Biosynthesis of CaO Nanoparticles using Cleome viscosa leaf Extract and Investigation of their Antioxidative and Cytotoxicity Activity
Hirdesh Sharma1,2, Roma Lal3*, Maneesha Pandey3 and Archana Shrivastav2
1School of Studies in Micro-Biology, Jiwaji University Gwalior-474009, Madhya Pradesh, India.
2College of Life Sciences, Cancer Hospital Campus, Gwalior-474009, Madhya Pradesh, India.
3Disciple of Biochemistry, School of Sciences, Indira Gandhi National Open University, New Delhi India.
Corresponding Author E-mail: lalroma80@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/390123
Article Received on : 10 Sep 2022
Article Accepted on : 11 Jan 2023
Article Published : 23 Jan 2023
Reviewed by: Dr. Prawit Nuengmatcha,
Second Review by: Dr. Ujjwal Das
Final Approval by: Dr. MGH Zaidi
Cleome viscose Linn. also known as the jakhya are widely utilised in traditional and ethnomedicine. Biosynthesis of calcium oxide nanoparticles has captured attention of many as due synthesis involve non-toxic and eco-friendly solvents and ingredients, is more environmentally friendly, least time taking and cost-effective, and simpler than the other alternatives. In the study CaCO3 was obtained from conch shell. CaONPs were biosynthesized in methanolic extract of Cleome viscosa leaves through precipitation and deposition of CaCO3. The synthesized CaO nanoparticle was having the average particle size of ~72 nm according to DLS and the particle was found to be stable with zeta potential of -21.6 mV. The SEM analysis of nanoparticle predicted the structure to be roughly round. The UV-Visible spectrophotometer analysis predicted the maximum absorption in the visible range of ~400-420 nm. The synthesized CaO nanoparticle was found to be quite effective against BT-474 breast cancer cell line of conc. 3.4 mg/ml having cell cytotoxicity of ~ 86% at this concentration and IC50 of nanoparticle was 1.359 mg/ml. The IC50 of Antioxidative assay was 282 µg/ml and 525 µg/ml for DPPH and ABTS free radicals respectively.
KEYWORDS:Antioxidative activity; Cleome viscose leaves; CaO Nanoparticle; Cell Cytotoxicity
Download this article as:| Copy the following to cite this article: Sharma H, Lal R, Pandey M, Shrivastav A. Biosynthesis of CaO Nanoparticles using Cleome viscosa leaf Extract and Investigation of their Antioxidative and Cytotoxicity Activity. Orient J Chem 2023;39(1). |
| Copy the following to cite this URL: Sharma H, Lal R, Pandey M, Shrivastav A. Biosynthesis of CaO Nanoparticles using Cleome viscosa leaf Extract and Investigation of their Antioxidative and Cytotoxicity Activity. Orient J Chem 2023;39(1). Available from: https://bit.ly/3iUv46X |
Introduction
Nanotechnology is a recently evolved discipline that strives to synthesize, modify and apply structures in the nanoscale size of 100 nm. These nanoparticles have been widely used in a variety of disciplines of public interest during the last few decades. Nanotechnology has the potential to transform the pharmaceutical sector by providing new tools for molecular illness therapy and quick disease diagnosis. Nanoparticles or nanostructures have higher surface area, large surface potential and distinct surface characteristics 1. When compared to bulk materials, these nanosized materials have high surface-to-volume ratios. Larger particles’ physical properties are stable, have a lower surface volume ratio, and restrict their use in many areas. Bulk materials display better and unique properties when treated at the nanoscale level due to their size, shape, and morphology 2. These nanostructures are beneficial in medical, solar energy, and material sciences 3-5. Calcium oxide (CaO) nanoparticles act as catalyst 6, help in adsorption 7, in purification of water 8, widely used as cosmetic industries 9, in medicines 10, and waste treatment 11. CaO nanoparticles have been synthesized by several methods like sol-gel 12, gas phase 13, microwave assisted synthesis [8], by chemical precipitation 7 and by electrochemical methods 14.
Green approaches are now mostly used approach to synthesize the nanomaterials. Synthesis by this approach is cost-effective, nontoxic as it does not require use of harmful chemicals, and are environmental favourable. and environmentally friendly 15. Secondary metabolites, phytochemicals of plant extracts have reducing properties and can reduce a calcium precursor like CaCl2, or CaCO3 16,11. CaO is considered to be safe for human beings and environment.
Cleome viscosa (CV) Linn., a type of weed widely distributed in the tropical region of the world including plains of India. The herb is a commonly used remedy for a variety of diseases, according to ethnobotanical research and traditional medicinal systems 17. Cleome viscosa has been studied scientifically against helminths, bacteria, fever, diarrhea, inflammation, liver diseases 18-22.
Therefore, objectives of our study are: 1) to obtain calcium oxide CaONP by the deposition and precipitation of CaCO3 in Cleome viscosa leaf extract; 2) to characterize CaO nanoparticles, and 3) to determine the antioxidant activity and its cytotoxicity against MCF-10A (normal) and BT-474 (breast cancer) cell lines.
Material and Methods
Material
Breast cancer cell line BT-474 and MCF-10A were purchased from NCCS, Pune.
Collection of plant sample
Cleome viscose’s leaves were collected from the local area in East nimar region (Khandwa) district (21°49’22.08”N, 76°21’8.244”E), Madhya Pradesh in the months of March-April, 2019 and was authenticated by Dr. Anamika, Department of Botany, Vardhman college Bijnor. After cleaning leaves were dried, grounded and stored in dry place. Nearly 200 gm of leaf powder was Soxhlet extracted for 3-4 cycles in methanol solvent. Crude extract was dried and weighed. Dried extract was used for further study.
Preparation of CaCO3 from conch shells
The conch shell was ground in a powerful grinder to produce a very fine powder containing nanoparticles of varying sizes. The powder was then sieved in a mechanical sieve, yielding particle sizes ranging from 43 to 150 microns. This powder was kept in a sterile container for further analysis (Figure 2).
Deposition or precipitation of CaO from CaCO3
CaO nanoparticles were prepared following the protocol of Maringgal et al., 2020 23. In distilled water, a 1M solution of CaCO3 derived from conch shell was prepared. The solution was maintained at room temperature on a magnetic stirrer for 5 hours before being filtered. CaCO3 was filtered, dried, and weighed before being mixed in distilled water with an equal amount of Cleome viscosa leaf extract to bind with molecules. The mixture was maintained at 600 C overnight followed by two to three days incubation at room temperature. Mixture was diluted in water and filtered overnight followed by thermal deposition at a temperature of ~7000 C and was calcinated at 9000 for 1 hr.
Characterization of synthesized CaO nanoparticle
The synthesized CaO nanoparticle was characterized by Uv-vis spectrophotometer, FTIR, DLS Zeta potential and SEM.
UV-vis spectrophotometer analysis of nanoparticles
The synthesized CaO nanoparticle was scanned from 200 to 800 nm. Band gap energy was calculated using tauc plot.
Fourier-Transform Infrared Spectroscopy (FT-IR)
Any possible alterations in the functional groups present in the cleome viscosa and CaO nanoparticles were investigated by FTIR spectroscopic measurements using Bruker Optik Alpha-T-FTIR Spectrometer, set in the range of 400-4000 cm-1.
Particle size and distribution
The particle size of synthesized CaO nanoparticle was estimated by dynamic light scattering using Malvern Mastersizer – 2000, Canada. Deionized water having refractive index of 1.330 was used as dispersion media. Zeta potential or surface charge of the CaO nanoparticle was estimated after proper dilution. A photon correlation spectrophotometer was used to measure the size distribution of CaO nanoparticles via dynamic light scattering.
SEM analysis
Scanning electron microscopic or SEM analysis was performed using JSM-7610F Schottky Field Emission SEM machine of Japan. The scale and structure of the nanoparticles were covered on the carbon coated copper grid, enabling the grid to be amplified to evaluate the structure and size of the nanoparticles.
Antioxidative activity of CaONP
ABTS assay
ABTS assay was performed according to Pellegrini et al., 1958 24. Briefly 7mM ABTS in water and 2.45 mM potassium persulphate mixed (1: 1) incubated in dark for 12-16 hrs. The mix is then diluted to get absorbance of ~0.7 at 734 nm. Samples were diluted to 1 mg/ml. Samples (Crude, NP) were further diluted in 100, 200, 300, 400 µg/ml concentration and volume make up with ABTS reagent up to 4 ml and incubated in dark for 10 min. Absorbance were taken at 734 nm. Control was prepared by taking absorbance of ABTS and methanol. Standard curve of Ascorbic acid was prepared simultaneously with standards dilution range of 100, 200, 300, 400 µg/ml. ABTS+ scavenging effect (%) was calculated as following equation.
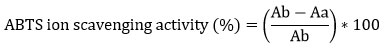
Ab=Absorbance of reagent control
Aa= Absorbance of sample/standard
IC50 of all samples were calculated with their respective standard curve equation.
DPPH assay
DPPH assay was performed using protocol of Blois et al., 1958 26. Samples were diluted to 1 mg/ml conc. Samples (Crude, NP) were further diluted to 100, 200, 300, 400 µg/ml concentrations and 1 ml 1.0 ml of 0.1 mM of DPPH in methanol was added. Ascorbic acid dilution was prepared in the range of 100, 200, 300, 400 µg/ml. Incubated the reaction mixture for 30 minutes at RT. Positive control was ascorbic acid. DPPH free radical scavenging activity was calculated as percentage inhibition of free radical and was calculated as follow:
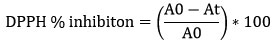
where A0 is the absorbance of control (blank without sample) and At is the absorbance of sample. All the tests were performed in triplicate and graph was plotted with mean values.
IC50 of all samples were calculated with their respective standard curve equation.
Cytotoxicity assay of CaONP:
Cytotoxicity of crude, CaCO3 and CaONPs was estimated by (3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide) assay following the protocol of Mossmann, 1983 26. The test samples had concentration levels of 1000, 1500, 2000, 2500, 3000, 3500, 4000 and 4500 µg/ml. The diluted samples were mixed together with the cell lines that had been grown for 24 hours in a confluent monolayer plate. After 24 hours of incubation at 37°C in a 5% CO2 incubator, the supernatant was collected, and 25 µl of MTT reagent (2 mg/ml) was applied to each well. Following a 2-hour incubation period at 37°C, each well received 125 µl of dimethyl sulphoxide to solubilize the formazan precipitate, and the wells were agitated for another 15 minutes. At a wavelength of 490 nm, the absorbance was measured using an ELISA reader. Control wells were medium only, DMSO control and Cell control (Well having BT-474 and MCF-10A cells) without the examined chemical.
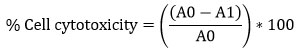
where;
A0 is the absorbance of the Cell control (BT-474, MCF-10A) and
A1 is the absorbance of the Cell with crude and CaONPs dilutions.
Cell morphology and cell count (trypan blue dye exclusion assay):
BT-474 cell lines were incubated at the IC50 concentrations of the crude, CaCO3 and CaONPs. Six well microplates were seeded with ~2.6*105 cells/ml. Incubated for 48 hrs. in CO2 incubator with 5% CO2, 95% air and 99% humidity at 370 C. Cells were visualized under inverted microscope to detect any morphological changes. Cells were then trypsin-zed and centrifuged for pelleting. Pellets were dissolved in 1 ml of DMEM media. Briefly 100-200 µl of the cells were mixed with equal volume of trypan blue (0.4%) and placed on haematocytometer. Observed under the compound microscope in 10X or 40X and counted the coloured (dead) and non-coloured (viable) cells. Counted all the four corners chamber of haematocytometer leaving the middle and below line and calculated the number of cells/ml using the formula:
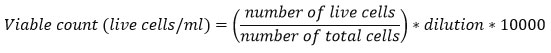
Characterization of CaO nanoparticles:
CaONPs were synthesised using dried Cleome viscosa leaf extract. When dark green methanolic leaf extract was added to CaCO3 followed by thermal deposition, it changed to brownish green, perhaps owing to capping of leaf extract compounds around CaO nanoparticle. (Figure 1).
 |
Figure 1: Biosynthesis of CaONP |
C. viscosa leaf extract displayed a maximal absorbance at 206-280 nm, according to UV-visible spectroscopic studies (Figure 2). The illustration shows that the absorption of CaONPs occurs in the visible area. The UV-Vis of CaONP demonstrates that the nanomaterial has high absorption capability in the visible range 27. The band gap mapping of CaO nanoparticles was computed approximately 2.673 eV, as shown in Figure 2C, and the absorption spectra corroborated this. A graph between (hv)2 and hv was used to explore the band gap energy.
(αhv)= B(hv – Eg)n
Where, B is constant, Eg is band gap energy of CaO and exponent ‘n’ is the nature of the transition and n = 1/2, 2
The secondary metabolites specially phenols, polyphenolic compounds and flavonoids significantly reduce the Ca ion formation by capping around the nanoparticle 28. The synthesized CaO nanoparticle through deposition and precipitation is found to have less toxic effect on environment and human due to lack of hazardous chemicals used in chemical synthesis of these nanoparticles 29 and secondary metabolites of extract increases the antioxidative capacity of nanoparticle 30.
 |
Figure 2: Uv-visible spectrum and band gap energy analysis: A: UV-vis spectrum of CaONP and Cleome viscosa leaf extract. |
Fourier-Transform Infrared Spectroscopy (FT-IR)
IR spectroscopy is a strong tool to get an idea on vibrational or rotational levels of a certain molecules. FTIR collects data in a wide range of wavelength and is dependent on the electromagnetic radiation. It describes the many functional groups on the surface of nanoparticles 31.
 |
Figure 3: FTIR spectra of A: Cleome viscosa leaf extract, B: CaONP. |
FTIR spectrum of Cleome viscosa, CaO nanoparticle are presented in Figure 3 to characterize and compare the two. The FTIR result of the Cleome viscosa leaf extract showed peaks at 3549, 2349, 1627, 1462, 1142, 1021 and 844 cm-1 indicating O-H bond present due water molecule present in leaf extract 32, N-H bond due to presence of amines in extract 33, C=C stretch due to presence of aromatic amines, C-N bends due to presence of amides, C-O bond due to environmental CO2, C-N or C-O stretch due to aliphatic amines and alcohol, C-Cl stretch due to halides 34 respectively. CaONPs IR spectra revealed key peaks at 1403, 874, 712, and 495 cm-1, with the peak at 1403 cm-1 indicating a C-O connection between carbonate and calcium. The existence of a high and sharp peak at 1403 indicates that CaCO3 has not been totally converted to CaO and that there is still a considerable amount of CaCO3. Calcium carbonate’s C-O stretching and bending modes feature two well-defined infrared peaks at 1403 and 874 cm-1, respectively 35. The sharp band at 712 cm-1 corresponds to Ca-O bonds, whereas the band at 495 cm-1 corresponds to the CaO band 36. The synthesized CaONP inherited some of the compounds of cleome viscosa leaf extract as indicated from FTIR spectra. There is also a broad peak at 3549 cm-1 due to presence of O-H bond and sharp peak of 2349 cm-1 due to amines of extract.
Particle Size and distribution (DLS)
Particle size of the synthesized CaONP is found to 72.20 nm with an intensity of 88.3% (Figure 4A). Some minor peaks indicating particle size of 8.91 nm was also seen. The results are consistent with SEM findings and near to previous studies of CaONP particle size of 62 nm 37. The zeta potential of the CaONP was found to be -21.6 mV (Figure 4B) and is in according to earlier reports 37. The zeta potential analysis indicates the stability of nanoparticle. Negative value of potential indicates its slightly anionic in nature.
 |
Figure 4: A: Particle size distribution of synthesized CaONP by intensity, B: Zeta potential of CaONP by DLS. |
Scanning electron microscopy (SEM)
SEM images of CaONP was analysed using ImageJ software to estimate the average particle size and morphology of synthesized CaONP. The average particle size estimated by SEM analysis was found to be 75.61 nm similar to the findings of particle size distribution. The mean area of the particles is found to be 4183.96 nm2. From the analysis it is revealed that maximum number of particles are in the range of 80 to 90 nm and are in 1000 to 2000 nm2 area. The nanoparticles were found to be irregular in shape (Figure 5 D). Surface of CaONPs is found to have dents and are not smooth.
 |
Figure 5: SEM analysis of CaONP (A, B, C); D: Frequency distribution plot of particle |
Antioxidative assay
Antioxidant activity and IC50 of cleome viscosa and CaONP was estimated by ABTS and DPPH free radicals. The IC50 of synthesized CaONP was found to be more effective than the cleome viscosa or CaCO3. IC50 for DPPH was estimated to be 533 µg/ml, 502 µg/ml and 283 µg/ml for CaCO3, cleome viscosa extract and CaONP respectively (Figure 6). While the IC50 for ABTS free radicals was found to be 5 mg/ml, 1.06 mg/ml and 525 µg/ml for CaCO3, cleome viscosa extract and CaONP respectively. The CaONP was more effective against DPPH than ABTS free radical.
 |
Figure 6: Antioxidative activity of CaCO3, Cleome viscosa leaf extract and CaONP by A: DPPH and B: ABTS assay. Data are mean ± std. dev. Where (n=3). |
Cell Cytotoxicity and cell viability assay
Cell cytotoxicity of synthesized CaONP, cleome viscosa crude and CaCO3 was estimated against BT-474 breast cancer cell lines and MCF-10A (control) by MTT assay (Figure 7). The IC50 concentration of CaONPs against BT-474 is found to be ~1.36 mg/ml and that of against MCF-10 is found to be 14 mg/ml. There is not much effect of CaONP on normal cell lines while it can kill ~88.76% of cancer cells at a concentration of 3.5 mg/ml. The IC50 of CaONP against lung cancer cell lines (A549) was found to be 92.08 µg/ml 38. The earlier reported findings and result obtained suggests its applicability against cancer.
The number of viable cells was greatly reduced after an incubation of 48 hrs. with IC50 concentrations of crude (Figure 8 B) and CaONPs (Figure 8 D) in BT-474 cell lines. While there is no visible decrease in cell viability in CaCO3 treated BT-474 cell lines. Although treatment of the BT-474 cell line with crude and CaONPs inhibited cell proliferation or growth, the number of dead cells as well as the number of viable cells increased in cells treated with CaCO3 (Figure 8 C). There are no morphological changes in the cells, and the cells are properly adhered to the substrate.
 |
Figure 7: A: Cell cytotoxicity assay (MTT) against MCF-10A (normal cell line) and BT-474 (breast cancer cell lines), |
 |
Figure 8: Cell morphology of BT-474 control (A) and after treatment of IC50 concentration of crude (B), CaCO3 (C) and CaONPs after 48 hrs of incubation. Click here to View figure |
Conclusion
Synthesized CaONP have good antioxidative and anticancer activity. The nanoparticle is quite effective against breast cancer cell line. The size of nanoparticle is less than 100 nm so it has good capacity to penetrate the cell membrane and diffuse into the cells.
Acknowledgement
I acknowledge ChemGeneics research foundation, Noida to let me use their lab and facility. I acknowledge Dr. Anamika Singh to help in authenticating the plant. I acknowledge Shraddha Analytical Services, Mumbai for SEM analysis and Lovely professional university for providing Zeta potential and Particle size analysis.
Conflict of interest
The authors state that they have no financial or other conflicts of interest.
Reference
- Roy, A.; Singh, V.; Sharma, S.; Ali, D.; Azad, A. K.; Kumar, G.; Emran, T. B. J. Nanomater. 2022, 2022, 3636481. https://doi.org/10.1155/2022/3636481.
CrossRef - Jeevanandam, J.; Barhoum, A.; Chan, Y. S.; Dufresne, A.; Danquah, M. K. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. https://doi.org/10.3762/bjnano.9.98.
CrossRef - Bashir, R.; Chisti, H. T.; Rangreez, T. A.; Mobin, R. In M. Bhat, I. Wani, & S. Ashraf (Ed.), Applications of Nanomaterials in Agriculture, Food Science, and Medicine. 2021, 310-329. IGI Global. https://doi.org/10.4018/978-1-7998-5563-7.ch017.
CrossRef - Khalfin, S.; Veber, N.; Dror, S.; Shechter, R.; Shaek, S.; Levy, S.; Kauffmann, Y.; Klinger, L.; Rabkin, E.; Bekenstein, Y. Adv. Funct. Mater. 2022, 32(15),2110421. https://doi.org/10.1002/adfm.202110421.
CrossRef - Khan, I.; Saeed, K.; Khan, I. Arabian J. Chem. 2019, 12(7), 908–931. https://doi.org/10.1016/j.arabjc.2017.05.011.
CrossRef - Banković–Ilić, I. B.; Miladinović, M. R.; Stamenković, O. S.; Veljković, V. B. Renewable Sustainable Energy Rev. 2017, 72(C), 746–760. https://doi.org/10.1016/j.rser.2017.01.076.
CrossRef - Khine, E. E.; Koncz-Horvath, D.; Kristaly, F.; Ferenczi, T.; Karacs, G.; Baumli, P.; Kaptay, G. J. Nanopart. Res. 2022, 24, 139. https://doi.org/10.1007/s11051-022-05518-z.
CrossRef - Roy A.; Bhattacharya J. TechConnect Briefs, 2011 3, 565–568. https://doi.org/10.1142/S0219581X11008150.
CrossRef - Raj, S.; Jose, S.; Sumod, U. S.; Sabitha, M. J Pharm Bioallied Sci. 2012, 4(3), 186-193. https://doi.org/10.4103/0975-7406.99016.
CrossRef - Butt, A.; Ejaz, S.; Baron, J. C.; Ikram, M.; Ali, S. Dig. J. Nanomater. Bios. 2015, 10(3), 799-809.
- Ferraz, E.; Gamelas, J. A. F.; Coroado, J.; Monteiro, C.; Rocha, F. Mater. Struc. 2018, 51(5), 115. https://doi.org/10.1617/s11527-018-1243-7.
CrossRef - Habte, L.; Shiferaw, N.; Mulatu, D.; Thenepalli, T.; Chilakala, R.; Ahn, J. W. Sustainability (Switzerland). 2019, 11(11), 3196. https://doi.org/10.3390/su11113196.
CrossRef - Popok, V.N.; Kylián, O. Appl. Nano. 2020, 1(1), 25-58. https://doi.org/10.3390/applnano1010004.
CrossRef - Li, N.; Bai, X.; Zhang, S.; Gao, Y. A.; Zheng, L.; Zhang, J.; Ma, H. J. Dispersion Sci. Technol. 2008, 29(8), 1059-1061. https://doi.org/10.1080/01932690701815606.
CrossRef - Ahmed, S. F.; Mofijur, M.; Rafa, N.; Chowdhury, A. T.; Chowdhury, S.; Nahrin, M.; Islam, A. B. M. S.; Ong, H. C. Environ. Res. 2022, 204, 111967. https://doi.org/10.1016/j.envres.2021.111967.
CrossRef - Osuntokun, J.; Onwudiwe, D. C.; Ebenso, E. E. IET Nanobiotechnol. 2018, 12(7), 888–894. https://doi.org/10.1049/iet-nbt.2017.0277.
CrossRef - Senthamilselvi, M. M.; Kesavan, D.; Sulochana, N. Org. Med. Chem. Lett. 2012, 2(1), 19. https://doi.org/10.1186/2191-2858-2-19.CrossRef
CrossRef - Devi, B. P.; Boominathan, R.; Mandal, S. C. Phytomedicine, 2002, 9(8), 739–742. https://doi.org/10.1078/094471102321621368.
CrossRef - Mali, R. G.; Mahajan, S.G.; Mehta, A. A. Pharm. Biol., 2007, 45(10), 766-768. https://doi.org/10.1080/13880200701585923.
CrossRef - Devi, B. P.; Boominathan, R.; Mandal, S. C. J Ethnopharmacol., 2003, 87(1), 11–13. https://doi.org/10.1016/s0378-8741(03)00099-0.
CrossRef - Gupta, N.K.; Dixit, V.K.; Indian J Pharmacol. 2009, 41(1), 36-40. https://doi.org/10.4103/0253-7613.48892.
CrossRef - Sudhakar, M.; Rao, Ch. V.; Rao, P. M.; Raju, D. B. Fitoterapia, 2006, 77(1), 47–49. https://doi.org/10.1016/j.fitote.2005.08.003. CrossRef
CrossRef - Maringgal, B.; Hashim, N.; Tawakkal, I. S. M. A.; Hamzah, M. H.; Mohamed, M. T. M. J. Mater. Res. Technol. 2020, 9(5), 11756–11768. https://doi.org/10.1016/j.jmrt.2020.08.054.
CrossRef - Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Free Radic. Biol. Med. 1999, 26(9-10), 1231–1237. https://doi.org/10.1016/s0891-5849(98)00315-3.
CrossRef - Blois, M.S. Nat. 1958, 181,1199-1200. http://dx.doi.org/10.1038/1811199a0.
CrossRef - Mosmann, T. J. Immunol. Methods. 1983, 65(1-2), 55-63.https://doi.org/10.1016/0022-1759(83)90303-4.
CrossRef - , S. S. T. H.; Moosavi, S. S.; Oskuee, R. K. Biomass Conv. Bioref. 2022. https://doi.org/10.1007/s13399-022-02643-6.
CrossRef - Mohanpuria, P.; Rana, N. K.; Yadav, S. K. J. Nanopart. Res. 2008, 10(3), 507-517. https://doi.org/ 10.1007/s11051-007-9275-x.
CrossRef - Singh, J.; Dutta, T.; Kim, K.-H.; Rawat, M.; Samddar, P.; Kumar, P. J. Nanobiotechnol. 2018, 16(1), 84. https://doi.org/10.1186/s12951-018-0408-4.
CrossRef - Filippi, A.; Mattiello, A.; Musetti, R.; Petrussa, E.; Braidot, E.; Marchiol, L. AIP Conf. Proc. 2017, 1873(1), 020004. https://doi.org/10.1063/1.4997133.
CrossRef - Dutta, A. Spectroscopic Methods for Nanomaterials Characterization. 2017, 2, 73–93. https://doi.org/10.1016/B978-0-323-46140-5.00004-2.
CrossRef - Mirghiasi, Z.; Bakhtiari, F.; Darezereshki, E.; Esmaeilzadeh, E. J. Ind. Eng. Chem. 2014, 20(1), 113–117. https://doi.org/10.1016/j.jiec.2013.04.018. CrossRef
CrossRef - Tizo, M.S.; Blanco, L.A.V.; Cagas AC, Q.; Dela Cruz BR, B.; Encoy, J.C.; Gunting, J.V.; Arazo, R.O.; Mabayo, V.I.F. Sustain. Environ. Res. 2018, 28, 326–332. https://doi.org/10.1016/j.serj.2018.09.002.
CrossRef - Pillai, L. S.; Bindu, R.; Nair. J Pharmacogn. Phytochem. 2014,2(6), 120-124.
- , A.; Monshi, A.; Salehi, R.; Fathi, M. H.; Golniya, Z.; Daniels, A. U. Ceram. Int. 2011, 37(7), 2311-2316. https://doi.org/10.1016/j.ceramint.2011.03.026.
CrossRef - , G.; We´ber, F.; Luka´cs, I.; To´th, A. L.; Horva´th, Z. E.; Miha´ly, J.; Bala´zsi, C. Ceram. Int. 2010, 36(2), 803–806. https://doi.org/10.1016/j.ceramint.2009.09.020.
CrossRef - Eram, R.; Kumari, P.; Panda, P. K.; Singh, S.; Sarkar, B.; Mallick, M. A.; Verma, S. K. J. Nanotheranostics. 2021, 2(1), 51–62. https://doi.org/10.3390/jnt2010004
CrossRef - Yoonus, J.; Resmi., R., Beena, B. Mater. Today: Proc. 2021, 41, 535–540. https://doi.org/10.1016/j.matpr.2020.05.246.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









