreich, M.; Peter, M. G.; Eyase, F. L.; Waters, N.C.; Walsh, D. S. Bull. Chem. Soc. Ethiop. 2009, 23, 3 doi: 10.4314/bcse.v23i3.47665.
CrossRef - Kumar, V. A.; Ammani, K.; Jobina, R.; Subhaswaraj, P.; Siddhardha, B. J. Photochem. Photobiol. B Biol. 2017, 171, pp. 1–8, https://doi.org/10.1016/j.jphotobiol.2017.04.022.
CrossRef - Takeda, Y.; Yano, K.; Ayabe, H.; Masuda, T.; Otsuka, H.; Sueoshi, E.; Shinzato, T.; Aramoto, M. J. Nat. Med., 2008, 62, 4 https://doi.org/10.1007/s11418-008-0263-y.
CrossRef - Jyoti, K.; Singh, A.; J. Genet. Eng. Biotechnol. 2016, 14, 2 https://doi.org/10.1016/j.jgeb.2016.09.005.
CrossRef - Cyril, N.; George, J. B.; Joseph, L.; Sylas, V. P. J. Clust. Sci., 2019, 30 https://doi.org/10.1007/s10876-019-01508-9.
CrossRef - Bera, R. K.; Raj, C. R. J. Photochem. Photobiol. A: Chemistry, 2013, 270 https://doi.org/10.1016/j.jphotochem.2013.07.005.
CrossRef - Babu, M. M. G.; Gunasekaran, P. Colloids Surf. B: Biointerfaces, 2009, 74, 1, 191-195 https://doi.org/10.1016/j.colsurfb.2009.07.016.
CrossRef - Hergert, W.; Wriedt, T. The Mie Theory: Basics and Applications. 2012, Springer.
CrossRef - Sathyavathi, R.; Krishna, M. B.; Rao, S. V.; Saritha, R.; Rao, D. N. Adv. Sci. Lett. 2010, 3, 2 https://doi.org/10.1166/asl.2010.1099.
CrossRef - Gole, A.; Dash, C.; Ramakrishnan, V.; Sainkar, S. R.; Mandale, A. B.; Rao, M.;. Sastry, M Langmuir, 2001, 17, 5 https://doi.org/10.1021/la001164w.
CrossRef - Tagad, C. K.; Kim, H. U.; Aiyer, R. C.; More, P.; Kim, T.; Moh, S. H.; Kulkarni, A.; Sabharwal, S.G. RSC Adv. 2013, 3 https://doi.org/10.1039/C3RA44547J.
CrossRef - Mohan, S.; Oluwafemi, O. S.; George, S. C.; Jayachandran, V. P.; Lewu, F. B.; Songca, S. P.; Kalarikkal, N.; Thomas, S. Carbohydr.Polym. 2014, 106 https://doi.org/10.1016/j.carbpol.2014.01.008.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









