Anti-Microbial Study and Synthesis of Schiff Bases of 3-Actyl 4-Hydroxy Quinolin-2-One
1Department of Chemistry, Sharadchandra College, Naigaon, District- Nanded MS- 431709, India.
2Department of Chemistry, S.G.B. College, Purna Jn., MS- 431511, India, (Affilated to Swami Ramanand Teerth Marathwada University, Nanded) India.
Corresponding Author E-mail: schandole@reddifmail.com
DOI : http://dx.doi.org/10.13005/ojc/390124
Article Received on : 25 Nov 2022
Article Accepted on : 14 Feb 2023
Article Published : 10 Feb 2023
Reviewed by: Dr. Bethala Sailu
Second Review by: Dr. Vetrivel Nadaraj
Final Approval by: Dr. Vandana Magarde
Quinoline based new Schiff bases were synthesized from 3-Actyl 4-Hydroxy Quinolin-2-(1H)-one and screened their antibacterial and antifungal activity. The Schiff bases 4-hydroxy-3-(1-((4-picolin-2-yl)imino)ethyl)quinolin-2-(1H)-one(L1),4-hydroxy-3-(1-((5-picolin-2-yl)imino)ethyl)quinolin-2(1H)-one(L2),4-hydroxy-3-(1-((6-picolin-2-yl)-imino)ethyl)quinolin-2(1H)-one(L3) and 4-hydroxy-3-(1-((3-nitro-4-picolin-2-yl)imino)ethyl)quinolin-2-(1H)-one (L4) were preparedfrom 3-Acetyl 4-Hydroxy Quinolin-2-One with 2-amino picolines.
The structures of Schiff bases were confirmed by Infrared, mass, proton-NMR and 13CNMR spectral analysis. In vitro studies of these Schiff bases were carried out for their antibacterial activity by Agar contact method and antifungal activity by the poison plate method. The bacterial species used were B. subtilis, E. coli, S. typhi, and S. aureus. Fungal species used were F. moneliforme, A.niger, A. flavus, and P. chrysogenum.
3-Actyl 4-Hydroxy Quinolin-2-One; Amino Picoline; Biological study; Spectral study; Schiff Bases
Download this article as:| Copy the following to cite this article: Anjanikar S. S, Chandole S. S. Anti-Microbial Study and Synthesis of Schiff Bases of 3-Actyl 4-Hydroxy Quinolin-2-One. Orient J Chem 2023;39(1). |
| Copy the following to cite this URL: Anjanikar S. S, Chandole S. S. Anti-Microbial Study and Synthesis of Schiff Bases of 3-Actyl 4-Hydroxy Quinolin-2-One. Orient J Chem 2023;39(1). Available from: https://bit.ly/3lsEHdK |
Introduction
Quinoline is an important fused heterocyclic aromatic compound and researchers seeking their major interest in 4-hydroxyquinolin-2(1H)-one due to their synthetic and medicinal values.1,2The importance of this structural moiety is due to its presence in many naturally occurring alkaloids such as Bucharidine and foliosidine inculcating quinoline moiety having estrogenic action3 while derivatives of 4-hydroxy quinolin-2(1H)-one shows Viral-RNA polymerase Inhibitory action to prevent the replication of the Hepacivirus C.4-7In addition to this their derivatives have shown many pharmacological,8-13various medicinal values like analgesic,14anti-inflammatory,15 diuretic,16 antiallergenic,17 orally active antagonists,18 cardiovascular agents,19anticonvulsant,20 antimicrobial (antibacterial and antifungal),21-23 antitubercular,24 dye-stuffs.25
The Schiff base are important due to wide spread pharmaceutical applications such as, anti-oxidant,26anti-cancer,27 antimicrobial,28anti-inflammatory.29
Contemplating the above evidences and their increasing significance of medicinal and biological values initiated to synthesize few new Schiff bases with substituted amino picolines and 3-acetyl-4-hydroxyquinolin-2-one and study their biological activity. All these Schiff bases were subjected to antibacterial and antifungal assessment competing with standard drugs.
Experimental
Material and Methods
2-aminobenzoate and ethyl 3-oxobutanoate were procured from Sigma-Aldrich, India. The melting points were determined in open capillaries at atmospheric pressure. The products were characterized by their spectral data. 1H NMR spectra were recorded on Varian Gemini in CDCl3 at 300 MHz using TMS as an internal standard. IR spectra were recorded on a Perkin-Elmer FTIR using KBr discs. Mass spectra were recorded on Micromass Quatrro-II using electrospray Ionization technique, showing (m+1) peak as a molecular ion peak. The test for the purity of products and the progress of the reactions were accomplished by TLC on Merck silica gel plates.
Synthesis of 3-Acetyl-4-Hydroxyquinolin-2-One
3-acetyl-4-hydroxyquinolin2-one is prepared by refluxing methyl 2-aminobenzoate (48 ml) and ethyl 3-oxobutanoate (42 ml) with catalytic amount of sodium (8.7 gms) in absolute ethanol (125 ml) for six hours. After completion of reaction, mixture was poured over ice and acidify with acetic acid, white colored solid formed was filtered and washed with water.Glacial acetic acid is used for the recrystallization of product.
Synthesis of Schiff Bases of 3-Acetyl-4-Hydroxyquinolin-2-One
100 ml of pure ethyl alcohol is combined with 0.05 moles of 3-acetyl-4-hydroxyquinolin-2-one (I), 0.05 moles of aromatic amine (IIa-d), and 0.2 gms of N,N-dimethylppridine as a catalyst. Over a heating mantle, the reaction mixture in the alcohol is heated for three hours at refluxing temperature. The mixture is cooled three hours later. The solid Schiff base is vacuum filtered after being washed with ethanol. The Schiff base is dried and recrystallized from ethanol. The purity of the Schiff bases was checked by m.p. and TLC.
Spectral data for the synthesized compound is given as below
L1: 4-hydroxy-3-(1-((4-picolin-2-yl)imino)ethyl)quinolin-2(1H)-one.
Yield: 72%; Colour: Yellow; Melting Point: 234-236O C
IR (KBr, cm–1):3500 Broad ─O─H and enolic, 3402Broad and weak >N─H, 1668(>C=O) of lactam, 1614 (>C=N) of imine, 1600 (>C=N) of picoline, 1570 and 1505 and 1450 aromatic (>C=C), 1242 (enolic ─O─H) inter action, 750 (>N-H).
1HNMR (CDCl3) (300 MHz): 2.23 (S, 3H, imine ─CH3), 2.40 (S, 3H, ─CH3 of substituted at Py- moiety), 7.23 S (1H) of Py- moiety, 8.23 (d, 2H of Py- moiety ), 7.23(d, 2H of Py- moiety ), 8.09-7.28 (Ar-H of Quinoline moiety), 16.75 (bs, S, 1H, O-H), 10.50 (bs, S,1H, N-H).
13CNMR (CDCl3) (300 MHz): 21 and 23 (imine─CH3 carbon and ─CH3 of substituted at picoline ring), 81for C3, 114-140 for aromatic carbons, 161 for C4, 162 for lactam carbon, and 175 for imine carbon.
Mass Spectra[M+1]+ : 294.27
L2: 4-hydroxy-3-(1-((5-picolin-2-yl)imino)ethyl)quinolin-2(1H)-one.
Yield: 75%; Colour: Yellow; Melting Point:220-222OC.
IR (KBr, cm-1):3506Broad ─OH and enolic, 3292Broad and weak >N─H, 1658(>C=O) of lactam, 1611 (>C=N) of imine, 1599 (>C=N) of picoline, 1566 and 1507 and 1445 aromatic (>C=C<), 1252 (enolic ─O─H) inter action, 773 (>N-H).
1HNMR (CDCl3) (300 MHz):2.23 (S, 3H, imine ─CH3), 2.49 (S, 3H, ─CH3 of substituted at Py- moiety), 8.36 S (1H) of Py- moiety, two doublets at 8.25-8.24 & 6.96-6.98 of 3C & 4C hydrogens of Py-moiety) 8.06-7.23 (Ar─H of quinolone moiety), 16.75 (bs, S, 1H, O-H), 10.50 (bs, S,1H, N-H).
13CNMR (CDCl3) (300 MHz):19 and 20 (imine─CH3 carbon and ─CH3 of substituted at picoline ring), 84for C3, 116-140for aromatic carbons, 160 for C4, 163 for lactam carbon, and 175 for imine carbon.
Mass Spectra[M+1]+: 294.27
L3: 4-hydroxy-3-(1-((6-picolin-2-yl)imino)ethyl)quinolin-2(1H)-one.
Yield: 74%; Colour: yellow; Melting Point:217-219OC.
IR (KBr, cm-1):3498 Broad -OH and enolic. 3402Broad and weak >N─H, 1668(>C=O) of lactam, 1607 (>C=N) of imine, 1600 (>C=N) of picoline, 1560 and and 1470 aromatic (>C=C>), 1236 (enolic ─O─H) inter action, 748 (>N─H).
1HNMR (CDCl3) (300 MHz): 2.23 (S, 3H, imine ─CH3), 2.23 (S, 3H, ─CH3of substituted at Py- moiety), 7.40-7.32 (m, 3H of Py- moiety), 8.06-7.23 (Ar-H of quinolone moiety), 16.75 (bs, S, 1H, O─H), 10.50 (bs, S,1H, N─H).
13CNMR (CDCl3) (300 MHz):21 and 23 (imine ─CH3 carbon and ─CH3of substituted at picoline ring), 84for C3, 139-114 for aromatic carbons, 162for C4, 165 for lactam carbon, and 176 for imine carbon.
Mass Spectra[M+1]+ :294.27
L4: 4-hydroxy-3-(1-((3-nitropicolin-2-yl)imino)ethyl)quinolin-2(1H)-one.
Yield: 70%; Colour: Green; Melting Point:239-2410C.
IR (KBr, cm-1):3507Broad ─O─H and enolic, 3349Broad and weak >N─H, 1663(>C=O) of lactam, 1617 (>C=N) of imine, 1598 (>C=N) of picoline, 1504 and 1450 1422 aromatic (>C=C<), 1269 (enolic ─O─H) inter action, 747 (>N-H).
1HNMR (CDCl3) (300 MHz): 2.38 (S, 3H, imine (─CH3), 2.65 (S, 3H, -CH3 of substituted at Py- moiety), two doublets at 8.23 and 7.08 of 5C & 6C hydrogens of Py-moiety),8.09-7.23 (m, Ar─H of quinolone moiety), 16.74 (bs, S, 1H, O─H), 10.64 (bs, S,1H, N─H).
13CNMR (CDCl3) (300 MHz):20 (─CH3 substituted at picoline moiety), 19 (imine ─CH3 carbon), 78 for C3, 114-156 for aromatic carbons, 161 for C4, 163 for lactam carbon, and 175 for imine carbon.
Mass Spectra[M+1]+: 339.82
Biological Activity
Antibacterial Activity
Anti-bacterial activity was performed by agar contact method.30B. subtilis and S. typhi were gram +ve bacteria that were utilized as test organisms, whereas S. aureus and E. coli were gram -ve microorganisms. Mueller Hinton Agar for bacteria was used for all tests for antibacterial activity. Ampicillin was used as positive control for bacteria. The solvent and positive control used was DMSO. Antibiotics and dehydrated media powder were brought from Hi-Media, India. Using sterile wire-loop, test organisms were aseptically added to sterile MH broth before being incubated at 37°C for 18 hours. This suspension was utilized as an inoculant. Wells in the media plates with a 10mm diameter were made using a sterile cork borer for the addition of compound solutions and controls. With the aid of a micropipette, 100 µl of the compound solution was aseptically poured to the wells to reach a ultimate strength of 10 g of compound in each well. As controls, the same quantity of DMSO and ampicillin solution were introduced. The plates were cooled for 30 minutes to allow solutions to diffuse through the agar substrate. Further, Plates were incubated at 37°C for a period of 24 hours. The zone margin should be regarded as the region that does not clearly display any expansion that the unaided eye can see. With a measuring scale in millimetres, the clean zone was measured
Antifungal Activity
The poison plate approach was used to provide antifungal activity. 31 For the evaluation of antifungal activity, Potato Dextrose Agar (PDA) media was utilized as a culture. The sterilization of the medium was archived by autoclaving at 120-125°C for 25-30 minutes under 15 psi of pressure. 20 mL of sterilized, melted PDA was added to sterilized petri plates with 2 ml of each component, and the mixture was then gently stirred in a circular motion to get homogenized. With positive Neomycin and negative DMSO controls, the identical process was followed. A. niger, A. flavus, F. moneliforme, and P. chrysogenum were chosen to assess the antifungal activities.The fungal spores from the slant culture were transferred to a test tube containing sterile saline and thoroughly mixed with a sterile wire loop. As an inoculant, this spore solution was employed. The plates were kept for incubation for 100 hrs at room temperature. Further, the growth of the infected fungi was monitored on the plates. The outcomes were noted.
Result and Discussion
All reactions were conducted using standard procedures. In the presence of sodium ethoxide, methyl anthranilate and ethyl 3-oxobutanoate were refluxed to produce the intermediate 3-acetyl-4-hydroxy-quinolin-2(1H)-one (I) needed for the synthesis of Schiff bases. The purity of the intermediate product (I) was assessed by TLC after it was recrystallized in ethanol. Various substituted 4-hydroxy-3-(1-(heteroarylimino)ethyl)quinolin-2-one(L1-L4) were prepared carrying out reaction in ethanol for 4 hours.
In the analytical results as detailed above, the significance of the peaks identified in the IR, 1HNMR, and 13CNMR spectra of the compounds (L1-L4) is clarified. The compound (L1-L4) IR spectra have shown a prominent band at 3507-3498 cm-1and is given to the ν(-OH) vibration, confirming the presence of enolic -OH group present in Schiff bases.32 1614-1607 cm-1 is predicted for the (C=N) vibration, confirming the formation of Schiff bases.33 The two bands at 1570-1504 cm-1 and 1470- 1422 cm-1 are designated to the aromatic ring. Strong band between 1668 and 1658 cm-1is assigned for lactam carbonyl.
Each of the (L1-L4) 1H NMR spectra showed a singlet(3H) in the range 2.23-2.38 ppm that was attributed to a methyl hydrogen bonded to imine group. A singlet (3H) in the region 2.22-2.65 ppm is given to picoline’s methyl substituent. The peaks observed in the region 8.2 and 7.0 ppm were ascribed for aromatic Hydrogen atoms. The existence of the 4-hydroxyl group is confirmed by a wide singlet at 15.63–15.92 ppm. The peak observed between 10.50 – 10.64 ppm reveals the presence of secondary amino group.34Lactam carbon revealed peaks in the range of 165–161 ppm, while imine carbon showed peaks in the range of 176–175 ppm. The explanation provided for other peaks found in 1HNMR, 13CNMR, and mass spectra, as well as molecular ion peaks, supports the structures of compounds (L1-L4).
The synthesized Schiff’s bases were investigated for anti-bacterial with Bacillus subtilis and Salmonella typhi(gram positive bacteria) while Staphylococcus aureus and Escherichia coli(gram negative bacteria). The results are reported in Table 1. All compounds have displayed good antibacterial activity with all bacterial species in the range of 10-14 mm diameter of zone of inhibition but lesser, except L4 which shown maximum zone of inhibition within range of 16-18 mm of diameter than reference used. The enhanced activity observed in L4 might be due to presence of nitro group in the moiety. The screening test for antifungal activity against F. moneliforme,A. niger, A. flavus, and P. chrysogenum. fungi revealed that (L1-L4) exhibit significant activity, especially L4 have shown minimum growth of all fungi.
Reaction Scheme
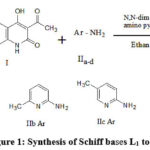 |
Figure 1: Synthesis of Schiff bases L1 to L4 |
Conclusion
New Schiff bases were synthesized by condensation of 3-acetyl-4-hydroxy-quinolin-2(1H)-one with substituted amino picoline. All the compounds were characterized by spectral study which favors the structure of targeted molecules. These synthesized Schiff’s bases were further subjected to their antimicrobial activity and reveled that they possess good antibacterial and antifungal activity.
Table 1: Anti- Bacterial study of Synthesized Schiff bases L1 to L4
|
Synthesized Schiff base
|
Zone of Inhibition(diameter measured in mm) Gram Positive Gram Negative |
|||
|
S. typhi |
B. subtilis |
E. coli |
S.aureus |
|
|
Ampicillin (Reference) |
19 |
16 |
18 |
17 |
|
L1 |
13 |
11 |
12 |
11 |
|
L2 |
14 |
12 |
13 |
14 |
|
L3 |
13 |
10 |
14 |
10 |
|
L4 |
17 |
16 |
18 |
18 |
 |
Figure 2: Graphical Representation of Antibacterial Study of Schiff Bases L1 to L4. |
Table 2: Anti- fungal study of Synthesized Schiff bases L1 to L4
|
Synthesized Schiff bases |
Growth of Fungi |
|||
|
A. niger |
A. flavus |
P. moniliforme |
P.m chrysogenum |
|
|
Neomycin(Reference) |
– |
– |
– |
– |
|
L1 |
+++ |
++ |
++ |
++ |
|
L2 |
++ |
++ |
++ |
++ |
|
L3 |
+++ |
+++ |
++ |
+++ |
|
L4 |
– |
– |
+ |
– |
Moderate growth (++), Reduced growth (+) and No growth (-) of fungi
Acknowledgements
The authors express their thanks to Principal, N.S.B. College, Nanded, Maharashtra for providing laboratory facility.
Conflict of Interest
There is no conflict of interest
References
- Shang X.F., Morris-Natschke S.L.,Liu Y.Q., Guo X., Xu X.S.,Goto M., Li J.C., Yang G.Z., and Lee K.H., Medicinal Research Reviews, 2018, (38)3, 775–828.
CrossRef - Shang X.F., Morris-Natschke S.L., Susan L.L.; Yang G.Z., Liu Y.Q., Guo X., Xu X.S., Goto M., Masuo L.; Li J.C., Zhang J.Y., and Lee.K.H., Medicinal Research Reviews. 2018, (38)5, 1614–1660.
CrossRef - Nazrullaev S.S., Bessonova I.A., Akhmedkhodzhaeva K.S., Chem. Nat. Compd., 2001, (37)6, 551-555.
CrossRef - Barreca M.L., Manfroni G., Leyssen P., Winquist J., Kaushik-Basu N., Paeshuyse J., Krishnan R., Iraci N., Sabatini S., Tabarrini O., Basu A., Danielson U.H.,Neyts J., Cecchetti V., J. Med. Chem., 2013, (56)6, 2270-2282.
CrossRef - Vicente J. de, Hendricks R.T., Smith D.B., Fell J.B., Fischer J., Spencer S.R., P.J., Stengel P. Mohr, RobinsonJ.E., Blake J.F., Hilgenkamp R.K., Yee C., Adjabeng G., Elworthy T.R., Tracy J., Chin E., Li J., Wang B., Bamberg J.T., Stephenson R., Oshiro C., Harris S.F., Ghate M., Leveque V.,Najera I., Le Pogam S., Rajyaguru S., Ao-Ieong G., Alexandrova L., Larrabee S., Brandl M., Briggs A., Sukhtankar S., Farrell R., Xu B.., Bioorg. Med. Chem. Lett., 2009, (19)13, 3642-3646,.
CrossRef - Hendricks R.T., Fell J.B.,Blake J.F., Fischer J.P.,Robinson J.E., Spencer S.R., Stengel P.J., Bernacki A.L., Leveque V.J.P., Le Pogam S., Rajyaguru S., Najera I., Josey J.A., Harris J.R., Swallow S., Bioorg. Med. Chem. Lett., 2009, (19)13, 3637-3641.
CrossRef - Tedesco R.,Chai D., Darcy M.G., Dhanak D., Fitch D.M.,Gates A., Johnston V.K., Keenan R.M., Lin-Goerke J., Sarisky R.T., Shaw A.N., Valko K.L., Wiggall K.J., Zimmerman M.N., and Duffy K.J., Bioorg. Med. Chem. Lett., 2009, (19)15, 4354-4358.
CrossRef - Hayashi, H., Miwa I.,Ichikawa S., Yoda, N., Miki I., Ishii A., Kono M., Yasuzawa T., Suzuki F. J. Med. Chem.,1993,36, 617–626.
CrossRef - Kulagowski J. J., Baker R., Curtis N. R., Leeson P. D., Mawer I. M., Moseley A. M., Ridgill M. P., Rowely M., Stansfield I., Foster A. C., Grimwood S., Hill,R. G., Kemp J. A., Marshall G. R., Saywell K. L., Tricklebank M. D. J. Med. Chem.,1994,37, 1402–1405.
- Chapman A. G., Duermueller N., Harrison, B. L., Baron B. M., Parvez N., Meldrum B. S. Eur. J. Pharmacol., 1995, (274)1-3, 83–88.
CrossRef - Kreimeyer A., Laube B., Sturgess M., Goeldner M., Foucaud B. J. Med. Chem., 1999, 42, 4394–4404.
CrossRef - Bessonova I.A., Chem. Nat. Comp., 2000, (36), 323.
CrossRef - Lager E., Andersson P., Nilsson J.,Pettersson I., Nielsen E.O., Nielson M., Sterner O., Liljefors T., J. Med. Chem., 2006, 49, 2526.
CrossRef - Ukrainets I. V., Gorokhova O.V., Taran S.G., Bezuglyi P.A., Turvov A. V., Marusenko N. A., Evtifeeva O. A., Khim. Geterotskl. Soedin., 1994, 7, 958.
- Ukrainets I. V., Bereznyakova N. L., Mospanova E. V., Chem. Heterocycl.Compd., 2007, 43, 856-862.
- Collin X., Robert J. M., Duflos M., Wielgosz G.,Le Baut G., Dubigeon C.R., Grimaud N., Lang F., Petit J. Y., J. of Pharm.and Pharmacology, 2001,(53)3, 417-423.
CrossRef - Ysoshizami S., Takai M., Abe M.,Fujisawa N., Jpn.Kokai Tokkyo Koho JP., 1990, 152, 996.
- Priya N., Singh P., Raj H.G., Gupta A., Chand K., Kathuria A., Parmar V.S., Parmar S.K., Bioorg. Med. Chem.,2010,(18)11, 4085-4094.
CrossRef - Meo S.K., Petratta B.,Scevers M.H., J. Pharmacol. Exp. Ther. 1949, 95, 207.
- Rowley M., Paul D.L., Grume I.S., Angela M.M., Ian S., Sanderson I., Lesley R., Baker F., Kemp J.A., George R.M., Alan C.F., Sarah G., Mark D.T., Kay I.S., J. Med. Chem., 1993,(36)22, 3386-3396.
CrossRef - Smiley J.A., Benkovic S.J., J. Am. Chem. Soc.,1995, 117, 3877.
CrossRef - O’Loughlin E.J., Sims G.K., Traina S.J.,Biodegradation,1999, 10, 93-104.
CrossRef - Arya K., Agarwal M., Bioorg. Med. Chem. Lett.,2007, 171, 86-93.
CrossRef - Dodia N., Shah A.,Indian J. Het. Chem., 1999, 9, 139.
- Ziegler F., Kappe T.,Salvador R., Monatshefte für Chemie und verwandte Teile anderer Wissenschaften, 1963, 94, 453–459.
- Bakir T. K., Lawag J. B., Res Chem Intermed, 2020, 46, 2541–2557.
CrossRef - Uddin N., Rashid F., Ali S., Tirmizi S. A., Ahmad I., Zaib S., Zubir M., Diaconescu L., Nawaz M. T., Iqbal J., Haider A., J. Biomolecular Stru. Dynam., 2020, 38:11, 3246-3259.
CrossRef - Fonkui N. B., Ikhile M. I., Njobeh P. B., Ndinteh D. T., BMC Chemistry, 2019, 13:127, 1-12.
CrossRef - Sahooa B. M., Dindaa S. C., Kumar R., Panda J., Brahmkshatriya P. S., Letters in Drug Design & Discovery, 2014, 11, 82-89.
CrossRef - M.Abou-Dobara A. El-Sonbati M., Diab A., El-Bindary S.,Morgan M., J. Microbial Biochem. Technol., 2014, S3, 006.
- Bagihalli G. B., Avaji P. G., Patil S. A., Badami P. S., European Journal of Medicinal Chemistry,2008, 43, 2639-2649.
CrossRef - MohamedE. A., Ismail M. M., Gabr Y., AbassM.,Chem. Papers, 1994, 48(4),285-292.
- Munzeiwaa W.A., Nyamoria V.O.,Omondi B., Inorganica Chimica Acta, 2019, 487, 264-274.
CrossRef - Stadlbauer W., HojasG., J. Heterocyclic Chem., 2004, 41, 681.

This work is licensed under a Creative Commons Attribution 4.0 International License.










