Synthesis, Spectral Characterization, and Antimicrobial Activity of Two Novel Schiff Bases Derived from Thiosemicarbazide and Mononuclear 3d Transition Metal Complexes
Karuna Chourasia1* , Bhanu Pratap Prajapati2
, Bhanu Pratap Prajapati2 , Mithun Kori1
, Mithun Kori1 , Kaushal Kumar1
, Kaushal Kumar1 and Ritu Yadav1
and Ritu Yadav1
1Department of Chemistry, Dr. Harisingh Gour University Sagar-470003, India.
2Department of Microbiology, Dr. Harisingh Gour University Sagar-470003, India.
Corresponding Author E-mail: karuchem128@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/380628
Article Received on : 10 Sep 2022
Article Accepted on :
Article Published : 08 Dec 2022
Reviewed by: Dr. Debajani Basumatary
Second Review by: Dr. Abhay N. Srivastava
Final Approval by: Vandana Magarde
Schiff bases are aldehyde or ketonic-like compounds in which an imine group replaces the carbonyl group and has been synthesized by condensing primary amines with an active carbonyl compound. Synthesis of two new Schiff base (NE)-N, 2-bis (2-methoxybenzylidene) hydrazine-carbothioamide and (2E)-N, 2-bis (3methoxybenzylidene) hydrazine- carbothioamide by Thiosemicarbazide with 2 methoxy benzaldehyde and 3 methoxy benzaldehyde via a condensation reaction. Metal complexes of synthesized Schiff bases have been synthesized via these Schiff base ligands with different metal ions. (Cu2+, Ni2+, Fe2+, Co2+etc) These compounds have been synthesized by the reflux method and characterized by a Spectroscopic Technique i.e., FT-IR, UV, 1H-NMR, and Mass (HRMS). The structure of the ligands was clarified by spectral studies which indicate the presence of two or four coordinating groups in ligands. Antimicrobial strain Bacillus subtilis fungi Aspergillus Niger is used for testing the antimicrobial activity. Results also indicate that the metal complexes are expected to be more biologically active as compared to the precursor. The structure of the ligands was clarified by the above spectral studies, which indicates the presence of two or four coordinating groups in ligands.
KEYWORDS:Antimicrobial activity; Benzaldehyde; Schiff base; Spectroscopic technique; Thiosemicarbazide
Download this article as:| Copy the following to cite this article: Chourasia K, Prajapati B. P, Kori M, Kumar K, Yadav R. Synthesis, Spectral Characterization, and Antimicrobial Activity of Two Novel Schiff Bases Derived from Thiosemicarbazide and Mononuclear 3d Transition Metal Complexes. Orient J Chem 2022;38(6). |
| Copy the following to cite this URL: Chourasia K, Prajapati B. P, Kori M, Kumar K, Yadav R. Synthesis, Spectral Characterization, and Antimicrobial Activity of Two Novel Schiff Bases Derived from Thiosemicarbazide and Mononuclear 3d Transition Metal Complexes. Orient J Chem 2022;38(6). Available from: https://bit.ly/3h9rODQ |
Introduction
Treatment modalities and strategies that utilize the latest antifungal medications are imperative [1]. Over a billion people are affected by fungi worldwide, killing more than 1.5 million. Since metallo- drugs can target multiple pathways to curb drug resistance,[2]. they seem worthy of consideration among the various methods being pursued [3]. Sometimes, ligands protect or stabilize the metal ion or the metal by undergoing redox events which enhances the overall efficacy of the complex, and sometimes the metal and the ligand(s) are mutually responsible for its biological activity [4]. Schiff bases are compounds containing the azomethine group (-HC=N-). They are condensation products of ketones or aldehydes with primary amines and were first reported by Hugo Schiff in 1864[5]. Formation of Schiff base generally takes place under acid or base catalysis or with heat. The common Schiff bases are crystalline solids, which are feebly basic but at least some form insoluble salts with strong acids. Schiff bases derived from Thiosemicarbazide were reported to possess antibacterial [6], antifungal [7], antiviral, antiprotozoal [8], and anthelmintic activities. They also exhibit significant anticonvulsant activity [9], apart from other pharmacological properties, and are used for the preparation of complex compounds [10]. Thiosemicarbazides are frequently used as intermediate compounds in the synthesis of bioactive heterocyclic compounds during several biological and clinical practices [11-12]. The importance of carbazides, carbazones, thiosemicarbazide, and their transition metal complexes has grown in a short period of time because of the discovery of their effects on flu, protozoa, variola (smallpox), certain types of tumors, and fungi [13-14] and their corresponding derivatives thiosemicarbazide are related to several essential antimicrobial activities [15]. Fungicidal, anthelmintic, anti-bacterial, activity [16]. Schiff bases derived from simple thiosemicarbazide and its derivatives are found to be very effective in the field of pharmacology and other imp medicinal fields [17]. Now a day fungal and bacterial infection is very common in every area of human and animal society, by using this simple base complexation makes it possible to overcome diseases induced to measure the biological activities of metal complexes [18], with different transition metals like Co (II), Ni (II), Cu(II), Zn (II), Pb(II), Cd (II), and, Ag (I). This type of tactic would conceivably give compounds [19]. With superior biological activity in biochemical research [20]. In prepared Schiff base, one sulfur-containing moiety, the base makes more effective towards microorganisms basically for bacterial and fungal infections [21-22]. We have synthesized a novel series of Schiff base results from thiosemicarbazide [23]. More specifically, in order to better understand the ligand-metal complexation, antimicrobial study studies [24], were used to assess the new molecules the findings demonstrated the important biological potential of these compounds.
Experimental
Materials and methods
All the reagents and chemicals used for the synthesis were of analytical grade and were purchased from commercial sources. They were used as received without further purification both aldehydes and thiosemicarbazide were purchased from Fluka, Sigma Aldrich, and metal nitrates were purchased from Merck and Spectrochem. Microanalyses (C, and N) were carried out with melting point apparatus used to determine the melting point of the synthesized compounds. The FT-IR IR spectra of the compounds were recorded using a BRUKER FT-IR Spectrophotometer, and all spectra of synthesized compounds were recorded as KBr pellets. The UV/vis spectra of synthesized Schiff bases and their metal complexes were detectedin Thermos Scientific (Multiskan Go) Spectrophotometer by using DMSO solvent under 200-800 nm wavelength. 1HNMR spectra were recorded on a Bruker (400 MHz) spectrometer. Chemical shifts were reported as δ in ppm relative to tetramethyl silane (TMS) (δ 0.00 singlet) in deuterated dimethyl sulfoxide (DMSOd6) for compounds and deuterated chloroform (CDCl3) for compound 1g. HRMS spectra were recorded. Solvents are used in analytical grades. For the preparation of metal complexes of corresponding Schiff bases, the nitrate salts of metals were used for the synthesis of metal complexes. By using silica-coated aluminum plates the progress of the reaction was monitored in the UV chamber. By using an open capillary, the melting point of all the compounds was resolute. Mass spectra were learned on Bruker Compass Data Analysis 4.0.
General synthetic procedure for ligands
By condensing 2Methoxybenzaldehyde and 3Methoxybenzaldehyde (2.0mmol) in ethanol (15 ml) with Thiosemicarbazide (1mmol) in ethanol (15ml) were dissolved separately and aldehyde was added to the amines under stirring. The resulting mixture was stirred for 30 minutes at room temperature. Additionally, for enhancing the rate of the reaction 2-3 drops of dilute acetic acid were added to the reaction mixture followed by refluxing at 70-800C for 5-8 hr, and the completion of the reaction was observed by TLC, using hexane and ethyl acetate (9:1v/v) as eluent. Under scheme-1 after completion of the reaction, the reaction mixture was allowed to cool at room temperature, the remaining precipitate was filtered, then wash with diethyl ether/ethanol (2:1) then put under a vacuum. For the synthesis of metal complexes of the above Schiff bases, the condensation refluxing method was acquired in which 0.01 M of metal (nitrate) ofCo (II), Ni (II), Fe (II), Cu (II), Ag (I), with 0.02 M of synthesized Schiff base in methanol is allowed to react in magnetic stirrer for 2-4 h. 1 Equiv. For making a slight basic medium a catalytic amount of KOH [1-2 drops] was added. Precipitation of complexes seemed which be filtered on, and washed with ethanol and petroleum ether.
Synthesis of KL-1: (NE)-N,2-bis(2-methoxybenzylidene) hydrazine- carbothioamide
MP;168 Yield (0. 024g) 76%; White powder, Chemical Formula C17H17N3O2S (MW, 327.40).; Elemental analyses: Calculated; C, 62.36; H, 5.32; N, 12.83;O,9.77; S,9.79 %; Observed; C, 63; H, 5.22; N, 12.78; S, 9.72; IR (selected vibrations; cm-1); FT-IR Spectroscopy(cm-1): 3402.21, 3283.77 , them obtained stretching frequency at 1684.38 which is basically responsible for the confirmation of Schiff base (stretch, -C=N- imino), lowing of this transition because of presence of Thio and methoxy electron-withdrawing groups, The IR spectra of Schiff base ligand (KL-1) exhibits strong band at 3402.21, 3283.77 , 1684.38 due to (stretch, -C=N- imino), the C-H stretching and bending vibrations appear at 1455.34, 1281.64 (aromatic -C=C-),1007cm-1(v (N-N) group,1234.86 (C-O-C stretch(E,E)-1,3-bis(2-methoxybenzylidene)thiourea. 1H NMR (DMSO- d6, δ, ppm) The 1HNMR spectra of ligand were recorded in DMSO. The 1HNMR spectrum of KL-1 shows a signal at 8.31 δ indicating the presence of azomethine (HC=N) proton. However, the multiple in the region 7.10-7.6 δ indicates the presence of phenolic protons; 6.6 (s. 1H) 7.4-7.3 (d, 1H), the important bands were observed at ~3.9δ assigned for methyl (O-CH3); Mass Spectroscopy(ESI-MS): 302 [M+ H2O+H] + anal for C15H12N2O3S. UV/vis; 301 nm, 270 nm.
Synthesis of KL-2: (2E)-N,2-bis(3methoxybenzylidene)hydrazine- carbothioamide
MP;158 0C Yield 0.022g (84%); light yellow powder, Chemical Formula: C17H17N3O2S (MW,327.40) Elemental analyses: 62.37; H, 5.28; N, 12.87;O,9.76; S,9.79 %; Observed; C, 63; H,5.22;N, 12.78; S, 9.72; IR (selected vibrations; cm-1); FT-IR Spectroscopy(cm-1): FT-IR spectrum of ligands showed a band at a region of 1587.78 which is due to (-C=N- stretching) frequency is a key feature of Schiff base. Some strong IR peaks are 1529.31(C=C aromatic) 1453.46 (CH3 bend), 1035.06 (C-O-C-Stretch) 1261.40. As the ligand shows particular bands at a particular region, the same band is obtained for complexes also, we can say generally, via coordination bonds ligand complex suggesting that ligands have combined with the metal through coordination. the bands in the region of 640-660 cm-1and 418 cm-1, are accredited to ν(M-O) and ν(M-N) stretching vibrations respectively, compatible coordination with Schiff bases.
 |
Scheme 1: Synthesis of KL-1 Ligand and synthesis of metal complexes |
General synthetic procedure for [M (KL1)2]
By using metal (II) nitrates complexes of (NE)-N, 2-bis (2-methoxybenzylidene) hydrazinecarbothioamide have been synthesized. To a stirring methanolic solution of KL-1and KL-2(1.0 mmol, 0.287g/0.332g) and KOH (1.0 mmol, 0.056g), a methanolic solution of metal (II) nitrates (1.0 mmol) were added dropwise at room temperature, then the resulting reaction mixture was extra stirred for 30 minutes. The reaction mixture was refluxed at 50-60°C for 2-4 h and the accomplishment of reaction was observed by TLC. Cooling the reaction content resulted in a precipitate which was filtered, washed with methanol (10 mL) .and finally dried at room temperature or vacuum. [25].
(2E,NE)-N,2-bis(2-methoxybenzylidene)hydrazinecarbothioamide- nickel(II) salt nickel [Ni(KL1)2](1)
have been synthesis, Green powder, yield: 0.308g,(80%),MP-136 °C, Chemical formula; [Ni(C17H17N3O2S2+], (MW; 386.1),Exact Mass; 385.0 Elemental Analysis: C, 52.88; H, 4.44; N, 10.88; Ni, 15.20; O, 8.20; S, 8.30.32; Found; C, 52.82; H, 4.42; N, 10.12; Ni, 14.30; O;8.13; S, 8.10; IR (selected vibrations; cm-1 ); 1619.54 (C=N), 3325.09 (N-H), 1364.33 ,604.13 ,817-811.21 (C=S) .UV/vis (λ max); 250-255 nm, Mass spectrometry (ESI-MS): 624.3 [M]+ anal for [Ni(C17H17N3O2S2+].
(2E, NE)-N, 2-bis (2-methoxybenzylidene)hydrazinecarbothioamid, copper(II) salt(2) [Cu(KL-1)2]
Dark green, Yield 0.504g, (78%) MP-160°C Chemical Formula;[C17H17CuN3O2S2; ]Exact Mass: 390.03 (Molecular Weight): 390.95, Elemental Analysis: C, 52.23; H, 4.38; Cu, 16.25; N, 10.75; O, 8.18; S, 8.20; Found; C, 52.12; H, 4.27; Cu, 16.22; N, 10.69 O, 8.16 S, 8.17; IR (selected vibrations; cm-1 ); UV/vis (λmax); 287 nm, Mass spectroscopy (ESI-MS):[M]+ anal for ;[C17H17CuN3O2S2;]m/z: 195.01 (100.0%), 196.01 (49.3%), 195.52 (18.7%), 196.52 (8.5%), 197.01 (2.2%), 196.02 (2.2%), 195.51 (1.9%), 196.51 (1.7%), 197.02 (1.0%)
(2E,NE)-N,2-bis (2-methoxybenzylidene) hydrazinecarbothioamide, cobalt(II) salt;[Co(KL-1)2]
Colour; Pink Yield;(0.316g) 82%Molecular Weight: (386.3), MP-168 °C Chemical Formula:[ C17H17CoN3O2S2+] ; Exact Mass: 386.0; Elemental Analysis: Calculated; C, 52.85; H, 4.44; Co, 15.25; N, 100.88; O, 8.28; S, 8.30; Found: C, 52.84; H, 4.43; Co, 15.23; N, 10.86; O, 8.26; S, 8.37; IR (selected vibrations; in cm-1) 3283, 2310.80, 1618.13, 1450.81, 1304.86, 1032.02 m/z: 193.0 (100.0%), 193.5 (20.6%), 194.0 (6.9%), 194.5 (1.1%).
(2E, NE)-N, 2-bis (2-methoxybenzylidene) hydrazinecarbothioamide, zinc (II) salt [Zn (KL-1)2] (3)
White powder obtained, Yield: 0.456g, (71%), MP-178°C. Chemical Formula: [Zn (C17H17N3O2S)2] (MW, 392). Elemental analyses: Calculated; C, 51.98; H, 4.36; N, 10.70; O, 8.15.; S, 8.16.; Zn, 16.65. Found; C, 5-1.90; H, 4.32; N, 10.55; O, 8.13; S, 8.15; Zn, 16.40.IR (selected vibrations; cm–1); 3337, 1630, 1327, 547. 1HNMR (DMSO- d6, δ, ppm); 8.59 (2H), 8.36 (2H), 8.18 (2H), 8.06 (2H), 7.91 (2H), 7.86 (2H), 7.61 (2H), 7.535(2H), 7.28 (2H); UV/vis (λmax); 279nm, Mass spectrometry (ESI-MS): m/z: 195.5 (100.0%), 196.5 (64.3%), 197.5 (44.4%), 197.0 (21.3%), 196.0 (20.6%), 198.0 (9.2%), 197.5 (4.1%)
(2E, NE)-N, 2-bis (2-methoxybenzylidene)hydrazinecarbothioamide, silver(I) salt(4)-[Ag(KL-1)]
Have been synthesis, Black colour; Yield (0.332g) 78%; MP-158°C. Chemical Formula: C17H17AgN3O2S2 Exact Mass: 434.0 Molecular Weight: (435.3), m/z: 217.0 (100.0%), 218.0 (99.8%), 217.5 (20.6%), 218.5 (20.2%), 219.0 (6.6%), 219.5 (1.0%)Elemental Analysis: Calculated; C, 46.91; H, 3.94; Ag, 24.78; N, 9.65; O, 7.35; S, 7.37 , Found; C, 46.81; H, 3.74; Ag, 24.68; N, 9.63; O, 7.32; S, 7.375 ,IR (selected vibrations; cm1); 2922.78, 1618.76 , 2851.23, 1524.20, 1777.64, 1034.07, 2366.76, 1361.50, 795.59, 547.24.
(2E,NE)-N,2-bis(2-methoxybenzylidene)hydrazinecarbothioamide,cadmium(II)Salt(5) salt(5)[Cd(KL-1)2]
Colour; White; Yield; (0.352g) 80%; MP-163°C Chemical Formula: [C17H17CdN3O2S2+]Molecular Weight:( 439.8); Exact Mass: 441.0; Elemental Analysis: calculated ; C, 46.42; H, 3.90; Cd, 25.56; N, 9.55; O, 7.28; S, 7.29; Found; C, 46.40; H, 3.90; Cd, 25.52; N, 9.54; O, 7.26; S, 7.27,;IR (selected vibrations; 2921.46, 1359.20, 1591.42, 1040.77, 1121.23, 855.24, 545.87 m/z: 220.5 (100.0%), 219.5 (83.5%), 220.0 (55.1%), 219.0 (46.5%), 218.5 (38.0%), 221.5 (29.2%), 221.0 (21.3%), 222.0 (5.7%), 216.5 (3.8%), 217.5 (3.0%), 222.5 (1.7%)
(2E,NE)-N,2-bis(2-methoxybenzylidene)hydrazinecarbothioamide, lead(II) salt [Pb(KL1)2] (6)
Colour; Creamy white; Molecular Weight: 534.6; Yield; (0.406)76%; MP176°Chemical Formula:[C17H17N3O2PbS2+]; Exact Mass: 535.1; Elemental Analysis: Calculated; C, 38.19; H, 3.21; N, 7.86; O, 5.99; Pb, 38.76; S, 6.00; Found.;: C, 38.17; H, 3.20; N, 7.84; O, 5.97; Pb, 38.75; S, 6.00; IR (selected vibrations; in cm-1)-3284, 1617, 1450,m/z: 267.5 (100.0%), 267.0 (46.2%), 266.5 (41.3%), 268.0 (21.5%), 268.5 (6.7%), 265.5 (2.4%), 269.0 (1.0%)
 |
Figure 1: Proposed structure of KL-1 (ligand) and their metal complexes. |
Scheme-2 Synthesis of KL-2 Ligand
 |
Scheme 2: Synthesis of KL-2 Ligand and synthesis of metal complexes. |
Synthesis of (2E)-N,2-bis(3-methoxybenzylidene)hydrazine carbothioamide, nickel(II) salt (2)
Colour; Dark green (powder),; Yield; (0.03gm)78,; MP-158℃;Chemical Formula:C17H17N3NiO2S2+,Mass;385.0,; Molecular Weight: 386.1; Elemental Analysis: Calculated – C, 52.88; H, 4.44; N, 10.88; Ni, 15.20; O, 8.29; S, 8.30 Observed; C,52.83; H,4.42; N,10.85, Ni, 15.20;O, 8.25; S,8.28; IR (selected vibrations; in cm-1)-m/z: 192.5 (100.0%), 193.5 (45.5%), 193.0 (20.6%), 194.0 (10.7%), 194.5 (8.5%), 195.5 (1.8%), 195.0 (1.7%)
Synthesisof(2E)-N,2-bis (3-methoxybenzylidene) hydrazinecarbothioamide, copper (II) salt-(1)
Colour; Green, (powder) Yield 80% (0.031gm),MP-145℃, Mass:390.0, Chemical Formula: C17H17CuN3O2S2+, Elemental Analysis: Calculated- C, 52.23; H, 4.38; Cu, 16.25; N, 10.75; O, 8.18; S, 8.20 Observed-C, 52.21; H, 4.37; Cu, 16.22, N, 10.72; O, 8.16; S, 8.18. m/z: 195.0 (100.0%), 196.0 (51.5%), 195.5 (20.6%), 196.5 (10.3%), 197.0 (3.2%)
(2E)-N,2-bis(3-methoxybenzylidene)hydrazinecarbothioamide, silver(I) salt(4)
Colour; dark black powder; Yield (0.036)85%, MP-190℃ Chemical Formula: C17H17AgN3O2S+2 Molecular Weight: 435.3;Elemental Analysis:Calculated- C, 46.91; H, 3.94; Ag, 24.78; N, 9.65; O, 7.35; S, 7.37, Observed-Elemental Analysis: C, 46.90; H, 3.92; Ag, 24.75; N, 9.64; O, 7.32; S, 7.36m/z: 434.0 (100.0%), 436.0 (99.8%), 435.0 (20.6%), 437.0 (20.2%), 438.0 (6.6%), 439.0 (1.0%) 1584.05,1487.97 ,1338.45, 1258.49,, 759.86 683.24 525.01
(2E)-N,2-bis(3methoxybenzylidene)hydrazinecarbothioamide, cadmium(II)salt-(5)
Colour; white powder; Yield (0.352gm) 80%,MP- 196 ℃; Mass: 441.0 Chemical Formula: C17H17CdN3O2 S2+, Elemental Analysis: (Calculated) C, 46.42; H, 3.90; Cd, 25.56; N, 9.55; O, 7.28; S, 7.29; Observed; C, 46.42; H, 3.82; Cd, 25.53; N, 9.54; O, 7.27; S, 7.27; IR (selected vibrations; in cm-1)- m/z: 220.5 (100.0%), 219.5 (83.5%), 220.0 (55.1%), 219.0 (46.5%), 218.5 (38.0%), 221.5 (29.2%), 221.0 (21.3%), 222.0 (5.7%), 216.5 (3.8%), 217.5 (3.0%), 222.5 (1.7%)
Synthesis of (2E)-N, 2-bis (3methoxybenzylidene) hydrazine- carbothioamide, lead (II) salt-(3)
Colour; Creamy white; Yield (0.041 gm.) MP- 200℃ 78,Chemical Formula: C17H17N3O2PbS2+, Mass: 535.1; Elemental Analysis: Calculated – C, 38.19; H, 3.21; N, 7.86; O, 5.99; Pb, 38.76; S, 6.00; Observed -C, 38.18; H, 3.19; N, 7.84; O, 5.94; Pb, 38.74; S, 6.00; IR (selected vibrations; in cm-1)- 1401.82,667.19, 505.91 m/z: 267.5 (100.0%), 267.0 (46.2%), 266.5 (41.3%), 268.0 (21.5%), 268.5 (6.7%), 265.5 (2.4%), 269.0 (1.0%)
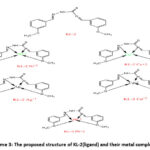 |
Scheme 3: The proposed structure of KL-2(ligand) and their metal complexes. |
Results and Discussion
Mixed ligand Complexes (Schiff bases) KL-1 and KL-2 were synthesized by reaction of 2-methoxy benzaldehyde and 3- methoxy benzaldehyde with thiosemicarbazide in a 2:1 ratio in Ethanol in the presence of the catalytic amount of dilute acetic acid (Scheme 1). Metal complexes of corresponding Schiff bases were prepared by using nitrate salt. The resulting metal complexes were neutral, colored, air-stable, and remarkably soluble in DMF and DMSO Table- 1 Summarize the Physicochemical properties of ligands and their metal complexes, and Table -2 and Table -3 summarize the antibacterial and anti-fungal activity of the ligands and corresponding metal complexes. By using the Spectroscopic technique and elemental analysis all synthesized compounds were characterized. i.e., IR, UV/Vis, 1HNMR, and mass spectrometry. FT-IR Spectroscopy (cm-1); 3402.21, 3283.77 they obtained stretching frequency at 1684.38 which is basically responsible for the conformation of Schiff base (stretching -C=N- imino), lowing of this transition because of the presence of Thio and methoxy electron-withdrawing groups.The IR spectra of Schiff base ligand (KL-1) shows strong band at 3402.21, 3283.77 , 1684.38 due to (stretch, -C=N- imino),1455.34 (CH3 bend) ,1281.64 (aromatic -C=C-), 1234.86 (C-O-C stretch)3402.21 (strong N-H stretch) ,3283.77(=CH-H stretch) 1H NMR: of KL-1 in (DMSO-d6, δ, ppm) 8.2-7.5 (m, 4H, J = 1.36 Hz), 6.6 (s. 1H) 7.4-7.3 (d, 1H), 3.68 (m, 5H), 2.54 (s, 3H), 2.48 (m, 4H) Mass Spectroscopy (ESI-MS): 302 [M+H2O+H] anal for C15H12N2O3S. UV/vis; 301 nm, 270 nm. Reaction progress was determined by using the thin-layer chromatographic technique [26]. Here we are using a conventional method which is to report an effectual practical technique (reflux) for the synthesis of a Schiff base (Scheme 1).
Table 1: Physico-chemical properties of ligand (L) and complexes
|
Comp.no. |
Colour |
Molecular formula |
Mo. Weight |
Yield % |
MP 0C |
|
KL-1 |
White powder |
[C17H17N3O2S] |
327.40 |
76% |
168 |
|
1- |
Green |
[Ni(C17H17N3O2S2+]. |
386.1 |
80 |
136 |
|
2- |
Dark green |
[CuC17H17N3O2S2;] |
390.95 |
78 |
160 |
|
3- |
Pink |
[CoC17H17N3O2S2+] |
386.3 |
82 |
169 |
|
4- |
White |
[Zn(C17H17N3O2S)] |
392 |
71 |
178 |
|
5- |
Black |
[AgC17H17N3O2S2] |
435.3 |
78 |
158 |
|
6- |
White |
[CdC17H17N3O2S2+] |
439.8 |
80 |
163 |
|
7- |
Creamy-white |
[PbC17H17N3O2S2+] |
534.6 |
76 |
176 |
|
KL-2 Light yellow |
Light yellow |
[C17H17N3O2S] |
327.40 |
84 |
158 |
Antimicrobial Activity
Antifungal Activity (in vitro)
Aspergillus Niger was obtained from the Department of Microbiology, Dr. Harisingh Gour University, Sagar, MP, and maintained on a Czapek dox medium at 28°C. Antifungal activity [27]. of all compounds was conducted on a solid Czapek dox medium using a well diffusion method against A. Niger. Spores of A. Niger was collected using a wet cotton swab followed by spreading these on the Czapek dox medium. Wells (6 mm in diameter) were formed in the solid agar plates containing Czapek dox medium using a sterilized cork borer. 100 µL test sample was poured into a well and plates were transferred to incubate at 30°C for 48 h. [28-29].
Antibacterial activity
Similarly, the antibacterial activity was assessed against Bacillus subtilis. B. Subtilis was grown and maintained on a nutrient agar medium. In vitro antibacterial activity was evaluated by the well diffusion method as described by Mukherjee et al. The overnight old bacterial culture ws centrifuged at 10000 g for 10 min. The bacterial pellet was washed by suspended in sterile distilled water (20 mL) followed by centrifugation at 10000 g then resuspended in sterilized distilled water and used for the assay. The suspension containing bacterial cells was spread on a solid agar plate and wells (6 mm in diameter) were formed in the solid nutrient agar plates using a sterilized cork borer. 100 µL test sample was poured into a well and plates were transferred to an incubator at 37°C for 24h. [30] After incubation, the zones of inhibition were recorded as the total diameter of the zone of inhibition minus the diameter of the well. All test runs were conducted in triplicate and the average value of inhibition was presented as activity.
 |
Figure 2: Anti-bacteria activity of KL-1 and KL-2 |
 |
Figure 3: Representation of antimicrobial activity of KL-1 and KL-2 and their metal complexes. |
 |
Figure 4: Anti-fungal activity of KL-1 and KL-2 |
Table 2: Antimicrobial data of [zone of inhibition] of KL-1
|
S. No. |
Dilution coding |
Zone of inhibition (in mm) |
|
|
Bacillus subtilis |
Aspergillus Niger |
||
|
1. |
KL-1 |
7 |
8 |
|
2. |
KL-1 Cu |
9 |
7 |
|
3. |
KL-1 Ni |
8 |
11 |
|
4. |
KL-1Ag |
10 |
14 |
|
5. |
KL-1 Pb |
9 |
9 |
|
6. |
KL-1 Cd |
11 |
15 |
|
7. |
Standard drug |
16 |
17 |
 |
Figure 5: Graphical representation of KL-1 and their metal complexes |
Table 3: Antimicrobial data of [Zone of inhibition] of KL-2
|
S. No. |
Dilution coding |
Zone of inhibition (in mm) |
|
|
Bacillus subtilis |
Aspergillus Niger |
||
|
1. |
KL-2 |
8 |
8 |
|
2. |
KL-2 Cu |
9 |
7 |
|
3. |
KL-2 Ni |
8 |
11 |
|
4. |
KL-2 Ag |
10 |
14 |
|
5. |
KL-2 Pb |
9 |
9 |
|
6. |
KL-2 Cd |
11 |
15 |
|
7. |
Standard drug |
16 |
17 |
 |
Figure 6: Graphical representation of KL-2 and their metal complexes |
Order of antimicrobial activity in used different metal complexes – Cd>Pb>Ag>Ni >Cu
Conclusion
An antifungal and anti-bacterial evaluation of a series of metal complexes formed from Schiff base tetradentate ligands was performed. Two novel Schiff base derivatives were synthesized from different aldehydes and thiosemicarbazide. The structures of Schiff base ligands and metal complexes were supported by different spectroscopic techniques such as FT-IR, 1H-NMR, and high-resolution mass spectrometry (HRMS). By using the conventional method [3]. We have synthesized some thiosemicarbazide-derived Schiff base and their metal complexes by the green conventional method [Reflux method]. Preparation of Schiff base by using reflux method was completed within 5-7 h, high yields and all the product obtained were moderate to moral yields, and such type of methodology condensed the time and creation of by-products. Synthesis is simple and eco-friendly and can be used as an alternative to the existing conventional heating method [31]. From the results of anti-bacterial and anti-fungal studies, [32]. It was concluded that the tested compounds exhibited significant antibacterial activities against both Bacillus subtilis fungi and Aspergillus Niger organisms. Among the tested compounds, compounds with Ag (I), Pb (II), and Cd (II) display promising antibacterial and antifungal activities [33]. Such analysis ascribed the greater electronic attractiveness which favors greater diffusion through the microbial membrane.The results of this study revealed that these complexes have a targeted activity, although, other modes of action cannot be ruled out because metal complexes are known to target multiple pathways to evade drug resistance.[34].
Acknowledgment
All The authors gratefully acknowledge the economic and financial support for Dr. Harisingh Gour [A Central University] Vishwavidyalaya Sagar, Department of Chemistry Sagar (MP) for laboratories facilities to carry out the work. We are thankful to the Head, Department of Chemistry, SIC of Dr. H. S. Gour University, for providing a laboratory and another facility, IISER Bhopal for spectral analysis [mass spectrometry]. We are also grateful to the Department of Microbiology for carrying out antimicrobial studies.
Conflict of Interest
Authors have no conflict of interest
References
- Wiederhold N., Infect. Drug Resist., 2017, 10, 249–259.
- Fisher M. C . Hawkins N. J, Sanglard D., and Gurr S. J., Science, 2018, 360, 739–742.
- 3 – Denning D. W., Philos. Trans. Soc R.., B, 2016, 371, 20150468.
- Pfaller M. A., Diekema D. J, Gibbs D. L, Newell V. A, J. F. Meis, I. M. Gould, W. Fu, A. L. Colombo and E. Rodriguez-Noriega, J. Clin. Microbiol., 2007, 45, 1735– 1745.
- O’Conner, MJ.: West BO (1967) Metal Complexes of Hydrogenated Schiff Bases. Aust J Chem 20:2077–2085. https://doi.org/10.1071/CH9672077
- Parham, S.; Kharazi AZ, Bakhsheshi-Rad HR, et al (2020) Antioxidant, antimicrobial and antiviral properties of herbal materials. Antioxidants 9:1–36. https://doi.org/10.3390/antiox9121309
- Mishra, N,.: Kumar K, Pandey .:H, et al (2020) Synthesis, characterization, optical and anti-bacterial properties of benzothiazole Schiff bases and their lanthanide (III) complexes. J Saudi Chem Soc 24:925–933. https://doi.org/10.1016/j.jscs.2020.09.009
- Josephus, RS; Nair MS (2008) Antibacterial and Antifungal Studies on Some Schiff Base Complexes of Zinc (II). Mycobiology 36:93. https://doi.org/10.4489/myco.2008.36.2.093
- Mishra, N; Gound, SS; Mondal, R; et al (2019) Synthesis, characterization and antimicrobial activities of benzothiazole-imino-benzoic acid ligands and their Co(II), Ni(II), Cu(II), Zn(II) and Cd(II) complexes. Results Chem 1:100006. https://doi.org/10.1016/j.rechem.2019.100006
- Mishra, AP; Sharma, N.; Jain RK (2013) Microwave Synthesis, Spectral, Thermal and Antimicrobial Studies of Some Ni (II) and Cu (II) Schiff Base Complexes. Open J Synth Theory Appl 02:56–62. https://doi.org/10.4236/ojsta.2013.22007
- 11-Tokalı, FS.; Taslimi, P.; Usanmaz, H.; et al (2021) Synthesis, characterization, biological activity, and molecular docking studies of novel Schiff bases derived from thiosemicarbazide: Biochemical and computational approach. J Mol Struct 1231 https://doi.org/10.1016/j.molstruc.2020.129666
- 12- Avasthi, K; Yadav.; Khan, T.; (2018) Greener and Efficient Synthesis of Some Novel substituted Azitidinones With 4- Amino Pyridine via Heterogenous catalyst
- 13-Govindula, A.;(2020) of Biomedical AND Pharmaceutical sciences. 7:174–190
- 14- Sahu, M; Manna AK, Rout K, et al (2020) Inorganica Chimica Acta A highly selective thiosemicarbazone based Schiff base chemo sensor for colorimetric detection of Cu 2 + and Ag + ions and turn-on fluorometric detection of Ag + ions. Inorganica Chim Acta 508:119633. https://doi.org/10.1016/j.ica.2020.119633
- 15-Mishra, N.; Kumar, K.; Pandey, H.; et al (2020) Synthesis, characterization, optical and anti-bacterial properties of benzothiazole Schiff bases and their lanthanide (III) complexes. J Saudi Chem Soc 24:925–933. https://doi.org/10.1016/j.jscs.2020.09.009
- 16- Patil, S.; Jadhav, SD.; Shinde, SK.; (2012) CES as an Efficient Natural Catalyst for Synthesis of Schiff Bases under Solvent-Free Conditions: An Innovative Green Approach. Org Chem Int 2012:1–5. https://doi.org/10.1155/2012/153159
- 17- Synthesis, SB.; Antioxidant I(2018) Cu (II) Complexes https://doi.org/10.3390/molecules23071581.
- 18- Raju, VV.; Balasubramanian, KP. ;(2011) Synthesis, spectral characterization, catalytic and biological studies of new Ru (II) carbonyl Schiff base complexes of active amines. 3:542–550
- 19- Jeewoth, T.; Bhowon, MG.; Li H.; Wah K (1999) Synthesis, characterization and antibacterial properties of Schiff bases and Schiff base metal complexes derived from 2, 3-diamino- pyridine. 448:445–448
- 20- Khan, T.;(2018) An Efficient Synthesis and Antibacterial Activity of Some Novel 2- Azetidinone Derivatives of 4H-1,2,4-Triazoles Under Mild Conditions. 2–7. https://doi.org/10.1002/jhet.3136
- 21-Sunil, K.; Kumara, TPP.; Kumar, BA.; Patel, SB.;(2021) Synthesis, Characterization and Antioxidant Activity of Schiff Base Compounds Obtained Using Green Chemistry Techniques. Pharm Chem J 55:46–53. https://doi.org/10.1007/s11094-021-02370-8
- 22- Manjula, SN.; Malleshappa Noolvi N, Vipan Parihar K, et al (2009) Synthesis and antitumor activity of optically active thiourea and their 2-aminobenzothiazole derivatives: A novel class of anticancer agents. European J Med Chem 44:2923–2929. https://doi.org/10.1016/j.ejmech.2008.12.002
- 23- Chetana, PR..; Somashekar, MN.; Srinatha, BS.; et al (2013) Synthesis, Crystal Structure, Antioxidant, Antimicrobial, and Mutagenic Activities and DNA Interaction Studies of Ni(II) Schiff Base 4-Methoxy-3-benzyloxybenzaldehyde Thiosemicarbazide Complexes. ISRN Inorg Chem 2013:1–11. https://doi.org/10.1155/2013/250791
- 24- Abdel-Rahman, LH.; Abu-dief, AM.; El-khatib, RM..; Abdel-Fatah, SM .;(2016) Bioorganic Chemistry Some new nano-sized Fe ( II ), Cd ( II ), and Zn ( II ) Schiff base complexes as a precursor for metal oxides : Sonochemical synthesis, characterization, DNA interaction, in vitro antimicrobial and anticancer activities. Bioorg Chem 69:140–152. https://doi.org/10.1016/j.bioorg.2016.10.009
- 25- Luo, Y.; Zhang, S.; Liu ZJ, et al (2013) Synthesis and antimicrobial evaluation of a novel class of 1,3,4-thiadiazole: Derivatives bearing 1,2,4-triazolo[1,5-a] pyrimidine moiety. Eur J Med Chem 64:54–61. https://doi.org/10.1016/j.ejmech.2013.04.014
- 26- Kosti, P.; Ahmad, G.; Ratnesh, N.; et al (2021) and Cd 2 + metal complexes of Schiff base ligand derived from 4 ‑ amino benzoic acid and isonicotinic hydrazide. J Iran Chem Soc 18:1773–1780. https://doi.org/10.1007/s13738-020-02148-x
- 27- Al-Hamdani, AAS.; Al Zoubi, W.; (2015) New metal complexes of N3 tridentate ligand: Synthesis, spectral studies, and biological activity. Spectrochim Acta – Part A Mol Biomol Spectrosc 137:75–89. https://doi.org/10.1016/j.saa.2014.07.057
- 28- AM A-D ;(2019) Azomethine Metal Chelates as an Efficient Catalyst for Oxidation of Organic Compounds. Ann Chem Sci Res 1:2–4. https://doi.org/10.31031/acsr.2019.01.000515
- 29- Kasare, MS.; Dhavan, PP.; Jadhav, BL.; Pawar SD (2019) Synthesis of Azo Schiff Base Ligands and Their Ni (II), Cu ( II ) and Zn ( II ) Metal Complexes as Highly-Active Antibacterial Agents. 10792–10797. https://doi.org/10.1002/slct.201901605
- 30- Magaldi, S.; Capriles, CH De.; Deibis L, et al (2001) “In vitro” Antifungal activity of protease inhibitors. 135–142
- 31- Luo Y, Zhang S, Liu ZJ, et al (2013) Synthesis and antimicrobial evaluation of a novel class of 1,3,4-thiadiazole: Derivatives bearing 1,2,4-triazole [1,5-pyrimidine moiety. Eur J Med Chem 64:54–61. https://doi.org/10.1016/j.ejmech.2013.04.014
- 32- Chetana PR, Somashekar MN, Srinatha BS, et al (2013) Synthesis, Crystal Structure, Antioxidant, Antimicrobial, and Mutagenic Activities and DNA Interaction Studies of Ni (II) Schiff Base 4-Methoxy-3-benzyloxybenzaldehyde Thiosemicarbazide Complexes. ISRN Inorg Chem 2013:1–11. https://doi.org/10.1155/2013/250791
- 33- K, SDR.; Sudhakumari. ;(2020) Synthesis, Characterization and Antimicrobial studies of Schiff base Ligand from the amino acid L-arginine and its Cu ( II ), Ni ( II ), Co ( II ) complexes. 13:1–8
- 34-Parikh, KS.; Vyas, SP.; (2012) Synthesis and spectral studies of some novel Schiff base derived with pyrimidines. Der Pharm Lett 4:638–640

This work is licensed under a Creative Commons Attribution 4.0 International License.









