Characterization, Pharmacology and In-silico Study of 2,4 Ditertiary Butylphenol Isolated from the Leaves of Ficus auriculata Lour.
Department of Chemistry, Scott Christian College (Autonomous), Nagercoil-629003. Affiliated to Manonmaniam Sundaranar University, Tirunelveli India.
Corresponding Author E-mail: premaisaac67@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/390122
Article Received on : 28 Oct 2022
Article Accepted on : 01 Jan 2023
Article Published : 31 Jan 2023
Reviewed by: Dr. Garima Kapoor Arora
Second Review by: Dr. Shahid Mirza
Final Approval by: Dr. B. K Sharma
Ficus auriculata belongs to the family of dicotyledonous plant. The general phytochemical screening for Ethanol, Hexane, Chloroform and Water extracts are done. Since ethanol shows the presence of maximum compounds, it is subjected to the isolation of phenolic compounds by TLC and Column Chromatography. On repeating this process, a white crystalline solid results, which on the basis of UV-Vis, FT-IR, GC-MS, 1H NMR, confirms that the isolated compound is 2,4 Ditertiary Butyl Phenol (2,4 DTBP). The isolated compound is further studied for its solvent effect and subjected to anti-microbial and Cytotoxicity studies with AGS cancer cell line and HIEC-6 (Human Normal Intestinal Epithelial cell line). Docking studies is carried out using Patch Dock server, with 2,4DTBP as guest and Occluding, which is a Tight Junction Protein (TJP) as host. The resulted structure is further subjected to Lipinski Rule of five. The present study concludes that 2,4 DTBP shows intermediate resistance against gram positive and gram negative bacteria like S. aureus, E. coli, Klebsiela pneumonia, strongly resistant to fungi Candida sps.
According to cytotoxic and in silico studies, the isolated compound has excellent anticancer properties and is thus used in the treatment of gastric cancer. From the Lipinski rule, it is confirmed that the drug can be administered orally.
2,4Ditertiary ButylPhenol; Ficus auriculata; Gastric cancer; Isolation; In-Silico studies; Phytochemicals; Pharmacology
Download this article as:| Copy the following to cite this article: Russell S. P Kumari J. P. Characterization, Pharmacology and In-silico Study of 2,4 Ditertiary Butylphenol Isolated from the Leaves of Ficus auriculata Lour. Orient J Chem 2023;39(1). |
| Copy the following to cite this URL: Russell S. P Kumari J. P. Characterization, Pharmacology and In-silico Study of 2,4 Ditertiary Butylphenol Isolated from the Leaves of Ficus auriculata Lour. Orient J Chem 2023;39(1). Available from: https://bit.ly/3wFIM0z |
Introduction
Medicinal plants act as a natural resource, providing opportunities for the discovery of new drugs. Plants used in traditional medicine contains a variety of substances that can be used for healing chronic and infectious diseases. Thousands of phytochemicals from plants are safer than other effective methods with minimal side effects.1Phytochemicals, commonly referred to as secondary metabolites, produced by plants through several chemical methods can be beneficial in the function of human cells.2 Polyphenols exhibit many protective functions such as hypolipidemic, antiproliferative, anti-inflammatory and antioxidative effects which reduce the onset of the disease.3 Extraction is a very important step in analyzing existing nutrients plant.1Ficusauriculata (F. auriculata) is a small, perennial evergreen tree which is cultivated in South and Southeast Asia and Brazil for its edible fruits.4 There are few studies on the benefits of F. auriculata to human health due to its phytochemical compounds.5 2,4 DTBP was also reported in different groups of plants, like Phaeodactylum tricornutum Bohlin (diatom), Marchantiapolymorpha L (liverwort), Osmundaregalis L. and Adiantumvenustum D (ferns).6 It has been reported that 2,4-Di-tert-butylphenol exhibit moderate cytotoxicity against HeLa and MCF-7, high percentage of antioxidant, anti-bacterial activity and induces senescence inhuman gastric adenocarcinoma.7,18 A global issue in the field of surgery is postoperative wound infections, which are linked to prolonged hospital stays. An infection in the tissues around the incision and the surgical site is referred to as a postoperative wound infection, and it typically develops five to thirty days following surgery. Streptococcus species, Proteus species, Enterobacter species, Klebsiella species, Coagulase negative and Pseudomonas species, are the most common pathogen.8
The effects of several solvents (including ethanol, water, butanol, ether, hexane, carbon tetrachloride, and chloroform) on2,4 DTBP using UV-Vis spectra were taken into consideration.
The findings revealed that the U.V region experienced majority of absorptions, and that the primary electronic transitions are connected to n -σ* and σ-σ*. The absorption values in different solvents are influenced by dielectric constants of the solvents. The solvent polarizability tends to move the absorption maximum towards lower wavelength.9 Molecular docking studies predict the preferred orientation of one molecule to another when they are bonded together to form a stable complex.20 The objective is to determine the correct interaction between two molecules.10 Lipinski’s rule of five, which has been used for nearly 20 years as a broad “rule of thumb “for valuing drug-like qualities, is a widely used way to forecast a drug’s performance, mostly for oral medications.11
The focus of my work is to identify the Phytochemicals present in various solvents, Isolation of bioactive phenolic component 2,4Ditertiary Butylphenol, its Characterization using various spectral analysis, and study the effect of various solvents using UV-Vis spectra. The compound is also tested for its anti-microbial activity, cytotoxicity and its drug-likeness is evaluated by in-silico study.
Materials and Methods
Plant leaves were collected from Nagercoil locality, washed well with double distilled water, shadow dried for 3-4 weeks, powdered and preserved for further work. Plant authentication was done by Dr. M. U Sharief, Scientist ‘E’ & Head of Office, Botanical Survey of India, Southern Regional Centre, Coimbatore. The voucher specimen no. BSI/SRC/5/23/2021/Tech-263.
Materials required
Ethanol, Chloroform, Hexane, Water, Lead Acetate, Neutral FeCl3, Fehling Solution (A & B),4% NaOH, 1% CuSO4, Acetic acid, Wagner Reagent, Dil. HCl, Conc.H2SO4, Distilled water. Preparation of extracts
The powdered plant material (5g) is extracted with solvents (500 ml) like Ethanol, Hexane, Chloroform, Water using Soxhletapparatusfor24hours. The solvent is evaporated using rotary evaporator.
Phytochemical Analysis
Isolation of Phenolic compound Column Fractions
Silica gel (mesh size 230-400) is used in column chromatography. The column is packed using wet packing method, washed with Ethyl acetate solvent. The plant extract is loaded into the packed column. Ethyl Acetate: Hexane(30:70) is used as the eluent, the extract is eluted and the fractions were collected in vials. This is further subjected to TLC.12
Thin Layer Chromatography
TLC ready-made sheet (Silicagel60F25420 cmx20 cm)is cut into equal sizes and thin mark of 0.5 Cm was made from the bottom to load the sample spots. Ethyl acetate: Hexane (6:14) is used as the mobile phase. The TLC sheet prepared is placed in the beaker containing the mobile phase. It is removed after the sample spot is raised above the level in mobile phase. This is dried, and placed under Iodine chamber and examined under UV for various spots. From the spot Rf value can be calculated by the formula:
Rf=Distance travelled by the solute/Distance travelled by the solvent. Gas chromatography-Mass spectrometry (GC-MS)
The JEOL GCMATE II GC-MS with Data system, a high resolution, double focusing instrument, was used to conduct the GC-MS analysis. 6000-pixel maximum resolution Maximum calibrated mass: 1500Daltonson acapillary column (300.25 mID 0.25 mdf)fused to an Elite-5MS (5% diphenyl/95% dimethyl poly siloxane). Utilizing the data bases of the National Institute of Standards and Technology (NIST) and Wiley Spectra Libraries, the interpretation of the mass spectrum GCMS was carried out. The name of the molecule was determined using the molecular weight, molecular formula, and the number of hits from the NIST and Wileyspectralibraries.13
HPLC
HPLC is recorded using SHIMADZU, LC-10AT VP, at ANJAC, Sivakasi.
UV-Vis Spectroscopy
The UV absorbtion spectra is recorded using Systronics Smart Double beamSpectrophotometer-2203,in Scott Christian College, Nagercoil.
FT-IR Spectroscopy
The FT-IR is recorded using Shimadzu FT-IR Spectrometer, in ANJAC, Sivakasi.
1H-NMR
1H-NMR spectroscopy was carried out using Bruker 300 MHz FT NMR Spectrometer, in Gandhi gram Rural University, Dindugal.
Anti-Microbial activity of 2,4 DTBP Anti-Bacterial activity
Sample Preparation
Test Organism
The given sample was dissolved in a mixture of aqueous and ethanol solvents at aconcentrationof0.1g/1ml.
Clinical samples were used to isolate Staphylococcus aureus, E. coli, Klebsiellapneumoniae, Enterococcus faecalis, Bacillus subtilis, and Pseudomonas aeruginosa to study theirantimicrobial properties.
The lack of zone inhibition was taken to mean that there was no activity. When the zoneof inhibition is smaller than 7 mm, the activities are classified as resistant moderate 8–10 mm,andsensitive greater than11 mm.14-16
Anti-Fungal activity
Test Organism
The test Fungi used Candidasps is isolated from the environment for antifungal analysis.
Antifungi assay is per formed by disc diffusion method.17
MTT Assay
Principle
MTT Assay Utilises cellular metabolic activity as a gauge of cell viability, proliferation,andcytotoxicity.Thiscolorimetricassayreliesonthetransformationofpurpleformazancrystalsinto a yellow tetrazolium salt (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, orMTT) by metabolically active cells. The MTT is converted to formazan by NAD(P)H-dependent oxidoreductase enzymes found in live cells.21 An ELISA plate reader is used to measure the absorbance at 570 nm after the insoluble formazan crystals have been dissolved using a solubilizing solution (100%DMSO).
The cytotoxic effect of the isolated compound is tested in both HIEC-6 (Human Normal Intestinal Epithelial cellline) and Gastric Adenocarcinoma(AGS) celllines.
All experiments were carried out in triplicates. The cell viability is determined using the following formula:
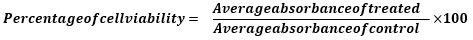
IC50 value
The sample’s half maximalin hibitory concentration is known as the IC50 value. The average absorbance of the various concentrations of the test sample (6.25-100 g/mL) were plotted in Microsoft Excel, and the equation for slope(y=mx+C) was derived.
Molecular Docking Studies
The 3D structural data of 2,4 DTBP was obtained from PubChem database in SDF format, it is translated into PDB format using PYMOL software and the 3D structural data of Occluding, a Tight Junction (TJ) protein is obtained from Protein Data Bank using the search interface. Using the Patch Dock server, the 2,4 DTBP ligand and Occluding, the receptor molecule, are uploaded along with the 3D coordinate data file in order to dock the guest 2,4 DTBP into the cavity of the host Occluding. Each conformation is given a docking score by the Patch Dock server.
Lipinski Rule of Five
Lipinski rule of 5 helps is used to distinguish the drug like and non-drug likeness of molecules. Lipinski’s rule states that, an orally active drug should not violate more than one of the following criteria:
★Molecularmass <500Dalton
★Lipophilicity(LogP<5)
★HydrogenBondDonors <5
★HydrogenBondAcceptors<10
★MolarRefractivity~40-130
Results and Discussion
Table 1: Column fractions eluted.
|
S.No |
Solvent System |
Ratio |
Volume |
Fractions |
|
1. |
Methanol:Chloroform |
4:16 |
20 |
6 |
|
2. |
EthylAcetate:Hexane |
5:95 |
100 |
5 |
|
3. |
EthylAcetate:Hexane |
6:14 |
20 |
7 |
Table 1. shows the Column fractions eluted using various solvents like Methanol :Chloroform, Ethyl Acetate: Hexane in various ratios.
Table 2: Thin Layer Chromatography using solvents of various ratios.
|
S.No |
Solventsystem |
Ratio |
Volume(ml) |
No ofSpots |
|
1. |
Methanol:Chloroform |
4:16 |
20 |
Nospot |
|
2. |
EthylAcetate:Hexane |
1:19 |
20 |
Nospot |
|
3. |
EthylAcetate: Hexane |
2:18 |
20 |
4 |
|
4. |
EthylAcetate:Hexane |
4:16 |
20 |
4 |
|
5. |
EthylAcetate:Hexane |
6:14 |
20 |
3 |
|
6. |
EthylAcetate:Hexane |
8:12 |
20 |
Nospot |
Table 2 shows the TLC of solvents Methanol: Chloroform and Ethyl Acetate : Hexane in various ratios. Out of which Fraction IV is subjected to further evaluation.
Table 3: Phytochemicals present in various solvents.
|
Phytochemical |
Ethanol |
Hexane |
Chloroform |
Water |
|
Tannin |
+ |
– |
– |
– |
|
Phenolic compound |
+ |
– |
– |
– |
|
Protein |
– |
– |
+ |
+ |
|
Flavonoids |
+ |
– |
+ |
+ |
|
Cardiac Glycoside |
+ |
– |
+ |
+ |
|
Coumarin |
+ |
+ |
– |
– |
|
Saponin |
+ |
+ |
– |
– |
|
Alkaloids |
+ |
– |
+ |
+ |
|
Carbohydrate |
+ |
– |
+ |
+ |
|
Steroid |
+ |
+ |
+ |
– |
Table 3 shows the phytochemicals present in various solvents, from the above data, it is concluded that, Ethanol is the most preferred solvent, since it shows the presence of majority of the components.
Structural elucidation of the Isolated compound
GC-MS of Fraction IV
 |
Figure 1: GC-MS Spectra of the isolated compound. |
The isolated compound’s molecular mass is determined to be 206.32 from the m/z value.
HPLC
 |
Figure 2: HPLC spectra of purity of the isolated compound. |
The retention time of 2,4 DTBP isolated from the ethanolic extract of Ficus auriculata leaves was about 3.077.
UV-Visible Spectroscopy
 |
Figure 3: UV-Visible Spectra of the isolated compound. |
The UV-Visible spectra of the isolated compound is performed over a wavelength range of 200-800 nm. Tertiary butyl group absorbs in the range 224 nm and 276.8 nm with intensities at 1.273and 0.75 respectively. This indicates the presence of phenolic functional group. These data provides additional support to the structure.
FT-IR Spectroscopy
 |
Figure 4: IR Spectra of the isolated compound. |
The IR Spectrum of the compound isolated shows various peaks. Out of which, O-H stretching is represented by 3519.85cm-1, C-H stretching is represented by 2962.46 cm-1, C-C stretching of aromatic compounds is represented by 1506.30–1604.66 cm-1, C-H bending in the tertiary butyl group may be responsible for 1362.61 cm-1, and C-O stretching frequency is represented by 1252.68 cm-1. Based on all these data, it is clear that the isolated compound contains aromatic ring, tertiary butyl group and phenolic group. Hence the compound maybe 2,4Ditertiary Butyl Phenol.
1H Nuclear Magnetic Resonance (1HNMR) Spectroscopy
 |
Figure 5: 1H-NMR Spectra of the isolated compound. |
Figure 5 shows the 1H NMR spectra of the compound isolated. The signals revealed the presence of phenolic proton (D) at 3.376, Methylene proton (R-CH2-R) (F) at 1.232, Methine proton (R3C-H) (E) at 1.348 and aromatic protons (A) (B)&(C) around7.147, 7.141and 7.006 respectively.
The existence of each of these signals supports the isolated compound’s structure.
The Phytochemical screening, Column Fractions, TLC Studies, GC-MS Spectra, UV-Vis, FT-IR and 1HNMR confirms that the isolated compound is 2,4DTBP and its structure is:
 |
Scheme 1 |
Effect of Solvent
 |
Figure 6: Effect of Solvent on isolated compound. |
The solvent effects on the electronic absorption is used to identify the electronic transitions in a molecule. The light absorbed by the compound shifts from low energy ,long wavelength to high energy, short wavelength as the solvents become more polar.
Table 4: Effect of Solvent on isolated compound.
|
Solvent |
Wavelength |
Absorbance |
|
Water |
221.6 |
1.237 |
|
Ethanol |
224 |
1.273 |
|
Ether |
231.2 |
1.351 |
|
Butanol |
224 |
1.282 |
|
Chloroform |
243.2 |
1.512 |
|
Carbon tetra chloride |
243.2 |
1.515 |
|
Hexane |
219.2 |
1.122 |
Fig.6 & Table 4. shows the Effect of Solvent on isolated compound. Saturated compounds with one hetero atom (ethanol, butanol, ether,CCl4, CHCl3, water) undergoes
n → σ* transition, whereas in Hexane only sigma bond is available and hence σ → σ* transition occurs, which requires higher energy and lower wavelength.
Anti-Microbial property of 2,4 DTBP
Table 5: Anti-Bacterial activity of 2,4 DTBP
|
Name of Bacteria Strains |
Samples Zone of inhibition (mm in diameter) |
|||||
|
S1 25µg |
S1 50µg |
S1 75µg |
S1 100µg |
Positive control |
Negative control |
|
|
E.Coli |
9 |
10 |
10 |
11 |
16 |
– |
|
Staphylococcus |
– |
– |
8 |
9 |
16 |
– |
|
Enterococcus |
– |
– |
– |
– |
17 |
– |
|
Pseudomonas aeruginosa(G-) |
– |
– |
– |
– |
10 |
– |
|
Klebsiella |
8 |
11 |
12 |
14 |
20 |
– |
 |
Figure 7: Anti-bacterial activity of the isolated compound on (a). E. coli (b) Staphylococcus aureus (c) Enterococcus faecalis (d) Pseudomonas aeruginosa (e) Klebsiella pneumonia Click here to View figure |
The anti-bacterial activity of 2,4 DTBP was analyzed with various bacteria like :E.coli, Staphylococcus aureus, Enterococcus faecalis, Pseudomonas aeruginosa, Klebsiella pneumonia. Enterococcus faecalis, Pseudomonas aeruginosa has no inhibition. E.coli, Staphylococcus aureus, Klebsiella pneumonia shows inhibition. Out of these three, Klebsiella pneumonia shows higher inhibition than E.coli, Staphylococcus aureus. Hence it is concluded that, 2,4 DTBP strongly inhibits the activity of bacteria Klebsiella pneumonia.
Table 6: Anti-Fungal activity of 2,4 DTBP
|
Fungai Name |
Samples Zone of inhibition(mm in diameter) |
|||||
|
S1 25µg |
S1 50µg |
S1 75µg |
S1 100µg |
Positive |
Negative |
|
|
Candida sps |
6 |
10 |
11 |
12 |
14 |
– |
 |
Figure 8: Anti-fungal activity of the compound |
Fig.8 depicts the anti fungal activity of the compound isolated, 2,4 DTBP with Candida albicans. This fungi shows dense zone of inhibition. The compound is very active against the fungi.
Cytotoxic Effect
MTT Assay
Table 7: % cell viability values of 2,4 DTBP against HIEC-6 cells after the treatment period of 24hrs.
|
Culture condition |
% cell viability |
IC50 conc (ug/ml) |
|
Untreated |
100 |
NA |
|
Std control (Dox-5uM) |
56.20 |
|
|
S1-10ug/ml |
99.41 |
|
|
S1-25ug/ml |
94.89 |
|
|
S1-50ug/ml |
89.08 |
|
|
S1-75ug/ml |
78.87 |
|
|
S1-100ug/ml |
69.50 |
 |
Figure 9: % cell viability values 2,4 DTBP against HIEC-6 cells after the incubation period of 24hrs. |
 |
Figure 10: Effect of Concentration of isolated compound on HIEC-6 cells (a)untreated 10μg/ml (c) 25μg/ml (d) 50μg/ml (e)75 μg/ml(f)100μg/ml. Click here to View figure |
Fig.9 is the graphical representation of the viability of HIEC-6 cells with the isolated compound,andFig.10shows the effect of concentration of the isolated compoundonHIEC-6 cells.
The observed results clearly confirmed the Non-toxic efficacy of the isolated compound on Normal human intestinal epithelial cells with % cell viability value of 69%, at the highestconcentrationof100ug/ml after the incubation period of24hours.
Table 8: Cell Viability of AGS cells with 2,4DTBP at various concentrations.
|
Samples |
Triplicate 1 |
Triplicate 2 |
Triplicate 3 |
Average |
Percentage of Viability |
IC50 |
|
Control |
0.679 |
0.668 |
0.653 |
0.66667 |
– |
83.18 |
|
6.25 |
0.633 |
0.625 |
0.617 |
0.625 |
93.75 |
|
|
12.5 |
0.584 |
0.571 |
0.565 |
0.57333 |
86 |
|
|
25 |
0.495 |
0.486 |
0.477 |
0.486 |
72.9 |
|
|
50 |
0.395 |
0.416 |
0.407 |
0.406 |
60.9 |
|
|
100 |
0.287 |
0.305 |
0.313 |
0.30167 |
45.25 |
 |
Figure 11: Cell Viability of AGS cell line with isolated compound. |
 |
Figure 12: Effect of Concentration of isolated compound on AGS cell line (a) untreated (b) 6.25 μg/ml (c) 12.5μg/ml (d) 25 μg/ml (e) 50μg/ml (f) 100μg/ml Click here to View figure |
Fig.11 is the graphical representation of the viability of AGS Cell line with the isolated compound, and Fig.12shows the effect of concentration of the isolated compound on AGS Cell line. As the concentration increases, the viability decreases. At 100 μg/ml concentration, the viability of the cancer cell is minimum. (ie) : 45.25%.
With varied quantities of the sample supplied to SKMEL cancer cells, a dose-dependent reduction in cell viability was seen. 83.18µg/mL is the IC50value obtained for the sample.
Molecular Docking Study
Table 9: Set of Patch Dock results showing the docking structures of Occluding with 2,4 DTBP.
|
S.No |
Score |
Area |
Atomic Contact Energy(ACE) |
Transformation |
|
1 |
2846 |
358.70 |
-57.23 |
-3.021.14 -1.87 6.06 15.72 0.80 |
|
2 |
2828 |
352.50 |
-101.00 |
0.081.24 -2.43 2.85 14.97 8.88 |
|
3 |
2790 |
331.70 |
-84.85 |
-0.49-1.03 0.30 2.75 14.84 9.02 |
|
4 |
2726 |
366.90 |
14.29 |
1.290.07 3.07 6.43 18.21 -6.28 |
|
5 |
2696 |
314.20 |
-73.90 |
-2.03-0.33 2.07 3.83 13.90 7.91 |
|
6 |
2684 |
368.00 |
7.26 |
-1.450.19 0.04 7.3618.54 -6.72 |
|
7 |
2680 |
304.10 |
-24.97 |
-1.67-0.35 1.68 1.29 12.73 17.69 |
|
8 |
2666 |
306.10 |
-1.60 |
-0.05-1.20 1.94 9.92 20.16 -14.58 |
|
9 |
2646 |
345.80 |
-18.61 |
-2.090.07 2.79 8.0016.19 -2.19 |
|
10 |
2632 |
372.20 |
-61.13 |
0.64-1.12 1.99 6.4816.07 -0.33 |
 |
Figure 13: 3-D Structure of (a) 2,4DTBP (b) Occluding (c) 2,4 DTBP Docked with Occluding |
Fig.13 (a), (b) shows the 3-D Structures of 2,4 DTBP, Occluding respectively. These structures are obtained in PDB format and viewed using PyMol software. Out of all the models, the one with score 2846 for the area 358.70 is chosen as a the most favourable model, which is represented in Fig.11 (c).
Lipinski Rule of Five
Table 10: Lipinski Rulefor2,4 DTBP with Occluding.
|
Mass |
206 |
|
Hydrogen Bond Donor |
1 |
|
Hydrogen Bond Acceptor |
1 |
|
Log P |
3.987 |
|
Molar Refractivity |
65.506 |
From the above results, it is clear that the compound 2,4 DTBP doesn’t violate any criteria, hence it can be orally administered.
Conclusion
Phytochemicals present in various extracts of Ficus auriculata leaf has been analyzed.
From the Chromatographic, GC-MS and Spectroscopic studies , and comparing the results from various literatures, it is found that the isolated compound is 2,4Ditertiary Butylphenol, which is a biologically active compound, and from the invitro & cytotoxicity studies, it is found to possess anti-microbial & anti-cancer activity. The 3-D structure obtained from Molecular docking studies, is subjected to Lipinski rule of 5,and from the data obtained it can be concluded that 2,4 DTBP can be administered orally.
Acknowledgement
The authors wish to thank the corresponding institutions for their instrumental support.
Conflict of Interest
There’s no conflict of interest, as stated by the authors.
References
- Sasidharan,S.;Chen,Y.;Saravanan,D.;Sundram,K.M.;Yoga Latha,L. Afr.J.Complement Altern Med.2011, 8(1), 1–10.
CrossRef - Sunyong Yoo.;Kwansoo Kim.;Hojung Nam.; DoheonLee.Nutr.J. 2018, 10(8), 1042.
CrossRef - Swapna Upadhyay.; Madhulika Dixit. Oxid.Med.Cell. Longev.2015,1-15.
- Vinay Kumar.;Nirmal Kumar.;Leirika Ngangom.;Kunal Sharma.;Manu Pant.; Syed Mohsin Waheed. Eco. Env.&Cons.2020,26(October Suppl.Issue), 108-113.
- Luísa Lima Bertoletti.;Everton Skoronski.;Liziane Schittler Moroni.;Aniela Pinto Kempka. Agric.conspec.Sci,2020,85(4),303-310.
- Fuqiang Zhao.; Ping Wang.;Rima,D.;Lucardi.;Zushang Su.; Shiyou Li.Toxins,2020, 12(35),1-26.
- Ei Aung.;Alfinda Novi Kristanti.;Nanik Siti Aminah.;Yoshiaki Takaya.;Rico Ramadhan. Ecol.Environ.Sci,2020,5(4),2573-2919.
- Reiye Esayas Mengesha.;Berhe Gebre-Slassie Kasa.;Muthupandian Saravanan.;Derbew Fikadu Berhe.; Araya Gebreyesus Wasihun.BMC Res.Notes, 2014,7, 575.
CrossRef - Fatimah Abdulsayid,A.; Hamad Adress Hasan,M.Int.J. Innov.Sci.Eng. Technol, 2020, 7(5),78-85.
- Rarey,M.;Kramer, B.;Lengauer,T.;Klebe,G.JMolBiol.,1996,261(3),470-489.
CrossRef - Thomas Karami,K.;Shumet Hailu.;Shaoxin Feng.;Richard Graham.; Hovhannes Gukasyan,J. JOcul Pharmacol Ther, 2022, 38(1), 43-55.
CrossRef - Soo Jung Choi.; Jae Kyeom Kim.; Hye Kyung Kim.; Keith Harris.; Chang-Ju Kim.; Gwi GunPark.;Cheung-Seog Park.;Dong-Hoon Shin. J.Med.Food,2013, 16(11),977-983.
CrossRef - Mihaela Pana.;Alexandru Pana.; Gabriella Rau.;George Dan Mogosanu.Farmacia,2011, 59(6),830-841.
- Kohner, P.C.; Rosenblatt, J.E.; Cockerill, F.R. J. Clin. Microbiol, 1994, 32, 1594 -1596.
CrossRef - Mathabe, M.C.; Nikolova, R.V.; Lall, N.; Nyazema, N.Z. J.Ethnopharmacol, 2006, 105, 286-293.
CrossRef - Assam,A.J.P.;DzoyemJ.P,Pieme,C.A.; PenlapV.B.BMC Complement.Altern.Med,2010,10(40),1-7.
- Bauer,A.W.; Kirby, W.M.M.; Sherris, J.C.;Turck M. Amer. I. C/in. Pathol, 1966, 45, 493-496.
CrossRef - Yeon Woo Song.;Yoongho Lim.;Somi Kim Cho.BBAMCR,2018,18241,1-36.
- Young-Min Kim.; In-Hye Kim.; Taek-Jeong.Mol.Med.Rep,2013,8, 11-16.
- Lengauer,T.;Rarey,M.Curr.Opin.Struct.Biol,1996,6(3), 402-406.
CrossRef - Mosmann,T,J.Immunol.Methods,1983,65(1-2),55-63.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.










