Pharmacognostic Characterization and Antacid Activity of Aqueous Extract of Desmodium Triflorum Linn and Pogostemon Heyneanus Benth
W.J.A. Banukie Jayasuriya1*, L. D. A. Menuka Arawwawala2, Shashani Gamage1, Himanshi Rathnasekara1, Bhavantha Dias1, Sugandhika Suresh3
1Department of Pharmacy and Pharmaceutical Sciences, Faculty of Allied Health Sciences, University of Sri Jayewardenepura, Gangodawila, Nugegoda, Sri Lanka.
2Industrial Technology Institute, Herbal Technology Section, R and D Complex, Halbarawa, Thalahena, Malabe, Sri Lanka.
3Department of Biochemistry, Faculty of Medical Sciences, University of Sri Jayewardenepura, Gangodawila, Nugegoda, Sri Lanka.
Corresponding Author E-mail: banukie@sjp.ac.lk
DOI : http://dx.doi.org/10.13005/ojc/390103
Article Received on : 18 Nov 2022
Article Accepted on : 17 Jan 2023
Article Published : 06 Feb 2023
Reviewed by: Dr.Asem Bimola Devi
Second Review by: Dr. Sanjeev Deshpande
Final Approval by: Dr. Bal Krishan Sharma
Present study was aimed to investigate the antacid potential of Desmodium triflorum Linn whole plant and Pogostemon heyneanus Benth leaves and to establish their diagnostic characteristics. Powder microscopy, detailed anatomical characteristics, analysis of phytochemicals and Ultra-Performance Liquid Chromatography were done. In addition, neutralizing effects on artificial gastric acid (AGA) by aqueous extracts (AE) of the plants, their ethyl acetate fractions (EAF) and residual aqueous fractions (RAF) were determined. Fordtran’s model (a titration method) was used to evaluate the in vitro neutralization capacity. A modified model of Vatier's artificial stomach was used in the assessment of in vitro acid neutralization duration. Statistical analysis was performed using SPSS 25.0. Pharmacognostic study aids in establishing the standardization parameters. Treatments including AE, RAF of D. triflorum and P. heyneanus showed significant acid neutralizing effects, duration for consistent neutralization and neutralization capacities. Findings of this study indicated that D. triflorum whole plant and P. heyneanus leaves possessed potent antacid effects.
KEYWORDS:Fabaceae; Fordtran’s model; Lamiaceae,, Vatier's artificial stomach
Download this article as:| Copy the following to cite this article: Jayasuriya W. J. A. B, Arawwawala L. D. A. M, Gamage S, Rathnasekara H, Dias B, Suresh S. Pharmacognostic Characterization and Antacid Activity of Aqueous Extract of Desmodium Triflorum Linn and Pogostemon Heyneanus Benth. Orient J Chem 2023;39(1). |
| Copy the following to cite this URL: Jayasuriya W. J. A. B, Arawwawala L. D. A. M, Gamage S, Rathnasekara H, Dias B, Suresh S. Pharmacognostic Characterization and Antacid Activity of Aqueous Extract of Desmodium Triflorum Linn and Pogostemon Heyneanus Benth. Orient J Chem 2023;39(1). Available from: https://bit.ly/3ldHRCg |
Introduction
An increase in the usage of herbal medicines in the developed world is observed in the last few years. The majority of the global population failed to afford medicines and medicinal products from Western Pharmaceutical Industry, which makes them rely solely or partly upon traditional medicines. Due to the notable reliance on medicinal plants in the treatment of ailments and their undetected potency in the process of drug discovery, it is timely to search for effective and safe plant medicines1.
Desmodium triflorum Linn (Family Fabaceae) is a well-known medicinal plant in Sri Lanka and called as ‘Heen-Undupiyaliya’ in Sinhala. The plant is available in many other tropical countries including Java, Philippine, India, and Taiwan. In Sri Lanka, the plant is commonly found in the low country2-3. Different parts of D. triflorum such as the leaves, roots, and sometimes even the whole plant is used in Ayurvedic medicine for various treatment objectives4-5. The plant is used in the treatment of headaches, eye disease, dysentery, bone fractures, and in snake biting2-3. Antiproliferative, anthelmintic, anticonvulsant, analgesic, hypoglycaemic, and anti-inflammatory activities of D. triflorum have also been demonstrated recently in modern studies6-9.
Pogostemon heyneanus Benth. (Family Lamiaceae), is an aromatic herb, commonly called as ‘Kollan kola’ or ‘Gan kollan kola’ in Sinhala. Shade-dried leaves of the plant yield a commercially important oil (patchouli oil) which is comprised of a spicy, herbaceous fragrance 10. The plant has been used widely in different traditional medicinal practices to treat many medical ailments. A decoction of the leaves is given for coughs and asthma, and poultices are applied for boils, headaches, jaundice and bilious fevers. It acts internally as an aromatic stomachic and carminative with astringent properties. Furthermore, the leaves are used for anorexia, chronic dyspepsia, and flatulence11. P. heyneanus is reported to possess antibacterial12, antifungal13, cytotoxic and anticancer14, antioxidant10, insecticidal15 and wound healing16 activities.
Pogostemon cablin, another species belonging to the genus of Pogostemon,is a well-known traditional Chinese medicinal herb for gastrointestinal diseases in South East Asia17. Moreover, the gastroprotective activity of P. cablin is well documented18. Despite the traditional use of P. heyneanus for gastric ailments, no studies were carried out to evaluate the antacid potency of P. heyneanus leaves so far.
Gastroprotective activity of Desmodium gangeticum has been reported19. However, D. triflorum has not been evaluated for its gastroprotective activity in-vitro. Considering all the above facts, the present study aimed to assess the gastroprotective activity of aqueous extracts of D. triflorum and P. heyneanus and their fractions in-vitro. Even though the study plants are of great medicinal importance yet there is no any study carried out for their pharmacognostic standardization as such data are required for authentication and quality control. Therefore, pharmacognostic characterization including macro and microscopic evaluation and the determination of phytochemical properties of D. triflorum whole plant and P. heyneanus leaves were also carried out.
Materials and Methods
Collection, identification, and preparation of plant material
Fresh P. heyneanus leaves were obtained from the Western Province of Sri Lanka, and fresh D. triflorum were collected from the Southern Province and Western Province of the island during the months of January to April, 2020. Plants were authenticated from the National Botanical Herbarium, Peradeniya, Sri Lanka, and the voucher specimens were deposited under reference numbers, DT Pharm 001(Desmodium triflorum) and PH Pharm 002(Pogostemon heyneanus).
Extraction of plant materials
Collected fresh plants were washed thoroughly, dried under shade, and then dried in a hot air oven at 40 ℃ until a constant weight was obtained and stored in air-tight polythene bags at a dry place. The dried plants were then ground to a coarse powder and this powder was stored in an air-tight and light-resistant container. The fresh leaves were used in the microscopic assessment. Small pieces of dried leaves (50 g) of P. heyneanus and dried D. triflorum whole plant(50 g) were weighed, respectively, and extracted with distilled water by refluxing. Crude aqueous extract (AE) of each plant was obtained and the extracts were fractionated using ethyl acetate. Fractions were concentrated and ethyl acetate fraction (EAF) and residual aqueous fractions (RAF) were obtained, respectively. Crude methanolic extracts of both plants were prepared and then used in the Ultra-performance liquid chromatography (UPLC) fingerprinting.
Pharmacognostic evaluations of Desmodium triflorum and Pogostemon heyneanus
Macroscopic evaluation
Macroscopic evaluations of the plants were done using 5 samples from each plant. Respective taxonomical descriptions were made according to the data given in the literature20.
Microscopic evaluation of stem and leaves of the plants
Microscopic evaluations of thestem and leaves of the plants were performed as per the methods described elsewhere21. Briefly, fresh cross-sections of the leaves were collected using a sharp blade. They were dehydrated in a graded alcohol series of 30%, 50%, and 70%, respectively, and transferred into a safranin solution (diluted in 70% alcohol). The anatomical observation was conducted under the microscope by mounting the selected cross-sectioned sample on a slide with 1-2 drops of chloral hydrate and then covering it with a cover slip. The prepared slides were examined under the light microscope.
Powder microscopy study
The powder microscopy study was performed according to the previously described method22. Shade dried leaves of P. heyneanus and leaves and stems of D. triflorum were finely powdered separately. Small quantities of powdered samples were placed on slides separately and each slide was mounted with 2-3 drops of chloral hydrate (75 %) solution. Each slide was covered with a cover slip and examined under the light microscope.
Qualitative and quantitative analysis of phytochemicals
Phytochemical analysis was carried out on the AEof each plant sample and their fractions (EAF and RAF), following standard procedures23. Phytochemical screening of phenolics was carried out using vanillin and lead acetate tests. To detect flavonoids, ammonia sulfuric acid test was employed. Tannins were detected with vanillin, lead acetate, and ferric chloride tests. The frothing test was performed for the detection of saponins in the sample. The steroids and terpenoids were detected by Liebermann-Burchard test and Salkowski test respectively. Vanillin in ethanol solution and conc. H2SO4 were used for the presence of monoterpenes. The extract was treated with a few drops of conc. H2SO4 to test for the presence of sesquiterpenes. For the screening of alkaloids, the picric acid test was carried out and observed for the presence of a yellow crystalline precipitate. Cardiac glycosides were detected by Glacial acetic acid with FeCl3 test.
The Total phenolic content (TPC) AE of D. triflorum whole plant and P. heyneanus leaves respectively, were analysed by Folin-Ciocalteu colorimetric method24. Stock solutions (100 mg/ml) were prepared from the two extracts. Twenty microliters of three different concentrations of each extract were mixed with 110 μL of freshly prepared and ten times diluted Folin-Ciocalteu reagent. After that, 10% sodium carbonate (70 μL) was added to each and the mixtures were kept at room temperature (25 ± 2 °C) for 30 minutes. The absorbances of the mixtures were recorded at 765 nm wavelength. Gallic acid was used as the standard. A calibration curve of gallic acid was prepared using different concentrations of gallic acid (mg/ml) in the same manner. The TPC was expressed as mg gallic acid equivalents (GAE)/g of the extract.
The Total flavonoid content (TFC) of the D. triflorum whole plant and AE of P. heyneanus leaves respectively, were analysed according to the aluminium chloride colorimetric method24. Stock solutions (100 mg/ml) were prepared from the two extracts. A volume of 100 μL of 2% aluminium chloride dissolved in methanol was mixed with three different concentrations of each extract (100 μL). The mixtures were allowed to stand at room temperature (25 ± 2°C) for 10 minutes and then absorbance values were recorded at 415 nm. Quercetin was used in the preparation of the standard curve. The TFC was expressed as mg quercetin equivalents (QE)/g of the extract.
Thin-layer Chromatographic separation and Ultra-performance liquid chromatography analysis
The Thin layer chromatography (TLC) of AE of D. triflorum whole plant, P. heyneanus leaves and all fractions was done according to the standard procedure25. TLC analyses were carried out using ALUGRAM Xtra SIL G/UV254 aluminium sheets (Sigma-Aldrich). The solvent system was made from ethyl acetate, dichloromethane, and cyclohexane in 3:2:1 (v/v/v). The bands were observed under UV light at 254 nm and 365 nm wavelengths. The vanillin solution (1 g vanillin + 100 ml conc. H2SO4) was used as the visualizing agent and the Rf values were determined.
Ultra-performance liquid chromatography (UPLC) fingerprint analyses were performed on Waters Acquity UPLC H class (Waters Corporation, USA) ultra-performance liquid chromatography machine with Waters Acquity UPLC BEH C18 column (2.1 mm × 100 mm, 1.7 µm). The mobile phase used in the analyses of both plants consisted of aqueous 0.2% formic acid (solvent A) and acetonitrile (solvent B). Gradient elution conditions in the analysis of P. heyneanus were: 0 – 5 min, 30% – 33% B; 5 – 8 min, 33% B; 8 – 14 min, 33% – 41% B; 14 – 25 min, 41% B; 25 – 29 min, 41% – 54% B; 29 – 35 min, 54% – 100% B; 35 – 40 min, 100% B. The flow rate was 0.3 mL/min. The injection volume was 0.5 μL. Analysis was done at 286 nm. Gradient elution conditions in the analysis of D. triflorum were: 0 – 4 min, 5% – 14% B; 4 – 7 min, 14% B; 7 – 12 min, 14% – 28% B; 12 – 18 min, 28% – 55% B; 18 – 19 min, 55% – 5% B. The flow rate was 0.3 mL/min. The injection volume was 0.5 μL. Analysis was done at 272 nm.
Determination of antacid activity
Neutralizing effect on artificial gastric acid
Neutralizing effects of AE and the fractions (EAF and RAF) of D. triflorum whole plantandP. heyneanus leaves were evaluated by previously reported in-vitro method26-27. In brief, 90 ml of test samples and distilled water each, and the standard, ENO, a dispersible antacid powder (90 ml) were added to the artificial gastric acid (100 ml) separately. The pH values of the test samples were recorded to determine the neutralizing effects. For the preparation of artificial gastric acid, 2.0 g of sodium chloride and 3.2 mg of pepsin enzyme (SIGMA) were dissolved in 500 ml of distilled water. HCl at a volume of 7.0 ml and an adequate amount of water were added to make up the solution to 1000 ml in volume. Then the pH of the solution was adjusted to 1.2.
Neutralizing capacity using the titration method of Fordtran’s model
Neutralizing capacity of AE and the fractions (EAF and RAF) of D. triflorum whole plant and P. heyneanus leaves was evaluated using Fordtran’s titration method27. The test samples and distilled water each, and the standard drug, ENO (90 ml) were put separately in a 250 mL beaker and this was maintained at 37 ℃. A magnetic stirrer was placed in it to run continuously at 30 rpm while imitating the gastric movements. Each test sample, distilled water, and the reference drug were titrated with 0.1N HCl to the end point of pH 3. The consumed volume of the HCl was measured as the parameter of acid-neutralising capacity.
Duration of consistent neutralization on artificial gastric acid using the modified model of Vatier’s artificial stomach
Duration of consistent neutralization on artificial gastric acid by AE and the fractions (EAF and RAF) of D. triflorum whole plantandP. heyneanus leaves was determined using the Vatier’s artificial stomach, a modified model 26-27. The test samples and distilled water at 90 ml of each, and the standard drug, ENO (90 ml) were mixed separately to 100 ml of artificial gastric juice in the artificial stomach at 37 ℃. Contents in the artificial stomach reservoir were continuously stirred at 30 rpm using a magnetic stirring apparatus. Artificial gastric juice at pH 1.2 was pumped in and out at 3 mL/min at the same time. A pH meter was connected to measure the changes in pH continuously in the artificial stomach. The duration of the neutralization effect was evaluated when the pH was returned to pH 1.2, the initial pH value.
Statistical analysis
All results were expressed as mean ± Standard error of the mean (SEM) and, where applicable, p-value < 0.05 was considered as significant. Statistical analysis of the results was performed by One-way ANOVA, using SPSS 25.0.
Results and Discussion
Desmodium triflorum and Pogostemon heyneanus are important medicinal plant materials extensively used in traditional medicine in Sri Lanka. In the present study, the in-vitro gastroprotective activities of AE of D. triflorum whole plant and P. heyneanus leaves and their fractions were evaluated. Pharmacognostic characterization of D. triflorum whole plant and P. heyneanus leaves was also carried out as pharmacognostic standardization is necessary for the identification and quality standardization of the plants.
The percentage yield for extraction from D. triflorum whole plant (50 g) was found to be: AE- 8.2 % w/w; EAF- 3.8 % w/w; RAF- 4.6 % w/w. Powder of P. heyneanus leaves (50 g) yielded AE- 7.8 % w/w; EAF- 4.5 % w/w; RAF- 3.3 % w/w.
Macroscopic and microscopic description of a plant is considered as the first step in identifying and examining its degree of purity28. In the macroscopic study, different parts of the plant are examined by the naked eye. It gives a morphological explanation of the relevant plant and helps to differentiate features among the species within a single genus29.
According to the macroscopic evaluation, D. triflorum is a very small, terrestrial prostate herb up to 50 cm long with rooting at nodes. Leaves of the plant are small, stipulate, alternate, and trifoliate. Terminal leaflets are 4-7.5 mm in length and 3-10 mm in width. Flowers are very small and bright purple in colour. P. heyneanus is an undershrub aromatic herb. The four-angled leaves of the plant are lanceolate, simple, opposite, without stipules, and slightly hairy on both sides. About 5-12.5 cm in length and petioles are 2.5-7.5 cm long.
The leaf anatomy of D. triflorum and P. heyneanus was evaluated microscopically. Figure 1a shows the transverse sections (T.S) of the leaf of D. triflorum. The cuticle was observed as the outermost layer. Just below the cuticle, epidermal cells were observed. Single layered upper epidermis contained few or no chloroplasts. Parenchymatous cells were compactly arranged and the outer walls of the cells were thick. Stomata could not be observed in the upper epidermis. Palisade parenchyma lies under the upper epidermis. There were 2-3 rows of long parenchymatous cells with plenty of chlorophyll and they were compactly packed without any intercellular spaces. Vascular bundles were centrally located and consisted of the xylem and phloem. Different cell arrangements and their anatomy were observed in the T.S of the leaf of P. heyneanus (Figure 1b). The outermost waxy cuticle layer called the epidermis consisted of trichomes. Just after the epidermis, cells called collenchyma consisting of thick deposits of cellulose in their cell walls were observed in a circular or oval shape. Lower epidermis was observed on the lower side of the leaf. Vascular bundles, consisting of the xylem and phloem were centrally located.
 |
Figure 1: Transverse sections of the leaf of Desmodium triflorum and Pogostemon heyneanus |
Stem anatomy of D. triflorum and P. heyneanus was observed microscopically. Figure 2a shows the T.S of the stem of D. triflorum. The T.S of the stem showed the cellular anatomy and the shape of the different cells. The outermost cell layer, the epidermis was observed as a compactly arranged single-cell layer without intercellular spaces. Below the epidermis, the endodermis was observed with compactly arranged cells. Vascular bundles were centrally located and arranged in a ring around the pith. Each bundle had a patch of xylem towards the centre, a patch of phloem towards the periphery, and a cambium in between them. The pith was the central portion of the stem. It was composed of thin-walled, rounded or polygonal parenchymatous cells with intercellular spaces. Xylem lies towards the pith of the vascular bundles. Figure 2b shows the T.S of the stem of P. heyneanus.The outermost layer, the epidermis consisted of trichomes. Just after the epidermis, the cortex was observed with collenchyma cells consisting of irregular thick cell walls that provide support to the structure. The cortex and pith together called ground tissue and it mainly consisted of parenchyma cells. Vascular bundles were observed and the vascular cambium could be identified in between the xylem and phloem in the vascular bundles. They were connected to form a continuous cylinder confirming the dicotyledon cell organization.
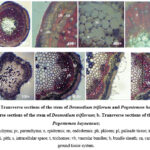 |
Figure 2: Transverse sections of the stem of Desmodium triflorum and Pogostemon heyneanus |
Transverse section examination of both plants showed special features of the cellular organization of the stem and leaf. Therefore, macroscopical and microscopical studies help to identify the diagnostic characters of both plants which can be used in the proper identification of the plants29.
In the identification of crude drugs, powder analysis plays a crucial role and would assist in the identification of the right variety and adulterants as well. Furthermore, powder microscopic evaluation can be used as the simplest and cheapest method to establish the correct identity of the source materials and an important approach in the standardization of the plant material and a path of pharmacological and therapeutic evaluation 30.
The prominent diagnostic features of the powder microscopy of D. triflorum revealed the presence of pitted vessels, calcium oxalate crystals, epidermal cells, simple fibres and starch granules (Figure 3).
 |
Figure 3: Powder microscopy of leaves of Desmodium triflorum and Pogostemon heyneanus |
The powder microscopy of D. triflorum stem powders indicated the presence of cork cells, epidermal cells, fibres, trichomes, calcium oxalate crystals, sclereids, pitted vessels and starch granules (Figure 4). Powder microscopical evaluation of dried leaf powder of P. heyneanus revealed the presence of trichomes, sclereids, calcium oxalate crystals, irregular shaped starch granules, lignified vessels, fibres etc. (Figure 3). A relatively high abundance of fibres was present in the samples collected from dry climatic geographical areas (Figure 3). Therefore, these powder microscopical features along with the observed anatomical features of the plants will aid in establishing the diagnostic characters that facilitate the correct identification of the species and provide useful parameters in quality control. These can be used as standards in authenticating the respective crude drugs.
 |
Figure 4: Powder microscopy of the stem of Desmodium triflorum; (a) cork cells (b) epidermal cells and crystals (c) fibres (d) trichomes |
The results of the preliminary phytochemical screening of D. triflorum whole plant and P. heyneanus leaves are represented in Table 1. Presence of tannins, phenolics, flavonoids, saponins, as well as cardiac glycosides in the AE of D. triflorum whole plant was observed. Phenolics, flavonoids, tannins, monoterpenes, and sesquiterpenes were present in both EAF and RAF. In addition, alkaloids and saponins were observed in RAF. Phytochemical screening of P. heyneanus leaves revealed the presence of tannins, phenolics, flavonoids, terpenoids, saponins, as well as cardiac glycosides in the AE. Phenolics, tannins, terpenoids, and cardiac glycosides were observed in both EAF and RAF. Flavonoids were only found in EAF. Alkaloids and saponins were only observed in RAF.
Table 1: Qualitative phytochemical analysis of aqueous extracts of Desmodium triflorum whole plant and Pogostemon heyneanus leaves and their fractions.
|
Phytochemicals |
D. triflorum whole plant |
P. heyneanus leaves |
||||
|
Aqueous extract |
Ethyl acetate fraction |
Residual aqueous fraction |
Aqueous extract |
Ethyl acetate fraction |
Residual aqueous fraction |
|
|
Phenolics |
+ |
+ |
+ |
+ |
+ |
+ |
|
Flavonoids |
+ |
+ |
– |
+ |
+ |
– |
|
Tannins |
+ |
+ |
+ |
+ |
+ |
+ |
|
Saponins |
+ |
– |
+ |
+ |
– |
+ |
|
Terpenoids |
– |
+ |
– |
+ |
+ |
+ |
|
Cardiac glycosides |
+ |
– |
– |
+ |
+ |
+ |
|
Alkaloids |
– |
– |
+ |
+ |
– |
+ |
|
Steroids |
– |
– |
– |
– |
– |
– |
+ Presence of the compound
– Absence of the compound
Presence of various phytochemicals in AE of D. triflorum and other organic extracts were reported6, 31. Similar to the present study, Singh et al., 2016, have not identified terpenoids, alkaloids, and steroids in the AE of D. triflorum. Medicinal effects of plants are widely varied with the region of cultivation. Therefore, it is essential to analyse the type of phytoconstituents present in D. triflorum grown in Sri Lanka. Similar to the present study, Dharmadasa et al., 2014, have reported the presence of saponins, alkaloids, tannins, and flavonoids in AE of Sri Lankan grown P. heyneanus leaves10. Moreover, the secondary metabolites observed in D. triflorum and P. heyneanus may be responsible for various pharmacological effects32-33. Biomarker compounds are considered as key compounds in the identification and determining the quality of plant materials. Phytochemical screening of plant materials will lead to establish such biomarker compounds34.
In the present study, TPC and TFC contents of AE of D. triflorum whole plant and P. heyneanus leaves were analysed. The TPC of AE of D. triflorum whole plant and P. heyneanus leaves were 0.69±0.16 mg GAE/ g and 0.45±0.00 mg GAE/ g respectively. The TFC of AE of D. triflorum whole plant and P. heyneanus leaves were 0.96±0.08 mg QE/ g and 1.34±0.00 mg QE/ g respectively. The TPC value of P. heyneanus leaves of the present study was lower than the reported data (0.83 ± 0.01 mg GAE/ g) 10. The TPC and TFC values of different extracts of D. triflorum were reported 35-36. However, the amount of secondary metabolites will depend on the season and the maturity of the plants.
The results of TLC of both plant extracts when viewed under UV 254 nm and UV 365 nm were shown in Figure 5. The solvent system was ethyl acetate, dichloromethane, and cyclohexane 3:2:1(v/v). When a TLC was carried out for AE, RAF, and EAF in the same TLC plate showed that there were no significant separations in AE and RAF compared to the EAF. The coloured bands were observed on the spraying detecting agent, 1% vanillin indicating the presence of alcoholic and carbonyl compounds. Proving the presence of different groups of compounds both EAFs of D. triflorum and P. heyneanus separated in upto 8 bands, at varying Rf values depicted in Table 2 and Figure 5. TLC of EAF depicts that the fraction contains a number of active constituents. Moreover, the characteristics patterns of TLC represented by EAF may be served as idiosyncratic fingerprints for qualitative evaluation. However, for the AE and RAF need to be separated by developing a suitable mobile phase in future studies.
Table 2: Thin layer chromatographic studies of ethyl acetate fractions of Desmodium triflorum whole plant and Pogostemon heyneanus leaves.
|
Fractions |
Solvents |
Ratio |
Rf values |
|
EAF of D. triflorum |
Ethyl acetate, dichloromethane and cyclohexane |
3:2:1(v/v) |
0.10,0.32,0.50,0.61,0.70,0.77,0.87,0.95 |
|
EAF of P. heyneanus |
0.11,0.20,0.31,0.47,0.56,0.65,0.73,0.80 |
 |
Figure 5: Thin layer chromatography of different extracts of Desmodium triflorum |
UPLC analysis of methanolic extracts of D. triflorum and P. heyneanus are shown in Figure 6a and Figure 6b respectively. The chromatogram of D. triflorum comprises three prominent peaks, which were observed between 0 to 20 min of the analysis. In the UPLC analysis of P. heyneanus, the chromatogram resulted in a greater number of prominent peaks between 0 to 42 min of analysis. The results of UPLC analysis comply with the HPLC observations of previous studies that the HPLC fingerprint of D. triflorum consists of less number of prominent peaks (due to the less number of compounds extracted to methanol in higher amounts) compared to Pogostemon spp36-37.
 |
Figure 6: Ultra-performance liquid chromatogram of crude methanolic extract of Desmodium triflorum and Pogostemon heyneanus. |
Peptic ulcer disease (PUD) has become one of the common causes of morbidity and mortality worldwide and can be characterized by erosions in the gastric and duodenal mucosal linings38. The imbalance between defensive factors (prostaglandin, mucin, nitric oxide, bicarbonate, and growth factors) and offensive factors (pepsin, acid, and Helicobacter pylori) causes this disease39. PUD is usually treated with proton pump inhibitors, H2-blockers, antacids, and anticholinergics. However, most of these therapeutic agents have limited efficacy, drug interactions, and side effects 38-39. Therefore, increasing attention towards botanical drug products can be seen to satisfy the requirement of novel therapeutic agents for gastric ulcers with favourable effectiveness, relatively low cost, and fewer adverse effects40. Consequently, D. triflorum and P. heyneanus are used traditionally for the treatment of gastrointestinal problems in Asian countries11. Therefore, the present study attempted to evaluate the in-vitro antacid activity of AE of D. triflorum, P. heyneanus, and its fractions comparatively.
The neutralizing effects of different concentrations of AE of each plant and their fractions were studied (Table 3).
Table 3: In vitro antacid activity of Desmodium triflorum whole plant and Pogostemon heyneanus leaves using aqueous extracts and their fractions
|
Sample |
Neutralizing effect |
Neutralizing capacity (Volume, ml) |
Duration of consistent neutralization(seconds) |
|
|
Initial pH |
End pH |
|||
|
Distilled water |
6.36±0.26 |
1.49±0.01 |
0.33±0.03 |
13.72± 3.33 |
|
Reference Drug (ENO) |
6.70±0.04 |
4.58±0.07† |
18.63±0.07† |
453.01±21.00† |
|
AE.D–58.00 mg/ml |
4.70±0.00 |
4.17±0.00† |
41.82±0.02† |
201.69± 0.34† |
|
AE.D – 29.00 mg/ml |
4.72±0.00 |
3.55±0.01† |
18.33±0.09† |
105.05± 5.53† |
|
AE.D – 14.50 mg/ml |
4.75±0.01 |
2.82±0.00† |
– |
82.72± 2.35* |
|
AE.P – 37.20 mg/ml |
5.05±0.00 |
4.02±0.01† |
33.90±0.06† |
196.37±12.47† |
|
AE.P -18.60 mg/ml |
5.08±0.01 |
3.49±0.01† |
17.87±0.07† |
114.41± 2.85† |
|
AE.P-9.30 mg/ml |
5.17±0.01 |
2.73±0.01† |
– |
87.06±13.03 * |
|
RAF.D – 42.00 mg/ml |
5.13±0.01 |
4.16±0.00† |
32.75±0.06† |
184.70± 1.46† |
|
RAF.D – 21.00 mg/ml |
5.23±0.00 |
3.57±0.00† |
16.60±0.04† |
120.36± 4.33† |
|
RAF.D – 10.50 mg/ml |
5.29±0.01 |
2.69±0.00† |
– |
91.03± 4.49* |
|
RAF.P – 43.00 mg/ml |
5.37±0.00 |
4.11±0.01† |
35.77±0.03† |
258.06± 4.61† |
|
RAF.P – 21.50 mg/ml |
5.31±0.00 |
3.62±0.00† |
17.70±0.06† |
125.73±18.35† |
|
RAF.P –10.75 mg/ml |
5.19±0.00 |
2.76±0.00† |
– |
90.03± 0.58 * |
|
EAF.D – 2.00 mg/ml |
4.94±0.16 |
1.31±0.01 |
0.33±0.04 |
49.39±17.84 |
|
EAF.P – 2.00 mg/ml |
4.76±0.22 |
1.31±0.01 |
0.39±0.33 |
108.68± 8.35† |
*p < 0.01 and †p < 0.001 compared to negative control (distilled water), ANOVA
Observed final pH values of plants at each concentration of AE, RAF, and the standard drug were significantly higher than negative control, demonstrating a significantly better neutralizing effect than distilled water (p<0.001). AE of D. triflorum (58 mg/ml) and AE of P. heyneanus (37.20 mg/ml) exhibited the highest neutralizing effects among the tested concentrations of AE of both plants. The concentrations of 42.00 mg/ml and 43.00 mg/ml possessed the highest neutralizing effects among the tested concentrations of RAF of D. triflorum and P. heyneanus, respectively. However, the standard drug expressed the highest neutralizing effect among the tested samples. However, EAF was not capable in neutralizing gastric acid when compared with distilled water. Moreover, the extracts which exhibited a final pH > 3 possessed excellent neutralizing effects when compared with distilled water (p< 0.001), thereby increasing the pH of the artificial gastric juice from pH 1.2 to a pH value more than 3. These results were comparable with the neutralizing effect expressed by the standard drug which showed the final pH 4.58±0.07. Furthermore, it can be observed that for all plant samples, the AE and RAF had consistently higher antacid potential than the EAF.
The neutralizing capacity of different concentrations of AE of D. triflorum and P. heyneanus and their fractions were performed by Fordtran’s in vitro titration model and compared with that of the standard and control. The volume of 0.1N HCl consumed to reach pH 3 was recorded for the test samples. According to Table 3, neutralizing capacity of tested concentrations of AE and RAF of each plant were significant (p < 0.001) when compared to distilled water. It was noted that AE of D. triflorum (58.00 mg/ml), AE of P. heyneanus (37.20 mg/ml), RAF of D. triflorum (42.00 mg/ml) and RAF of P. heyneanus (43.00 mg/ml) have exhibited the potent neutralization capacity (p < 0.001) among the tested samples, and were able to consume 41.82±0.02 ml, 33.90±0.06 ml, 32.75±0.06 ml and 35.77±0.03 ml of 0.1N HCl respectively. The neutralization capacity of the standard was 18.63±0.07 ml and it was found to be less (p < 0.001) when compared to the above-mentioned samples. Moreover, AE of D. triflorum at 29.00 mg/ml and AE of P. heyneanus at 18.60 mg/ml showed good neutralizing capacities which were comparable with the neutralizing capacity of the standard drug. EAF of both plants at 2 mg/ml did not exhibit significant neutralizing capacity (Table 3).
According to the results represented in Table 3, the duration of neutralization was highest for RAF of P. heyneanus at 43.00 mg/ml which was found to be 258.06±4.61 seconds. AE of D. triflorum at 58.00 mg/ml concentration also demostrated a relatively similar duration of neutralizing effect of 201.69±0.34 seconds, whereas the standard drug ENO showed the best activity with a duration of neutralization of 453.01±21.00 seconds. The duration of consistent neutralization effect of tested AE and RAF doses of both plants and EAF of P. heyneanus were lesser than the reference drug but significantly (p < 0.01) better than the negative control, distilled water. EAF of D. triflorum did not exhibit significant effect when compared to the distilled water. Hence, it can be suggested that the possibility of a relapse in acidity will be delayed with AE and RAF of both plants and EAF of P. heyneanus.
Fordtran’s titration model and Vatier’s artificial stomach model, which mimic some of the regular physiological functioning of a human stomach, were mainly used in this study to determine the in-vitro antacid activity of the selected plants. These models are commonly adapted in research studies to discover the gastroprotective potential of herbal remedies or plants41- 42. Fordtran’s model mimics the regular physiological conditions of the stomach by maintaining the temperature at 37 ℃ and inducing stomach movements by stirring at 30 rpm 26, 41. The artificial stomach model consists of three parts which include a a pH recording system, stomach and a peristaltic pump. The stomach is devised with three parts as the reservoir, secretory flux and gastric emptying flux. The secretory flux corresponds to acid secretion and emptying flux corresponds to gastric emptying. It mimics gastric secretion and empties with a 3ml/min rate. Furthermore, the reservoir of the artificial stomach is maintained at 37 ℃ and stirred continuously (30 rpm) for providing the physiological situations 43.
In the present study, the highest concentrations of AE and RAF of each plant exhibited the highest neutralizing capacities and were higher than that of the positive control, ENO. Incorporation of AE and RAF of both plants and EAF of P. heyneanus to the gastric reservoir caused an increase of pH and significantly longer lag times for initial pH recovery than the negative control, indicating consistent antacid effects. Therefore, AE and RAF of both plants were found to have potent antacid activity in vitro. The positive control, ENO demonstrated the highest duration of consistent neutralization when compared to that of AE and RAF doses of both plants and EAF of P. heyneanus in the present study. This may be due to the crude nature of the plant extracts. A similar type of finding was observed in the study conducted by Panda and Shinde, 2016. The standard drug, ENO comprised of NaHCO3, citric acid, and sodium carbonate. Owing to the dispersible nature, powder exhibits a fast dissolution in gastric juice and high alkaline nature has brought a quick rise in pH and a longer antacid activity.
Moreover, the investigation showed that the polar solvent extracts and fractions of both plants have more antacid activity than nonpolar solvent fractions. It can be attributed that bioactive compounds which are responsible for antacid activity may present in the polar extracts than in the nonpolar extract.
Antacids act either by neutralizing the gastric HCl content through releasing anions into the medium or chemically reacting with buffer quantities in the gastric content without any direct effect on the ultimate output and relief of the hyperacidity condition in the stomach by reducing acid concentration 44. Antacids are known to express some side effects such as constipation, nephrolithiasis, diarrhoea, hypercalcemia, milk alkali syndrome, and intestinal blocking of phosphorus absorption. Drug interactions of antacids are also a major clinical issue 45. Considering the interactions and side effects of antacids, D. triflorum, P. heyneanus are suitable alternatives for the treatment of PUD.
In conclusion, the diagnostic characters discussed may be of potential application in the identification of the D. triflorum whole plant and P. heyneanus leaves, and to establish standardization parameters. D. triflorum whole plant and P. heyneanus leaves exhibited potent antacid activity and the plants further needed to be explored for accountable bioactive constituents. Evaluation of the antacid potential of both plants using a suitable in vivo model and elucidation of its mechanism of action is recommended in future studies.
Acknowledgements
Financial assistance by the University of Sri Jayewardenepura, Sri Lanka, is gratefully acknowledged (Grant no.: ASP/01/RE/AHS/2021/88).
Conflicts of Interest
The authors declare that there is no conflict of interest. The authors alone are responsible for the content of the paper.
References
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Neurol. 2014; 4: 1–10.
CrossRef - Ilandara, R.; Chandrapala, R.; Jayasuriya, W.J.A.B.N.; Suresh, T.S. Phytochemical and ethno-pharmacological properties of Desmodium triflorum: A Review. Pharmaceutical Journal of Sri Lanka. 2015; 5: 34-38.
CrossRef - Ayurvedic medicinal plants of Sri Lanka compendium. Ayurvedic medicinal plants. [Accessed November 6, 2021]. Available from: http://www.instituteofayurveda.org/plants/.
- Warrier, P.K.; Nambiar, V.P.K.; Ramankutty, C. Indian Medicinal Plants, A Compendium of 500 Species. vol 1. Hyderabad: Orient Black Swan, 1993.
- Deshpande, D.J. A handbook of herbal remedies: A source book of medicinal herbs chemical constituents biological activities and usage. India: Agrobios, 2008.
- Bhosle, V. Anticonvulsant and antioxidant activity of aqueous leaves extract of Desmodium triflorum in mice against pentylenetetrazole and maximal electroshock induced convulsion. Rev. Bras. Farmacogn. 2013; 23(4): 692–698.
CrossRef - Lai, S.C.; Peng, W.H.; Huang, S.C.; Ho, Y.L.; Huang, T.H.; Lai, Z.R.; Chang, Y.S. Analgesic and anti-inflammatory activities of methanol extract from Desmodium triflorum DC in mice. Am. J. Chinese Med. 2009; 37(3): 573–588.
CrossRef - Lai, S.C.; Ho, Y.L; Huang, S.C.; Huang, T.H.; Lai, Z.R.; Wu, C.R.; Lian, KY.; Chang, Y.S. Antioxidant and antiproliferative activities of Desmodium triflorum (L.) DC. Am. J. Chinese Med. 2010; 38(2): 329–342.
CrossRef - Gavalapu, V.R.; Kolli, P.; Korra, S.K.; Kavuri, M.K.; Avagadda, C.; Singam, V.; Vanumu, Y.; Kudirella, H. Preliminary phytochemical screening and anthelmintic activity of Desmodium Triflorum (L.) DC leaf and root extracts. Int. J. Pharma. Sci. 2013; 3(1): 156-158.
- Dharmadasa, R.M.; Rathnayake, R.M.D.H.; Abeysinghe, D.C.; Rashani, S.A.N.; Samarasinghe, K.; Attanayake, A.L.M. Screening of local and introduced varieties of Pogostemon heyneanus Benth. (Lamiaceae), for superior quality physical, chemical and biological parameters. World J. Agric. Res. 2014; 2(6), 261-266.
CrossRef - Jayaweera, D.M.A. Medicinal Plants (Indigenous and Exotic) Used in Ceylon. part 3. Sri Lanka: National Science Council of Sri Lanka, 1981.
- Adhavan, P.; Kaur, G.; Princy, A.; Murugan, R. Essential oil nano emulsions of wild patchouli attenuate multi-drug resistant gram-positive, gram-negative and Candida albicans. Ind. Crops. Prod. 2017; 100: 106-116.
CrossRef - Karpiński, T.M. Essential oils of Lamiaceae family plants as antifungals. Biomolecules. 2020; 10(1): 103.
CrossRef - Rai, V.; Pai, V.R.; Kedilaya, P. A preliminary evaluation of anticancer and antioxidant potential of two traditional medicinal plants from Lamiaceae – Pogostemon heyneanus and Plectranthus amboinicus. J App Pharm Sci. 2016; 6(8): 73-78.
CrossRef - Anjana, S.; Thoppil, J.E. Chemical composition of the essential oils of four Pogostemon spp and their larvicidal activity against Aedes albopictus Skuse (Diptera : Culicidae). International Journal of environmental biology. 2013; 3(1): 26–31.
- Thomas, B.; Arumugam, R.; Veerasamy, A,; Ramamoorthy, S. Ethnomedicinal plants used for the treatment of cuts and wounds by Kuruma tribes, Wayanadu districts of Kerala, India. Asian Pac. J. Trop. Biomed. 2014; 4(1): 488–491.
CrossRef - Chen, X.Y.; Chen, H.M.; Liu, Y.H,; Zhang, Z.B.; Zheng, Y.F.; Su, Z.Q.; Zhang, X.; Xie, J.H.; Liang, Y.Z.; Fu, L.D.; Lai, X.P.; Su, Z.R.; Huang, X.Q. The gastroprotective effect of pogostone from Pogostemonis Herba against indomethacin-induced gastric ulcer in rats. Exp Biol Med. 2016; 241(2): 193–204.
CrossRef - Wu, J.Z.; Liu, Y.H.; Liang, J.L.; Huang, Q.H.; Dou, Y.X.; Nie, J.; Zhuo, J.Y.; Wu, X.; Chen, J.N.; Su, Z.R.; Wu, Q.D. Protective role of β-patchoulene from Pogostemon cablin against indomethacin-induced gastric ulcer in rats: Involvement of anti-inflammation and angiogenesis. Phytomedicine. 2018; 39: 111-118.
CrossRef - Mahesh, A.; Jeyachandran, R.; Rao, D.M.; Thangadurai, D. Gastroprotective effect of Desmodium gangeticum roots on gastric ulcer mouse models. Rev. Bras. Farmacogn. Braz. J. Pharmacogn. 2012; 22(5): 1085-1091.
CrossRef - Aslam, I.; Afridi, M.S.K. Pharmacognostic characterization of Beaumontia grandiflora (Roxb.) Wall. leaf for taxonomic identification for quality control of a drug. J. Appl. Res. Med. Aromat. Plants. 2018; 8: 53-59.
CrossRef - Peng, Y.L.; Shishiyama, J.; Yamamoto, M. A whole-leaf staining and clearing procedure for analysing cytological aspects of interaction between rice plant and rice blast fungus. Ann. Phytopath. Soc. Japan. 1986; 52(5): 801–808.
CrossRef - Akbar, S.; Hanif, U.; Ali, J.; Ishtiaq, S. Pharmacognostic studies of stem, roots and leaves of Malva parviflora L. Asian Pac. J. Trop. Biomed. 2014; 4(5): 410-415.
CrossRef - Goveas, S.W.; Abraham, A. Extraction and secondary metabolite analysis of Coscinium fenestratum (Gaertn.) Colebr: An important medicinal plant of Western Ghats. Int. J. Pharm. Sci. Res. 2014; 5(8): 3484–3489.
- Perera, P.K.; Subasinghe, U.R.; Arawwawala, M. Evaluation of physico-chemical properties and antioxidant capacity of leaf powder of Moringa (Moringa Oleifera, Lam) grown in Sri Lanka. Asian J Med Health Res. 2017; 2(5): 1-9.
- Abubakar, N.; Shehu, K.; Yahaya, M.M.; Tafinta, I.Y.; Imonikhe, M.A. Phytochemical screening and Thin Layer Chromatographic studies of Guierasenegalensis G. F Gmel (Egyptian mimosa). Ann. Biol. Sci. 2016; 4(1): 26–30.
- Sandhya, S.; Venkata, R.K.; Vinod, K.R. A comparative evaluation of in vitro antacid activity of two Tephrosia species using modified artificial stomach model. Hygeia. J. D. Med. 2015; 7(2): 9–17.
- Fordtran, J.S; Morawski, S.G.; Richardson, C.T. In vivo and in vitro evaluation of liquid antacids. N. Engl J Med. 1973; 288(18): 923-928.
CrossRef - World Health Organisation. Quality control methods for herbal materials. Geneva: World Health Organisation, 1998.
- Chanda S. Importance of pharmacognostic study of medicinal plants: An overview. J. Pharmacogn. Phytochem. 2014; 2(5): 69–73.
- Yadav, P.; Adhikari, B.P.; Yadav, S.; Khadaka, S.; Bhandari, D.P.; Khanal, C. Microscopic, UV- Fluorescence and FT-IR analysis of the powder of five medicinal plants of Nepal. J. Pl. Res. 2018; 16(1): 70-77.
- Singh, N.; Tailang, M.; Mehta, S.C. Pharmacognostic and phytochemical screening of Desmodium triflorum linn. Int. J. Pharmacogn. 2016; 3(1): 43-49.
- Vedpal.; Dhanabal, S.P.; Dhamodaran, P.; Chaitnya, M.V.N.L.; Duraiswamy, B.; Jayaram, U.; Srivastava, N. Ethnopharmacological and Phytochemical profile of three potent Desmodium species: Desmodium gangeticum (L.) DC, Desmodium triflorum Linn and Desmodium triquetrum Linn. J. Chem. Pharm. 2016; 8(7): 91-97.
- Rai, V.; Pai, V.R.; Kedilaya, P. A preliminary evaluation of anticancer and antioxidant potential of two traditional medicinal plants from Lamiaceae-Pogostemon heyneanus and Plectranthus amboinicus. J App Pharm Sci. 2016; 6(8): 73-78.
CrossRef - Kumar, S.M.; Azamthulla, M.; Saravanan, K.S. Pharmacognostical evaluation and anti-convulsant property of Annona reticulata Linn. (Annonaceae) root. Futur J Pharm Sci. 2021; 7:173.
CrossRef - Lai, S.C.; Ho, Y.L.; Huang, S.C.; Huang, T.H.; Lai, Z.R.; Wu, C.R.; Lian, K.Y.; Chang, Y.S. Antioxidant and antiproliferative activities of Desmodium triflorum (L.) DC. Am J Chin Med. 2010; 38(2): 329-342.
CrossRef - Tsai, J.C.; Huang, G.J.; Chiu, T.H.; Huang, S.S.; Huang, T.H.; Lai, S.C.; Huang, T.H.; Lai, S.C.; Lee, C.Y. Antioxidant activities of phenolic components from various plants of Desmodium species. Afr. J. Pharm. Pharmacol. 2011; 5(4): 468-476.
CrossRef - Li, P.; Yin, Z-Q.; Li, S-L.; Huang, X.J.; Ye, W.C.; Zhang, Q.W. Simultaneous determination of eight flavonoids and pogostone in Pogostemon cablin by high performance liquid chromatography. Journal of Liquid Chromatography & Related Technologies. 2014; 37: 1771–1784
CrossRef - Park, J.U.; Kang, J.H.; Rahman, M.A.A.; Hussain, A.; Cho, J.S.; Lee, Y.I. Gastroprotective Effects of Plants Extracts on Gastric Mucosal Injury in Experimental Sprague-Dawley Rats. BioMed. Res. Int. 2019; doi:10.1155/2019/8759708
CrossRef - Zakaria, Z.A.; Zainol, A.S.N.; Sahmat, A.; Salleh, N.I.; Hizami, A.; Mahmood, N.D.; Nasir, N.; Mamat, S.S.; Hidayah, F.K.; Mohtarrudin, N.; Hamid, S.S.A.; Tohid, S.F.; The, L.K.; Salleh, M.Z. Gastroprotective activity of chloroform extract of Muntingia calabura and Melastoma malabathricum leaves. Pharm. Biol. 2016; 54(5): 812–826.
CrossRef - Kim, Y.S.; Lee, J.H.; Song, J.; Kim, H. Gastroprotective Effects of Inulae Flos on HCl/Ethanol-induced gastric ulcers in rats. Molecules. 2020; 25: 5623.
CrossRef - Sandhya, S.; Venkata, R.K.; Vinod, K.R.; Chaitanya, R. Assessment of in vitro antacid activity of different root extracts of Tephrosia purpurea (L) Pers by modified artificial stomach model. Asian Pac J of Trop Biomed. 2012; 2(3): 1487–1492.
CrossRef - Thabrew, M.I.; Arawwawala, L.D.A.M. An Overview of in vivo and in vitro models that can be used for evaluating anti-gastric ulcer potential of medicinal plants. Austin Biol. 2016; 1(2): 1007.
- Panda, V.S.; Shinde, P.M. A comparative study of the antacid effect of raw spinach juice and spinach extract in an artificial stomach model. J Complement Integr Med. 2016; 13(4): doi:10.1515/jcim-2016-0032.
CrossRef - Rang, H.P.; Dale, M.M.; Ritter, J.M.; Flower, R.J.; Hendorson, G. Rang and Dale’s Pharmacology. 9th edition, London: Churchill Livingstone, 2018.
- Wu, T,.H.; Chen, I.C.; Chen, L.C. Antacid effects of Chinese herbal prescriptions assessed by a modified artificial stomach model. World J Gastroenterol. 2010; 16(35): 4455-4459.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









