Evaluation of Secondary Metabolites of Ageratina adenophora and Synthesis of Silver Nanoparticles for its Antibacterial and Antioxidant Activity
Latha Maheswari B1 , Mani N1*
, Mani N1* , Kavikala N1
, Kavikala N1 , Karthika S1
, Karthika S1 and Rajasudha V2
and Rajasudha V2
Department of Chemistry, A.V.V.M Sri Pushpam College, Autonomous, (Affiliated to Bharathidasan University ),Poondi-613 503,Thanjavur (Dt), Tamil Nadu, India.
Department of Chemistry, Annai Vailankanni Arts and Science College, (Affiliated to Bharathidasan University), Thanjavur-613007 Tamil Nadu, India.
Corresponding Author E-mail: maniavvm@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/390112
Article Received on : 03 Nov 2022
Article Accepted on :
Article Published : 15 Feb 2023
Reviewed by: Dr. Fasi Ulla
Second Review by: Dr. Yashas S R
Final Approval by: Dr.Ammar A. Razzak Mahmood
Synthetic antibiotics have been successfully utilized for decades against pathogenic bacteria to control infectious diseases. However, the continuous and overuse has resulted in multidrug resistant (MDR) bacterial species. Further, the negative side effects caused by commercial antibiotics also hindered their usage. The phytochemicals produced by plants in response to adverse biotic and abiotic conditions possess significant pharmacological properties and can be an effective alternative to synthetic antibiotics. The phytochemicals of Ageratinaadenophora, served the role of reducing and stabilizing agent. Ageratinaadenophora mediated silver nanoparticles (Aa-AgNPs) were characterized using advanced spectroscopic instrumentation. The qualitative analysis by GC-MS showed Methyl ionone, 2(3H)-Naphthalenone, 4, 4a, 5, 6, 7, 8- hexahydro-4a,7,7-trimethyl-(R), Isolongifolone as the major compounds. The quantitative estimation showed leaves were rich in total phenol, flavonoids, alkaloids and tannins. The Aa-AgNPs were effective in inhibiting bacterial pathogens. Further, A.adenophora mediated nanoparticles possessed strong antioxidant activity.
KEYWORDS:Ageratinaadenophora; Antibacterial agent; Antioxidants; Isolongifolone; Silver nanoparticles
Download this article as:| Copy the following to cite this article: Maheswari B. L, Mani N, Kavikala N, Karthika S, Rajasudha V. Evaluation of Secondary Metabolites of Ageratina adenophora and Synthesis of Silver Nanoparticles for its Antibacterial and Antioxidant Activity. Orient J Chem 2023;39(1). |
| Copy the following to cite this URL: Maheswari B. L, Mani N, Kavikala N, Karthika S, Rajasudha V. Evaluation of Secondary Metabolites of Ageratina adenophora and Synthesis of Silver Nanoparticles for its Antibacterial and Antioxidant Activity. Orient J Chem 2023;39(1). Available from: https://bit.ly/3Ipf4DT |
Introduction
Bacterial infections pose a significant threat to humans around the world 1,2. The factors that contribute to microbial infections in emerging and underdeveloped countries include lack of public awareness about public hygiene, environmental cleanliness, effective sanitation, lack of critical primary health care, and rapid population growth rate 3. Escherichia coli, Streptococcus pneumonia, Staphylococcus aureus, Pseudomonas aeruginosa, and Klebsiella pneumoniae are the important pathogenic bacterial species that cause frequent infection in humans. These microorganisms cause childhood infections and premature deaths. 4. Synthetic antibiotics are effective against bacterial pathogens. However, the emergence of bacterial resistance to commercial antibiotics hinders the efficacy of treatment. Furthermore, negative side effects of antibiotics such as allergies, hypersensitivity, and immunosuppression also play critical role in treating infectious disorders. The overuse and improper application of antibiotics against microbes led to the emergence of multidrug-resistant microorganisms. 5-7. This crisis would worsen in the near future if alternative strategies that are secure are not developed.
Medicinal plants are preferred, as they produce phytochemicals (secondary metabolites) that possess effective pharmacological properties. The common people still prefer medicinal plants as they are safe nature and lack of side effects, despite technological advancements in contemporary antibiotic drugs and treatment approaches. Phytochemicals such as alkaloids, flavonoids, terpenoids, saponins, phenolic compounds, and sterols efficiently attenuate the side effects and can be a safe alternative to synthetic antibiotics [8]. Previous research has demonstrated the efficacy of phytochemicals against antibiotic-resistant bacterial pathogens 8-12. Recent technological advances have paved the way for the identification of diverse range of metabolites that play an effective role in the survival of plants under extreme biotic and abiotic environments 13. Since more than 60,000 years ago, traditional medicine in India uses phytochemicals 14,15. In comparison to conventional antimicrobials, plant-derived bioactive chemicals have multiple mechanisms of action against microbial infections, including binding affinity for numerous targets.
Reactive oxygen species (ROS) is required for normal cellular activity. But when the intracellular concentration of ROS rises, it causes a slew of negative consequences, including cancer, diabetes, inflammation, premature ageing, and atherosclerosis. Antioxidants are molecules that protect the body from the negative effects of reactive oxygen species (ROS). Secondary metabolites produced including flavonoids, anthocyanins, and carotenoids, are effective free radical scavengers.Among phytochemicals found in plants, phenolic compounds are antioxidative agents. 16. They breakdown peroxides, donate hydrogen, quench singlet and triplet oxygen and trap free radicals.
A.adenophora(Asteraceae)is an invasive plant. The plant originated in Mexico, although it can also be found in nations in South and Southeast Asia. Larvicidal 17, antimicrobial 18, anti-inflammatory 19, antipyretic 20, wound-healing 21, antioxidant 22, and analgesic 23 have been demonstrated.E. coli, Bacillus subtilis, S. aureus, K. pnuemoniae 24, and Proteus mirabilis were effectively inhibited by A. adenophora25.
Nanotechnology, dynamic field of science involves biology, physics and chemistry of nanoscale materials that has unplugged new avenues in the diagnosis and treatment of diseases 26. Silver is among the most effective metallic nanoparticles used in medicine. Silver nanoparticles(AgNPs) exhibit biological activity, including antibacterial, antioxidant, antifungal, DNA fragmentation 27 and cytotoxicity 28 properties. This study focuses on fabricating AgNPs from A. adenophora aqueous extract of leaves as an alternative strategy for enhancing the biological activity of phytochemicals in the control of microbial pathogens and in quenching free radicals.
Materials and Methods
Plant material
Fresh healthy A. adenophoraleaves collected from Kodaikanal hills, Tamilnadu, India. The washed leaves were shade dried for 10 days and powdered using mechanical blender.
Preparation of solvent extracts
About 20 g of powdered plant material was packed into a thimble and extracted with 250 mL of different solvents (water, petroleum ether, benzene, chloroform, acetone and methanol) in a soxhlet’s apparatus. After 24 hours of extraction the solvent was evaporated under reduced pressure. Crude extract was collected and stored at 4°C26.
Determination of Ash values
The physicochemical parameters such as percentage of ash and extractive values and weight loss on drying were carried out using the official methods29-30.
Water soluble extracts
A 5% aqueous plant extract was prepared and filtered using Whatmann filter paper. An empty evaporating dish was weighed, and 25ml of a 5% plant extract was added and heated until a damp mass was formed. The damp mass was cooled and weighed. The weight of empty dish to that ofdamp mass gives the value of water-soluble extract.
Alcohol soluble extracts
A 5% methanol plant extract was prepared and filtered using Whatmann filter paper. An empty evaporating dish was weighed, and 25ml of a 5% plant extract was added and heated until a damp mass was formed. The damp mass was cooled and weighed. The difference in weight of empty dish and with damp mass gives the value of alcohol-soluble extract.
Phytochemical analysis
The initial phytochemical screening assays were conducted with the established techniques of Kokate 31 and Harborne 32. Approximately 100g of leaf powder was extracted using the Soxhlet apparatus for 24 hours with water, benzene,petroleum ether, acetone,chloroform, and methanol. The filtratewas concentratedand diluted in respective solvents.Solvent extracts were analysed for phytochemicals.
GCMS analysis for identification of compounds
Plant powder and methanol (1:25 ratio) was subjected to GC-MS analysis for the identification of compounds using the procedure adapted by [33]. 1µl of the sample was loaded into fused silica column coated with poly dimethyl siloxane (stationary phase) and the mobile phase, helium was applied (1 ml/min). Injector temperature was set to 250oC and the oven temperature was increased to 250oC from its initial temperature of 60oC at the rate of 2oC/ min and maintained for 5 min. The ionization voltage used was 70 eV with a spilt ratio of 1:25. Retention time/mass spectra of known compounds and published data were utilised to identify compounds.
Determination of total phenol content
Methanol extract of A.adenophoraleaves was used to quantify phytochemicals. The total phenol 34 and tannins 35 were determined by Folin-ciocalteau method. Total flavonoid concentration was determined using a modified calorimetric method 36. Following the protocol of 37 the alkaloid content was determined.
Ageratina adenophora mediated silver nanoparticles (Aa-AgNPs)
Slight adjustments were made to the methodology of Gautam et al. 38 in the preparation of Aa-AgNPs. Leaf powder (5g) and distilled water (100 ml) was heated for 30 minutes at 70° C, cooled and filtered (Whatman filter paper.No.1). For the reduction reaction, 50 ml of aqueous leaf extract was added to 50 ml of 3 mM AgNO3 solution and stirred vigorously with a magnetic stirrer for 30 minutes. The variation in colour of the reacting solution indicated Aa-AgNPs synthesis. The resulting solution was then centrifuged at 15,000 revolutions per minute for 10 minutes. The pellets obtained were dispersed in deionized water to eliminate uncoordinated biological molecules. The pellets were then dried in a lyophilizer.
Fourier transform infra-red spectroscopy
FTIR spectroscopy was used inidentifyingthe functional phytochemical groups. The nano solution was centrifuged at 60,000 rpm for 40 minutes, after which the pellets were dissolved in deionized water and filtered through 0.45 m Millipore filter paper. 1 mg of Aa-AgNPsand 10 mg of KBr pellets were, finely crushed, and formed into a pellet using hydraulic pressure. The spectra were captured in the region of 4000 to 400 cm-1.
X Ray diffraction analysis
XRD analysis confirms the synthesis of Aa-AgNPs(PAN analytical X pert PRO Model)with operating conditions of voltage of 40 kV; current of 30 mA with Cu Kα radiation. Particles size (L) was calculated with Debye-Scherrrer’s equation.
L = 0.9λ /β h θ
λ = wavelength of the X-ray, β = full width and half maximum and θ = the Bragg’s angle.
Scanning Electron Microscopic (SEM) analysis
The morphology and distribution of Aa-AgNPs were investigated with a FESEM, Carl Zeiss – Sigma model, Germany. Aa-AgNPs were placed on a double-sided metal stub and sputtered with gold (Au) for SEM investigation.
Antibacterial activity of Aa–AgNPs
E. coli, K. pneumoniae (MTCC1610), S. aureus (MTCC96) and S. pneumoniae (MTCC1610) were procured from IMTECH, India. Antibacterial activity was determined using agar well diffusion assay [39]. The nutrient broth medium was seeded with selected bacteria, and cultures were adjusted to 0.5 McFarland standards (1 108 CFU mL-1) and plated on sterile nutrient agar. The plates were dried for 15 minutes before to sensitivity testing. Aa-AgNPs (40, 50, and 60 µg/ml) and a standard antibiotic (20 µL) were evaluated for their efficacy. Amoxicillin was employed as a positive control. The plates were then incubated at 37oC for 24 h and bacteriostatic zone was measured.
in-vitro antioxidant assay
DPPH (2,2-diphenyl-1-picrylhydrazyl), ABTS (2, 2′-azino-bis (3-ethylbenzothiazole-6-sulphonic acid), nitric oxide scavenging assays, and total reducing power assays were utilised to test the antioxidant property of the synthesised nanoparticles. The DPPH and total reducing power analysis were conducted according to Syed Ismail et al.,40. The nitric oxide was investigated following Hashemi and Ebrahimzadeh 41. The inhibition of ABTS by Aa-AgNPs was determined using Hajebi et al 42 technique.
The inhibition (%) or scavenging of free radicals was calculated by the formula:
% inhibition = (A0-A1) / A0∗100
A0= control and A1= sample
Result and Discussion
Percentage of yield obtained with solvent extracts was presented in Table (1). The aqueous extract had a high yield percentage of 4.105% and low yield was recorded with benzene (1.215%). The yield by different solvent extract was as follows: aqueous > methanol > petroleum ether > acetone > chloroform > benzene. Mazumder et al., 43 reported that methanol extract of leaves had high yield (11.47%) followed by petroleum ether (5.55%) and chloroform (3.06%). The yield was related to the weight of plant material used for extraction, geographic distribution and the environmental factors. Table (2) represents the yield of water-soluble extract and alcohol soluble extracts. High water-soluble extract yield of 14.15% was recorded while, the alcohol soluble extract yield was 12.50%. High water-soluble yield indicates that leaves are rich in carbohydrates, phenolic compounds and acids. Water soluble and alcohol soluble yield of A. adenophora leaves reported by Negi et al., [44] showed low percentage of yield in water soluble (13.71%) and alcohol soluble (11.84%) extracts. The inorganic composition and other substances (impurities) in a plant material can be determined with ash values. The total ash value was higher (10.31%) compared to acid insoluble (8.2%) and water soluble (4.1%) ash values (Table 3). The low acid insoluble ash value was because of siliceous matter in minute quantity. The ash value was consistent with the findings of Negi et al., 44. The quantitative estimation of phytochemicals was evaluated to explore the plant’s biological significance. A. adenophora leaves had a total phenol of 155.22 mg/g. The flavonoids were 134.85 mg/g, alkaloids and tannins content were 43.8 mg/g and 78.83 mg/g respectively (Table 4 and Fig 2). The total phenol and flavonoid content of 30 mg/g and 510 mg/g respectively were reported in A. adenophora. Though the phenol content was low, the flavonoid content was high 45.
Table 1: Percentage of yield obtained from different solvent extracts of A.adenophora leaves
|
S.No |
Solvent |
Colour of the yield |
% of yield |
|
1) |
Petroleumether |
Darkgreen |
2.495 |
|
2) |
Benzene |
Black |
1.215 |
|
3) |
Chloroform |
Black |
1.457 |
|
4) |
Acetone |
Darkgreen |
2.315 |
|
5) |
Methanol |
Darkgreen |
3.540 |
|
6) |
Aqueous |
Darkgreen |
4.105 |
Table 2: Overall percentage of yield obtained from A.adenophora leaves
|
S.No |
Extract |
% of yield |
|
1) |
Water soluble extract |
14.15 |
|
2) |
Alcohol soluble extract |
12.50 |
Table 3: Percentage of ash values obtained from A.adenophora leaves
|
S.No |
Ash content |
% of ash |
|
1) |
Total ash |
10.31 |
|
2) |
Acid insoluble ash |
8.2 |
|
3) |
Water soluble ash |
4.1 |
|
4) |
Loss due drying |
0.14 |
Table 4: Quantitative estimation of secondary metabolites in A. adenophora leaves
|
S.No |
Phytochemicals |
Quantity (mg/ml) |
R2 |
|
1) |
Phenol |
155.22±2.66 |
0.9870 |
|
2) |
Flavonoids |
134.85±0.36 |
0.9836 |
|
3) |
Alkaloids |
43.88±0.73 |
0.9996 |
|
4) |
Tannins |
78.83±1.25 |
0.9984 |
 |
Figure 1: Fresh leaves of Ageratina adenophora |
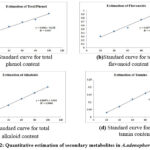 |
Figure 2: Quantitative estimation of secondary metabolites in A. adenophora leaves |
The qualitative estimation of aqueous leaf extract showed carbohydrates, glycosides, flavonoids, steroids, tannins and terpenoids. Tannins are the only secondary metabolite that was not detected with petroleum ether. Similarly, glycosides, tannins and steroids were absent in chloroform extract. Alkaloids, steroids, tannins, and flavonoids were present in benzene extract. Glycosides, tannins, steroids and terpenoids were present in acetone extract. Methanol extract did not report for steroids (Table 5) Saponins in methanol extract 43 and tannins in petroleum ether 44 contradicted with this study. The phytochemicals aqueous extract as reported by Negi et al., 44 was in concurrence with present finding. The difference in the phytochemicals as reported by previous investigation was because of geographical distribution of the plant and the phytochemicals indicates the pharmacological significance of the plant.
Table 5: Phytochemicals identified from A. adenophora leaves using different solvent extracts.
|
Phytochemicals |
Solvent extracts |
|||||
|
Water |
Pet.ether |
Benzene |
Chloroform |
Acetone |
Methanol |
|
|
Carbohydrates |
+ |
+ |
– |
+ |
– |
+ |
|
Proteins |
+ |
+ |
– |
+ |
– |
+ |
|
Saponins |
+ |
+ |
– |
+ |
– |
+ |
|
Alkaloids |
+ |
+ |
+ |
+ |
– |
+ |
|
Glycosides |
+ |
+ |
– |
– |
+ |
+ |
|
Flavonoids |
+ |
+ |
+ |
+ |
– |
+ |
|
Terpenoids |
+ |
+ |
– |
+ |
+ |
+ |
|
Steroids |
+ |
+ |
+ |
– |
+ |
– |
|
Tannins |
+ |
– |
+ |
– |
+ |
+ |
GC-MS analysis showed 20 different compounds (Fig.3 and Table 6) with maximum proportion (76.17%) being occupied by four different compounds namely isolongifolene (22.73%), methyl ionone (22.26%), 2(3H) napthalenone 4,4a,5,6,7,8-hexahydro-4a,7,7-trimethyl (R)- and D (-) quinic acid (14.20%). Isolongifolene is a polycyclic hydrocarbon and quinic acid is a polyphenol. 2(3H) napthalenone 4,4a,5,6,7,8-hexahydro-4a,7,7-trimethyl (R)- and methyl ionone are sesquiterpenoids. Spathulenol, α-bisabolol and 4,6,6, trimethyl-2-(3-methyl-buta-1,3-dienyl)-3-oxa-tricyclo [5.1.0.02,4] octane has been previous reported by Poudel et al., 18. Rangasamy and Namasivayam[46] reported that isolongifolene isolated from Muurayakoenigii exhibited effective invitro antioxidant property via scavenging DPPH, ABTS, nitric oxide, hydroxyl, and hydrogen peroxide super oxide free radicals. Quinic acid was reported for its antioxidant, hepatoprotective and anti-inflammatory activity 47. Bai et al.,48 reported quinic acid with antibacterial activities. The chromatogram showed the major compounds were terpenoids and alcohol.
 |
Figure 3: GC-MS spectrum of A.adenophora leaves. |
Table 6: Identification of bioactive compounds of A. adenophora leaves
|
Peak |
Area% |
R.Time |
M.formula |
Compoundname |
|
1) |
0.69 |
6.092 |
C12H20O2 |
alpha-Fenchylacetate |
|
2) |
0.608 |
9.996 |
C15H24O |
(-)-Spathulenol |
|
3) |
14.20 |
10.643 |
C7H12O6 |
D-(-)-QuinicAcid |
|
4) |
1.19 |
11.133 |
C15H26O |
alpha-Bisabolol |
|
5) |
22.26 |
12.612 |
C14H22O |
MethylIonone |
|
6) |
0.73 |
12.727 |
C22H34O3 |
Kauran-18-al,17-(acetyloxy)-,(4.beta.)- |
|
7) |
16.98 |
12.835 |
C13H20O |
2(3H)-Naphthalenone, 4,4a,5,6,7,8-hexahydro-4a,7,7-trimethyl-,(R)- |
|
8) |
1.00 |
13.168 |
C15H24 |
Thujopsene |
|
9) |
22.73 |
13.437 |
C15H24 |
Isolongifolene |
|
10) |
2.05 |
13.812 |
C15H22O |
4,6,6-Trimethyl-2-(3-Methyl-Buta-1,3-Dienyl) -3-Oxa-Tricyclo[5.1.0.02,4]Octane |
|
11) |
3.04 |
13.973 |
C16H32O2 |
Palmiticacid |
|
12) |
1.21 |
14.087 |
C15H24 |
Cedr-8-Ene |
|
13) |
1.52 |
14.225 |
C16H32O2 |
Hexadecanoicacid |
|
14) |
0.82 |
14.396 |
C15H24 |
1-Methyl-4-Methylene-2-(2-Methyl-1-Propenyl)-1-Vinylcycloheptane |
|
15) |
1.66 |
14.706 |
C15H22O2 |
6-(1-Hydroxymethyl-Vinyl)-4,8A-Dimethyl-3,5,6,7,8,8A-Hexahydro-1H-Napthalen-2-One |
|
16) |
1.10 |
15.553 |
C20H40O |
Phytol |
|
17) |
1.67 |
15.910 |
C19H32O2 |
Methyllinolenate |
|
18) |
1.08 |
16.167 |
C20H36O2 |
Ethyllinoleate |
|
19) |
4.24 |
18.267 |
C15H24 |
Cycloheptan4-Methylen-1-Methyl-2-(2-Methyl-1-Propen-1-yl)-1-Vinyl-(Humulen-”V |
|
20) |
1.25 |
20.006 |
C20H34O |
Thunbergol |
Aa-AgNPs was synthesized with 5% plant extract (50ml) and 3mM AgNO3 (50ml) was added with pH adjusted to 8.0. Plant extract when added to aqueous AgNO3 altered the colour from light brown to greenish black (Fig.4). It was then left in the dark for 18 hours to ensure total nanoparticle saturation. FTIR identified the functional groups on Aa-AgNPs. The absorbance peaks at 3937.98 cm-1, 3769.14 cm-1, and 3206.64 cm-1 in the FTIR spectrum (Fig. 5) showed OH-stretch of alcohol. CH-alkane and aldehyde were identified at 2974.18 cm-1.The absorbance peak at 2901.59 cm-1 corresponded to the C-H stretch of the methyl group . C=C aromatic alkanewas identified at 1581.21 cm-1 . Similarly, the asymmetric stretch of a methyl molecule was identified at 1257.67 cm-1 . The peaks at 1030.42 cm-1 and 799.57 cm-1 respectively indicated the C-O stretch of alcohol and the C=C bend . Bromo and iodo compounds were represented with peaks at 687.94 cm-1 and 571.67 cm– . Alcohol, aldehyde, alkane, methyl, aromatic amines, bromo and iodo chemicals contained in alkaloids, phenolic compounds, aminoacids, carbohydrates, and tannins were implicated in Aa-AgNPs synthesisand stability.
 |
Figure 4: A.adenophora leaf extract mediated silver nanoparticles (Aa-AgNPs) synthesis |
 |
Figure 5: FTIR absorbance spectrum of A. adenophora leaf extract mediated AgNPs |
The XRD investigation demonstrated the crystallinity of Aa-AgNPs (Fig.6). The crystal plane displayed a significant peak at 2 angles of 38.23, 44.51, 64.41, and 77.51, which correspond to miller indices of (111), (200), (220), and (311). (JCPDS No: 04-0783). These index planes confirmed the fabricated silver nanoparticles were crystalline and had a face-centred cubic structure. In addition, Scherrer equation analysis of peaks revealed an average particle size of 84 nm. A diffraction spectrumforAgNPs mediated through Andrographis paniculataleaf extract was consistent with the XRD pattern found in this investigation.
 |
Figure 6: XRD spectrum of Aa–AgNPs |
SEM examinations validated the morphology, size, and form of the produced AgNPs. The SEM scans demonstrated that Aa-AgNPs were devoid of aggregation and were spherical (Fig.7). AgNP synthesis fromButea monosperma flower extract showed spherical AgNPs 26. The antibacterial activity mediated by plant extracts depends on phytochemicals 49 . The antibacterial activity of Aa-AgNPswas due to surface coated phytochemicals and its synergistic activity withAa-AgNPs. The phytochemical investigation of A. adenophora leaf extract showed alkaloids, saponins, steroids, tannins and terponoidsthat are reported with antibacterial activities 50 .The antibacterial activity of Aa-AgNPs (Fig.8 and Table 7) was highly effective against gram negative bacterial species, exhibiting high bactericidal activity measuring 18mm against K.pneumonia and 16 mm against E.coli. A decrease in the zone of inhibition against positive bacterial species, namely S. pneumonia and S.aureus measuring 15 mm and 13 mm was observed. The antibacterial potential of Aa-AgNPs may have been caused by the adhesion of AgNPs to the cell wall of bacteria, as suggested by the hypothesis. This binding of AgNPs to bacterial cell wall is caused by a difference in charge between the nanoparticles and the bacterial cell wall.Further, the high surface area of nanoparticles, size of the nanoparticles and the ability to generate free radicals might have influenced the effective antibacterial efficacy of nanoparticles 51,52 .The binding of AgNPs to a bacterial cell wall stimulates conformational changes in membrane proteins, which results in an increase in membrane permeability. Subsequent AgNPs penetration causes cellular content leakage and cell death. The binding affinity of AgNPs towards sulphur and phosphorus in proteins and damaged DNA together contributes to its effective antibacterial potential. Das &Devkota 53, reported that aqueous extract of A. adenophora leaves showed bactericidal activity against Klebsiella pneumonia, Enterococcus faecalis, Escherichia coli, Bacillus subtilis and Staphylococcus aureus.
 |
Figure 7: SEM images of Aa–AgNPs |
 |
Figure 8: Antibacterial activity of Aa–AgNPs |
Table 7: Bactericidal activity of Aa-AgNPs
|
Bacterial species |
Antibiotic Amoxicillin |
Zone of Inhibition of AgNPs (mm) |
||
|
40µg/mL |
50µg/mL |
60µg/mL |
||
|
Staphylococcus aureus |
9 |
12.5 |
12.5 |
13 |
|
Streptococcus pneumoniae |
14 |
13.5 |
14 |
15 |
|
Escherichia coli |
18.5 |
14 |
14.5 |
16 |
|
Klebsiella pneumoniae |
15 |
15 |
16.5 |
18 |
Inhibition of DPPH activity of Aa-AgNPs was 76.90%, while ABTS radical inhibition was 73.71%. The Aa-AgNPs were effective in quenching nitric oxide (83.21%) and ferric reducing power (74.87%). The Aa-AgNPswere more effective in scavenging nitric oxide(Fig.9 and Table 8). The methanol extract A. adenophora exhibited an IC50 value of 92.791% against DPPH. Rajalakshmi et al., [54]reported ethanol and aqueous extract of A. adenophora to be effective against nitric oxide. The results indicated that aqueous extract exhibited higher scavenging activity (55.16%) compared to ethanolic extract (40.48%). The reduction of ferric ions by aqueous extract was 339.97 mg/g and ethanolic extract was 326.48 mg/g [54]. The effective scavenging of A. adenophora might be attributed to polyphenolic compounds and flavonoids. Also, the major compounds isolongifolene, quinic acid and methyl ionone, reported for their antioxidant activity might have played an effective role.
 |
Figure 9: Antioxidant activity of Aa–AgNPs |
Table 8: Antioxidant activity of Aa-AgNPs
|
Assay |
Antioxidant activity of AgNPs |
||||
|
10µL |
20µL |
30µL |
40µL |
50µL |
|
|
DPPH |
35.00±2.55 |
43.89±3.12 |
59.25±3.29 |
66.31±2.07 |
76.90±2.59 |
|
ABTS |
32.75±2.62 |
38.91±3.92 |
54.79±2.94 |
63.64±2.79 |
73.71±3.14 |
|
NO |
38.72±1.02 |
43.84±1.88 |
64.41±3.71 |
73.45±3.50 |
83.21±2.53 |
|
Red. power |
34.12±1.52 |
43.77±4.22 |
56.17±1.89 |
60.49±1.89 |
74.87±4.38 |
Conclusion
The present study revealed that the A. Adenophora leaves was rich in phytochemicals such asalkaloids, phenolic compounds, tannins, carbohydrates, proteins and aminoacids. The quantitative estimation of phytochemicals showed high amount of phenols, flavonoids, alkaloids and tannins in the leaves. Further, the phytochemicals are effective in the reducing metal ions in the synthesis of Aa-AgNPs and its stabilization. The phytochemicals bound to surface of Aa-AgNPs might be attributed to their biological activity. Hence, A. adenophora might be a possible drug candidature and the study suggest further clinical trials before considering as a drug.
Conflict of Interest
There is no conflict of interest.
References
- Hotez, P.J. Aboriginal Populations and Their Neglected Tropical Diseases. PLoS Neglected Tropical Diseases2014, 8, doi:10.1371/journal.pntd.0002286.
- Holt, D.C.; McCarthy, J.S.; Carapetis, J.R. Parasitic Diseases of Remote Indigenous Communities in Australia. International Journal for Parasitology 2010, 40.
- Tankeo, S.B.; Damen, F.; Awouafack, M.D.; Mpetga, J.; Tane, P.; Eloff, J.N.; Kuete, V. Antibacterial Activities of the Methanol Extracts, Fractions and Compounds from FagaraTessmannii. Journal of Ethnopharmacology2015, 169, doi:10.1016/j.jep.2015.04.041.
- Namita, P.; Mukesh, R. Issn 2230 – 8407 Medicinal Plants Used As Antimicrobial Agents : A Review. International research journal of pharmacy2012, 3.
- Bassetti, M.; Righi, E. Multidrug-Resistant Bacteria: What Is the Threat? Hematology / the Education Program of the American Society of Hematology. American Society of Hematology. Education Program 2013, 2013.
- Medina, E.; Pieper, D.H. Tackling Threats and Future Problems of Multidrug-Resistant Bacteria. Current Topics in Microbiology and Immunology2016, 398, doi:10.1007/82_2016_492.
- Abdallah, E. Beyond Antibiotics, Is There Any Hope for New Alternatives? American Journal of Preventive Medicine and Public Health2017, doi:10.5455/ajpmph.281398.
- Nath, D. Bottle Gourd (Lagenaria Siceraria) in Fruit and Vegetable Phytochemicals: Chemistry and Human Health. Phytochemistry Reviews2017, 16, 491–478.
- Prasannabalaji, N.; Muralitharan, G.; Sivanandan, R.N.; Kumaran, S.; Pugazhvendan, S.R. Antibacterial Activities of Some Indian Traditional Plant Extracts. Asian Pacific Journal of Tropical Disease2012, 2, doi:10.1016/S2222-1808(12)60168-6.
- Sukalingam, K.; Ganesan, K.; Xu, B. TrianthemaPortulacastrum L. (Giant Pigweed): Phytochemistry and Pharmacological Properties. Phytochemistry Reviews2017, 16, doi:10.1007/s11101-017-9493-5.
- Tariq, H.; Arshad, M.; Khan, S.; Sattar, H.; Qureshi, M.S. In Vitro Screening of Methanol Plant Extracts for Their Antibacterial Activity. Pakistan Journal of Botany2011, 43.
- Arun, P.; Purushotham, K.G.; Johnsyjayarani, J.; Kumari, V. In Vitro Antibacterial Activity and Flavonoid Contents of LawsoniaInermis(Henna). International Journal of PharmTech Research2010, 2.
- Fang, C.; Fernie, A.R.; Luo, J. Exploring the Diversity of Plant Metabolism. Trends in Plant Science 2019, 24.
- Hemalatha, K.; Madhumitha, G.; Vasavi, C.S.; Munusami, P. 2,3-Dihydroquinazolin-4(1H)-Ones: Visible Light Mediated Synthesis, Solvatochromism and Biological Activity. Journal of Photochemistry and Photobiology B: Biology2015, 143, doi:10.1016/j.jphotobiol.2014.12.028.
- Shi, Q.W.; Li, L.G.; Huo, C.H.; Zhang, M.L.; Wang, Y.F. Study on Natural Medicinal Chemistry and New Drug Development. Chinese Traditional and Herbal Drugs2010, 41.
- Lorrain, B.; Ky, I.; Pechamat, L.; Teissedre, P.L. Evolution of Analysis of Polyhenols from Grapes, Wines, and Extracts. Molecules 2013, 18.
- Niu, H.B.; Liu, W.X.; Wan, F.H.; Liu, B. An Invasive Aster (Ageratina Adenophora) Invades and Dominates Forest Understories in China: Altered Soil Microbial Communities Facilitate the Invader and Inhibit Natives. Plant and Soil2007, 294, doi:10.1007/s11104-007-9230-8.
- Poudel, R.; Neupane, N.P.; Mukeri, I.H.; Alok, S.; Verma, A. An Updated Review on Invasive Nature, Phytochemical Evaluation, & Pharmacological Activity of Ageratina Adenophora. International Journal of Pharmaceutical Sciences and Research2020, 11.
- Chakravarty, A.K.; Mazumder, T.; Chatterjee, S.N. Anti-Inflammatory Potential of Ethanolic Leaf Extract of Eupatorium AdenophorumSpreng. Through Alteration in Production of TNF-α, ROS and Expression of Certain Genes. Evidence-based Complementary and Alternative Medicine2011, 2011, doi:10.1093/ecam/neq033.
- Ringmichon, C.L.; Gopalkrishnan, B. Antipyretic Activity of Eupatorium Adenophorum Leaves. International Journal of Applied Biology and Pharmaceutical Technology2017, 8.
- Kumar, N.; Singh, A.; Sharma, D.; Kishore, K. Evaluation of Wound Healing Activity of Ageratina Adenophora (Spreng.) Rm King & h. Rob. Int J Pharma Res Health Sci2017, 5, 1873–1876.
- Khazeo, P.; Mazumder, M.U.; Puro, K.N.; Jyrwa, R.; Jamir, N.; Sailo, L. In Vitro Antioxidant Activity of Methanolic Extracts of Ageratum Conyzoides and Ageratina Adenophora Leaves.; 2018.
- Jin, Y.; Hou, L.; Zhang, M.; Tian, Z.; Cao, A.; Xie, X. Antiviral Activity of Eupatorium Adenophorum Leaf Extract against Tobacco Mosaic Virus. Crop Protection2014, 60, doi:10.1016/j.cropro.2014.02.008.
- Baral, B.; Maharjan, B.L. Antagonistic Characteristics and Phytochemical Screening of Invasive Alien Species of Nepal Himalaya. nternational Journal of Pharmaceutical & Biological Archives2011, 2, 1444–1450.
- Bhattarai, N.; Shrestha, G. Antibacterial and Antifungal Effect of Eupatorium AdenophorumSpreng against Bacterial and Fungal Isolates. Nepal Journal of Science and Technology1970, 10, doi:10.3126/njst.v10i0.2834.
- Ananth, S.; Thangamathi, P. Larvicidal Efficacy of Fabricated Silver Nanoparticles from Butea Monosperma Flower Extract against Dengue Vector, Aedes Aegypti .Biotech Today : An International Journal of Biological Sciences2018, 8, doi:10.5958/2322-0996.2018.00004.2.
- Gulbagca, F.; Ozdemir, S.; Gulcan, M.; Sen, F. Synthesis and Characterization of Rosa Canina-Mediated Biogenic Silver Nanoparticles for Anti-Oxidant, Antibacterial, Antifungal, and DNA Cleavage Activities. Heliyon2019, 5, doi:10.1016/j.heliyon.2019.e02980.
- Safaepour, M.; Shahverdi, A.R.; Shahverdi, H.R.; Khorramizadeh, M.R.; Gohari, A.R. Green Synthesis of Small Silver Nanoparticles Using Geraniol and Its Cytotoxicity against Fibrosarcoma-Wehi 164. Avicenna J Med Biotechnol2009, 1.
- WHO Quality Control Methods for Medicinal Plant Materials. World Health Organization1998.
- Pharmacopoeia 1996, I, 764.
- Kokate, C. Preliminary Phytochemical Analysis; 1st ed.; Vallabh Prakashan: New Delhi, 1986;
- Harborne, J. Methods of Extraction and Isolation. Phytochemical methods1998, 3, 60–66.
- Abbasipour, H.; Mahmoudvand, M.; Rastegar, F.; Hosseinpour, M.H. Fumigant Toxicity and Oviposition Deterrency of the Essential Oil from Cardamom, Elettaria Cardamomum, against Three Stored – Product Insects. Journal of Insect Science2011, 11, doi:10.1673/031.011.16501.
- Rasool, N.; Rizwan, K.; Zubair, M.; Naveed, K.U.R.; Imran, I.; Ahmed, V.U. Antioxidant Potential of Different Extracts and Fractions of Catharanthus Roseus Shoots. International Journal of Phytomedicine2011, 3.
- Afify, A.E.M.M.R.; El-Beltagi, H.S.; Abd El-Salam, S.M.; Omran, A.A. Biochemical Changes in Phenols, Flavonoids, Tannins, Vitamin E, β -Carotene and Antioxidant Activity during Soaking of Three White Sorghum Varieties. Asian Pacific Journal of Tropical Biomedicine2012, 2, doi:10.1016/S2221-1691(12)60042-2.
- Atanassova, M.; Georgieva, S.; Ivancheva, K. Total Phenolic and Total Flavonoid Contents, Antioxidant Capacity and Biological Contaminants in Medicinal Herbs. Journal of the University of Chemical Technology and Metallurgy2011, 46.
- Rao, T.M.; Ganga Rao, B.; Venkateswara Rao, Y. International Journal of Phytopharmacology ANTIOXIDANT ACTIVITY OF SPILANTHES ACMELLA EXTRACTS. International Journal of Phytopharmacology2012, 3.
- Gautam, S.K.; Baid, Y.; Magar, P.T.; Binadi, T.R.; Regmi, B. Antimicrobial Study of Green Synthesized Silver Nanoparticles (AgNPs) by Using Ageratina Adenophora and Its Characterization. International Journal of Applied Sciences and Biotechnology2021, 9, doi:10.3126/ijasbt.v9i2.37822.
- Syed Ismail, T.; Gopalakrishnan, S.; Hazeena Begum, V.; Elango, V. Anti-Inflammatory Activity of Salacia Oblonga Wall. and AzimaTetracantha Lam. Journal of Ethnopharmacology1997, 56, 145–152, doi:10.1016/S0378-8741(96)01523-1.
- Hashemi, Z.; Ebrahimzadeh, M.A. Evaluation of Three Methods for the Extraction of Antioxidants from Vicia Faba L. Bean and Hulls. Latin American Applied Research2014, 44, doi:10.52292/j.laar.2014.442.
- Hajebi, S.; Tabrizi, M.H.; Moghaddam, M.N.; Shahraki, F.; Yadamani, S. Rapeseed Flower Pollen Bio-Green Synthesized Silver Nanoparticles: A Promising Antioxidant, Anticancer and Antiangiogenic Compound. Journal of Biological Inorganic Chemistry2019, 24, doi:10.1007/s00775-019-01655-4.
- Rao, G.V.; Kumar, S.; Islam, M.; Mansour, S.E. Folk Medicines for Anticancer Therapy-a Current Status Review Article. Cancer Therapy2008, 6.
- Mazumder, M.U.; Khazeo, P.; Puro, K.N.; Jyrwa, R.; Jamir, N.; Sailo, L. Qualitative and Quantitative Analysis of Phytochemicals of Crude Extracts of Ageratina Adenophora Leaves.; 2018.
- Negi, A.; Upadhyay, A.; Semwal, A.; Wahi, A.K. Pharmacognostical Studies on the Leaves of Eupatorium AdenophorumSpreng. Pharmacognosy Journal2010, 2, doi:10.1016/S0975-3575(10)80071-9.
- Lallianrawna, S.; Muthukumaran, R.; Ralte, V.; Gurusubramanian, G.; Kumar, N.S. Determination of Total Phenolic Content, Total Flavonoid Content and Total Antioxidant Capacity of Ageratina Adenophora (Spreng.) King & h. Rob. Sci Vis2013, 13, 149–156.
- Rangasamy, K.; Namasivaya, E. In Vitro Antioxidant and Free Radical Scavenging Activity of Isolongifolene. Asian Journal of Biological Sciences2013, 7, doi:10.3923/ajbs.2014.13.23.
- Xiang, Q.; Nomura, Y.; Fukahori, S.; Mizuno, T.; Tanaka, H.; Fujiwara, T. Innovative Treatment of Organic Contaminants in Reverse Osmosis Concentrate from Water Reuse: A Mini Review. Current Pollution Reports 2019, 5, 294–307.
- Bai, J.; Wu, Y.; Zhong, K.; Xiao, K.; Liu, L.; Huang, Y.; Wang, Z.; Hong, G.A.O. A Comparative Study on the Effects of Quinic Acid and Shikimic Acid on Cellular Functions of Staphylococcus Aureus. Journal of Food Protection2018, 81, doi:10.4315/0362-028X.JFP-18-014.
- 49.Bhalodia, N.; Shukla, V. Antibacterial and Antifungal Activities from Leaf Extracts of CassiaFistula l. Journal of advanced pharmaceutical teachnology and research 2011, 2.
- Hedberg, I.; Hedberg, O.; Madat, P.J.; Mshigeni, K.E.; Mshiu, E.N.; Samuelsson, G. Inventory ofPlants Used in Traditional Medicine in Tanzania. II. Plants of the Families Dilleniaceae-Opiliaceae. Journal of Ethnopharmacology 1983, 9, doi:10.1016/0378-8741(83)90030-2.
- Guzmán, K.; Kumar, B.; Vallejo, M.J.; Grijalva, M.; Debut, A.; Cumbal, L. Ultrasound-AssistedSynthesis and Antibacterial Activity of Gallic Acid-Chitosan Modified Silver Nanoparticles.Progress in Organic Coatings 2019, 129, doi:10.1016/j.porgcoat.2019.01.009.
- Adibelli, M.; Ozcelik, E.; Batibay, G.S.; Arasoglu, T.O.; Arsu, N. A Facile and Versatile Route forPreparation AgNp Nanocomposite Thin Films via Thiol-Acrylate Photopolymerization:Determination of Antibacterial Activity. Progress in Organic Coatings 2020, 143,doi:10.1016/j.porgcoat.2020.105620.
- Das R.K. &Devkota A. Activity Test of Crude Extracts of Invasive Plants Ageratina Adenophora and Ipomoea Carnea Ssp. Fistulosa against Human Pathogenic Bacteria. Annals of Plant Sciences 2020, 9.
- Rajalakshmi, P.; Sumathi, V.; Pugalenthi, M. Antioxidant Activity of Erigeron Karvinskianus Dc. and Ageratina Adenophora (Spreng.) King (Leaves). International Journal of Food Science and Nutrition2016, 1, 64–68.

This work is licensed under a Creative Commons Attribution 4.0 International License.









