Sonochemical Synthesis, Characterization and Molecular Docking of Thiazole and Triazole Tethered Tetra-Substituted Imidazoles.
Himanshu Pandey* , Kaushal Kumar
, Kaushal Kumar , Neha Mishra
, Neha Mishra , Ritu Yadav
, Ritu Yadav and S. P. Shrivastava
and S. P. Shrivastava
Department of Chemistry, Dr. Harisingh Gour Vishwavidyalaya(Central) Sagar-470003, India.
Corresponding Author E-mail: himpan5040@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/380616
Article Received on : 21 Jun 2022
Article Accepted on :
Article Published : 15 Nov 2022
Reviewed by: Dr. Ammar A.Razzak Mahmood
Second Review by: Dr. Shameran Jamal Salih Salihy
Final Approval by: Prof. Dr. Nenad Ignjatovic
Sonochemical synthesis of tetra-substituted imidazole derivatives tethered with thiazole and triazole moieties carried out by condensation reaction, involving aldehyde, benzil, ammonium acetate and selected amino azole moiety with brick clay as catalyst. The synthesized tetra-substituted imidazole derivatives were characterized using FT-IR, NMR spectroscopy. Molecular docking studies of the synthesized tetra-substituted imidazole derivatives for their antimicrobial potency were also performed. These derivatives scored satisfactorily and can be the possible lead for the future drug candidate against microbial pathogens
KEYWORDS:Benzothiazole; Green synthesis; Imidazole; Molecular docking; Thiazole; Ultrasonic irradiation
Download this article as:| Copy the following to cite this article: Pandey H, Kumar K, Mishra N, Yadav R, Shrivastava S. P. Sonochemical Synthesis, Characterization and Molecular Docking of Thiazole and Triazole Tethered Tetra-Substituted Imidazoles. Orient J Chem 2022;38(6). |
| Copy the following to cite this URL: Pandey H, Kumar K, Mishra N, Yadav R, Shrivastava S. P. Sonochemical Synthesis, Characterization and Molecular Docking of Thiazole and Triazole Tethered Tetra-Substituted Imidazoles. Orient J Chem 2022;38(6). Available from: https://bit.ly/3tOlhB1 |
Introduction
The prime quest for synthetic organic chemist is to develop such protocol which favors both economy and environment. In the field of synthetic organic chemistry, green protocols are crucial for saving the depleting environment. Greenness of reaction conditions is a comparative phenomenon and always assessed by comparative analysis of protocols with the reported ones. Endeavors of organic chemist continuously guided towards making the synthesis protocols more and more environmentally benign.
Imidazoles have shown their relevance in the structure of molecules that are active in biological systems and exhibited some prominent biological activities.1-3 They act as inhibitors of B-Raf kinase4, p38 MAP kinase5, and plant growth regulators.6 Some imidazole derivatives are also used as glucagon receptors7, antibacterial agents8 and antitumor agents.9 The versatile character of imidazole moiety highlighted the need for such protocols which are efficient and environmentally benign. The prime objective in front of the synthetic organic chemist is to simplify the workup by following the green protocols.
In our previous work we have synthesized terta-substituted imidazole derivatives by conventional method using red brick clay as catalyst.10 In recent decades, ultrasound irradiated organic synthesis protocol has attracted tremendous attention due to high efficiency and enhanced greenness associated with it. It is an important and greener protocol, which results in the rapid organic synthesis with maximum yield of the desired product and minimum byproduct.11 Ultrasonic-assisted organic synthesis is extremely efficient and powerful technique frequently used to accelerate organic reactions.12-14 The prominent features of this protocol are formation of desired products in high yields, enhanced reaction rates and energy efficiency as compared with conventional method proving it as a greener alternative.15-16 However, the application of ultrasound in the synthesis of heterocyclic structure is not fully explored.17-19
Ultrasound irradiation proved itself as an alternative energy source for synthetic organic synthesis, which proceeds by the generation of acoustic bubbles, their subsequent growth and sudden burst of gaseous micro-bubbles in the liquid reaction mixture.20Ultrasound irradiation assisted organic synthesis employs power ultrasound with frequency ranging from 20 kHz to 2 MHz capable of initiating chemical, thermal and physical effects.21The application of ultrasound initiate the cavitation phenomenon which results intense localized heat, high pressure, acoustic microcurrent and diverging shock waves.22-23
Material and Methods
All the chemical reagents utilized in our research were purchased through commercial sources- Sigma Aldrich and Merck. TLC Silica gel aluminum plates were used to observe the reaction progress. 1H NMR and 13C NMR analysis performed with JEOL ECX500 while Bruker FT-IR instrument used for FT-IR analysis.
Synthesis of tetra-substituted imidazole derivatives tethered with thiazol / triazol / benzothiazole mioeties.
General procedure
All the reactants namely the selected aldehyde, ammonium acetate, aromatic amine and benzil were mixed in equimolar amount and brick clay catalyst (45 mg) in ethanol (10 ml) in a 100 ml flask. This mixture was then irradiated with 50 kHz ultrasound waves at 70°C for the 25 min. TLC was used to monitor the reaction accomplishment, the synthesized product was then allowed to attain room temperature followed by evaporation of solvent and further recrystallization from acetone–water mixture resulting in tetra-substituted imidazole derivatives.
 |
Scheme 1 Click here to View figure |
(HP7) 2-(2-(3,4-dimethoxyphenyl)-4,5-diphenyl-4,5-dihydro-1H-imidazol-1-yl)thiazole yield 95% ; IR(υ max, cm−1) : 3026(=C–H), 1659(C=C), 1576(C=N); 1H NMR (400 MHz, DMSO-d6) δ 7.6-7.8(m, 10H, Ar-H), 7.02-7.05 (d, 3H, Ar-H), 3.9 (s, 6H, O-CH3); 13C NMR (100 MHz, DMSO-d6) δ 191, 187, 176, 171, 156, 149, 142, 138, 137, 136, 135, 132,131, 130, 125, 123, 115, 112, 95, 74, 58, 56, 48. MP 198-201 °C.
(HP8) 4-(4,5-diphenyl-1-(thiazol-2-yl)-4,5-dihydro-1H-imidazol-2-yl)-N,Ndimethylaniline yield 93% ; IR(υ max, cm−1) : 3020(=C–H), 1667(C=C), 1583(C=N); 1H NMR(400 MHz, DMSO-d6) δ 7.4-7.8(m, 10H, Ar-H), 7.03-7.05 (d, 3H, Ar-H),; 13C NMR (100 MHz, DMSO-d6) δ 190, 188, 173, 171, 156, 149, 142, 136, 135, 132, 131, 130, 127, 125, 123, 115, 112, 109, 98, 95, 58, 56, 42. MP 192-195 °C
(HP9) 4-bromo-2-(4,5-diphenyl-1-(thiazol-2-yl)-4,5-dihydro-1H-imidazol-2-yl)phenol yield 91% ; IR(υ max, cm−1) : 3024 (=C–H), 1666 (C=C), 1564(C=N); 1H NMR(400 MHz, DMSO-d6) δ 7.6-7.9(m, 10H, Ar-H), 7.02-7.05 (d, 3H, Ar-H),; 13C NMR(100 MHz, DMSO-d6) δ 198, 191, 172, 171, 156, 149, 142, 132, 131, 130, 129, 128, 127, 125, 123, 115, 112, 109, 98, 95, 58, 56, 47. MP 186-189 °C
(HP10) 2-(2-(3,4-dimethoxyphenyl)-4,5-diphenyl-4,5-dihydro-1H-imidazol-1-yl)-6-methylbenzothiazole yield 89% ; IR(υ max, cm−1) : 3032 (=C–H), 1676 (C=C), 1585(C=N); 1H NMR(400 MHz, DMSO-d6) δ 8.02-8.05(d, 3H, benzothiazole), 7.4-7.8(m, 10H, Ar-H), 7.02-7.04 (d, 3H, Ar-H), 3.7 (s, 6H, O-CH3); 13C NMR(100 MHz, DMSO-d6) δ 172, 171, 156, 149, 142, 138, 137, 136, 135, 132, 131, 130, 129, 128, 127, 125, 123, 115, 112, 109, 98, 95, 57. MP 197-200 °C
(HP11) N,N-dimethyl-4-(1-(6-methylbenzo[d]thiazol-2-yl)-4,5-diphenyl-4,5-dihydro-1H-imidazol-2-yl)aniline yield 88% ; IR(υ max, cm−1) : 3020 (=C–H), 1645 (C=C), 1545(C=N); 1H NMR(400 MHz, DMSO-d6) δ 8.01-8.50(d, 3H, Ar-H), 6.71-7.6 (m, 4H, Ar-H), 7.27-7.42 (d, 10H, Ar-H), 3.06 (s, 6H, Ar-H); 13C NMR(100 MHz, DMSO-d6) δ 171, 159, 156, 153, 144, 141, 138, 135, 133, 131.8, 130, 126, 125, 123, 122, 121, 120, 119, 117, 112, 111, 110, 49. MP 210-213 °C
(HP12) 2-(1-(benzo[d]thiazol-2-yl)-4,5-diphenyl-4,5-dihydro-1H-imidazol-2-yl)-4-bromophenol yield 86% ; IR(υ max, cm−1) : 3035 (=C–H), 1684 (C=C), 1577(C=N); 1H NMR(400 MHz, DMSO-d6) δ 8.03-8.07(d, 3H, benzothiazole), 7.4-7.9(m, 10H, Ar-H), 7.03-7.06 (d, 3H, Ar-H),; 13C NMR(100 MHz, DMSO-d6) δ 197, 191, 172, 171, 156, 149, 142, 138, 137, 136, 135, 132, 131, 130, 129, 128, 127, 125, 123, 115, 112, 109, 98, 95, 56. MP 192-195 °C
(HP13) 4-(2-(3,4-dimethoxyphenyl)-4,5-diphenyl-4,5-dihydro-1H-imidazol-1-yl)-4H-1,2,4-triazole yield 91% ; IR(υ max, cm−1) : 3027 (=C–H), 1694 (C=C), 1563(C=N); 1H NMR(400 MHz, DMSO-d6) δ 7.6-7.8(m, 10H, Ar-H), 7.02-7.05 (d, 3H, Ar-H), 3.6 (s, 6H, O-CH3); 13C NMR(100 MHz, DMSO-d6) δ 198, 171, 156, 149, 142, 138, 137, 136, 135, 132, 131, 130, 129, 128, 127, 125, 123, 115, 112, 109, 98, 95, 58, 56, 48. MP 181-184 °C
(HP14) 4-(4,5-diphenyl-1-(4H-1,2,4-triazol-4-yl)-4,5-dihydro-1H-imidazol-2- yl)-N,N-dimethylaniline yield 87% ; IR(υ max, cm−1) : 3030 (=C–H), 1674 (C=C), 1583(C=N); 1H NMR(400 MHz, DMSO-d6) δ 8.02-8.05(d, 3H, benzothiazole), 7.4-7.7(d, 10H, Ar-H), 7.03-7.05 (d, 3H, Ar-H), 3.4 (s, 6H, O-CH3); 13C NMR (100 MHz, DMSO-d6) δ 192, 172, 171, 156, 149, 142, 138, 137, 136, 135, 132, 131, 130, 129, 128, 127, 125, 123, 115, 112, 109, 98, 95, 58. MP 196-199 °C
(HP15) 4-bromo-2-(4,5-diphenyl-1-(1,2,4-triazol-4-yl)-4,5-dihydro-1Himidazol-2-yl)phenol yield 91% ; IR(υ max, cm−1) : 3038 (=C–H), 1661(C=C), 1587(C=N); 1H NMR(400 MHz, DMSO-d6) δ 7.6-7.8(m, 10H, Ar-H), 7.02-7.05 (d, 3H, Ar-H); 13C NMR(100 MHz, DMSO-d6) δ 192, 171, 156, 142, 138, 137, 136, 135, 132, 131, 130, 129, 128, 127, 125, 123, 115, 112, 109, 98, 95, 58, 56. MP 191-194 °C
 |
Table 1: Synthesized imidazole derivatives with reaction time and yield. |
Mechanism
The proposed mechanism for the reaction assumes that the activation of carbonyl group on aldehyde by the catalyst initiated the reaction. Due to the increased electrophilicity of the aldehyde carbonyl group, it results in the formation of intermediate A. This intermediate carried out nucleophilic attack on carbonyl group on benzil, resulting in the formation of intermediate B. Their cyclization occurs due to intramolecular interaction that leads to the formation of intermediate C, which further dehydrates to form the desired tetra-substituted imidazoles.
 |
Scheme 2: Mechanism Click here to View Scheme |
Evaluation of antibacterial, antifungal activity and molecular docking of the synthesized compounds.
The synthesized compounds in this work HP-7 to HP-15 and our previous work compounds HP1 to HP6 were screened for antibacterial and antifungal activities using pathogens namely A. niger, C. albicans, S. aureus, E. coli. The antibacterial activity was evaluated by applying methods like, well diffusion and disk diffusion.24-25 200 μg/mL concentration of each synthesized compound solution was prepared in DMSO and screened against selected strains comparatively with standard drug Ampicillin and Fluconazole respectively. After 24 hours, the inhibition zone that appeared around the well in each plate was measured in mm. Calculation of standard deviation zone by triplication of the experiment. Table 2 elaborates the obtained results.
Table 2: Antibacterial and antifungal activity result of the synthesized compounds.
|
Sr. No. |
Tetra-substituted Imidazole derivatives |
Zone of inhibition (mm) |
|||
|
S. aureus |
E. coli |
A. niger |
C. albicans |
||
|
1 |
HP1 |
11 |
09 |
10 |
13 |
|
2 |
HP2 |
10 |
12 |
11 |
09 |
|
3 |
HP3 |
10 |
09 |
08 |
10 |
|
4 |
HP4 |
26 |
20 |
19 |
17 |
|
5 |
HP5 |
23 |
19 |
11 |
14 |
|
6 |
HP6 |
12 |
12 |
13 |
09 |
|
7 |
HP7 |
07 |
09 |
07 |
12 |
|
8 |
HP8 |
22 |
19 |
08 |
09 |
|
9 |
HP9 |
21 |
18 |
11 |
08 |
|
10 |
HP10 |
14 |
11 |
11 |
10 |
|
11 |
HP11 |
09 |
10 |
09 |
11 |
|
12 |
HP12 |
24 |
21 |
07 |
08 |
|
13 |
HP13 |
22 |
18 |
16 |
14 |
|
14 |
HP14 |
19 |
17 |
10 |
12 |
|
15 |
HP15 |
17 |
19 |
13 |
09 |
|
Fluconazole |
– |
– |
24 |
25 |
|
|
Ampicillin |
24 |
22 |
|||
Molecular docking studies
All the structures were prepared and minimization of energy of structures done using Chemdraw Software package. The synthesized compounds were docked against selected target proteins obtained from the database of Protein data bank (PDB). Autodock Vina and Discovery studio software have been used for performing the docking studies. These are automated program used for predicting the interaction of ligand with biomacromolecular target. The comparative docking were performed using Penicillin binding protein 4 (PBP4) from S. aureus (PDB ID – 3HUN) and Penicillin binding protein 6 (PBP6) from E.coli (PDB ID – 3ITA) with compounds HP1 to HP15.
 |
Figure 1: Structure of Penicillin binding protein 4 (PBP4) from S. aureus (PDB ID – 3HUN) and Penicillin binding protein 6 (PBP6) from E.coli (PDB ID – 3ITA). |
Results and Discussion
The new tetra-substituted imidazole derivatives containing various azole moieties have been successfully synthesized in this work with ultrasonic irradiation as the energy source. This protocol enhanced the greenness by reducing the reaction time and minimizing the energy loss during the reaction. The yield of synthesized imidazole derivatives clearly indicates the effectiveness of this protocol.
In our investigation, we found that the use of ultrasonic irradiation as energy source significantly enhanced the yield of desired product and the reaction time reduced from hours to few minutes. All the reactions were irradiated with ultrasound waves (50 kHz) at 70°C and the reaction got accomplished within 25 min. The yield of tetra-substitutd imidazoles primarily depends on the nature of functional group present on the aldehyde and results in high product yield when it is an electron-withdrawing group. Imidazole moiety’s characteristic vibrational bands were observed at 1659 cm−1 (C=C) and 1576 cm−1 (C=N) in the FT-IR spectral analysis of the synthesized compounds.
 |
Graph 1: FT-IR Spectra of HP-7 |
All the synthesized compounds were evaluated for their antimicrobial potential and exhibited activity but HP4 and HP14 came out as more potent compounds. The in vitro studies were confirmed by the results obtained through molecular docking analysis. In molecular docking analysis, we docked all our synthesized compounds against the Penicillin binding protein 4 (PBP4) from S. aureus (PDB ID – 3HUN) and Penicillin binding protein 6 (PBP6) from E.coli (PDB ID – 3ITA).
All the synthesized compounds were evaluated for their antimicrobial potential and exhibited activity but HP4 and HP14 came out as more potent compounds. The in vitro studies were confirmed by the results obtained through molecular docking analysis. In molecular docking analysis, we docked all our synthesized compounds against the Penicillin binding protein 4 (PBP4) from S. aureus (PDB ID – 3HUN) and Penicillin binding protein 6 (PBP6) from E.coli (PDB ID – 3ITA).
The comparison of docking scores of our synthesized compounds with standard drug used against the selected pathogens shown in table 3.
Table 3: Docking score of the synthesized compounds
|
S.No. |
Dock score (K cal/mol) |
|
|
S. aureus (3HUN) |
E. coli (3ITA) |
|
|
HP1 |
-8 |
-4.2 |
|
HP2 |
-7.9 |
-3.6 |
|
HP3 |
-7.9 |
-3.8 |
|
HP4 |
-9.7 |
-4.3 |
|
HP5 |
-8.4 |
-4.1 |
|
HP6 |
-7.9 |
-3.2 |
|
HP7 |
-7.1 |
-3.5 |
|
HP8 |
-8.2 |
-3.3 |
|
HP9 |
-8.3 |
-3.8 |
|
HP10 |
-7.6 |
-3.7 |
|
HP11 |
-7.6 |
-3.1 |
|
HP12 |
-8.8 |
-4.1 |
|
HP13 |
-8.3 |
-3.9 |
|
HP14 |
-8.2 |
-3.7 |
|
HP15 |
-8.3 |
-3.8 |
|
Ampicillin |
-7.7 |
-4.8 |
Docked pose of HP4 in Penicillin binding protein 4 (PBP4) from S. aureus (PDB ID – 3HUN) active binding site. Showing in fig.2 and fig.3 the interaction with ASP130, LYS227, ASP224, ALA129, LEU96.
 |
Figure 2: 2D Docked pose of HP4 in the binding site of Penicillin binding protein 4 (PBP4) from S. aureus (PDB ID – 3HUN). |
 |
Figure 3: 3D Docked pose of HP4 in the binding site of Penicillin binding protein 4 (PBP4) from S. aureus (PDB ID – 3HUN). |
Docked pose of HP14 in the active binding site of Penicillin binding protein 4 (PBP4) from S. aureus (PDB ID – 3HUN). Showing interaction in fig.4 and fig.5 with ASP256, LYS227, ASP256, ALA235, LEU223.
 |
Figure 4: 2D Docked pose of HP14 in the binding site of Penicillin binding protein 4 (PBP4) from S. aureus (PDB ID – 3HUN). |
 |
Figure 5: 3D Docked pose of HP14 in the binding site of Penicillin binding protein 4 (PBP4) from S. aureus (PDB ID – 3HUN). |
The synthesized compound HP4 showing very good docking score due to its interactions in the active binding site of Penicillin binding protein 4 (PBP4) from S. aureus (PDB ID – 3HUN). The carbon hydrogen bond interaction of HP4 with ASP130, ASP224. Pi-cation and Pi-anion interaction with ASP130, LYS221, ASP97. Pi-alkyl interaction with ALA129, LEU96, LYS227. These interactions highlight the enhanced potency of benzimidazole tethered imidazole moiety. The compound HP14 also exhibited good interactions in the active binding site of Penicillin binding protein 4 (PBP4) from S. aureus (PDB ID – 3HUN). The conventional hydrogen bond interaction with ALA235 and LYS227. Carbon hydrogen bond interaction with ASP256. Pi-cation and Pi-alkyl interaction with LYS227 and LEU223 respectively. The conventional hydrogen bond interaction is very crucial for potent antimicrobial activity and this interaction is carried out by triazole tethered imidazole moiety of HP14 compound.
Docked pose of HP4 in Penicillin binding protein 6 (PBP6) from E.coli (PDB ID – 3ITA) protein active site. Showing interaction in fig.6 and fig.7 with ASP130, LYS227, ASP224, ALA129, LEU96.
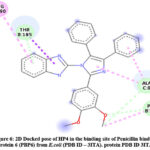 |
Figure 6: 2D Docked pose of HP4 in the binding site of Penicillin binding protein 6 (PBP6) from E.coli (PDB ID – 3ITA). protein PDB ID 3ITA. |
 |
Figure 7: 3D Docked pose of HP4 in the binding site of Penicillin binding protein 6 (PBP6) from E.coli (PDB ID – 3ITA) protein PDB ID 3ITA. |
Docked pose of HP14 in Penicillin binding protein 6 (PBP6) from E.coli (PDB ID – 3ITA) protein active site. Showing interaction in fig.8 and fig.9 with ARG242, LEU197, ARG196, ASN246, PHE245, GLU249.
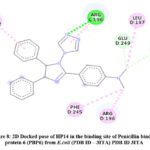 |
Figure 8: 2D Docked pose of HP14 in the binding site of Penicillin binding protein 6 (PBP6) from E.coli (PDB ID – 3ITA) PDB ID 3ITA. |
 |
Figure 9: 3D Docked pose of HP14 in the binding site of Penicillin binding protein 6 (PBP6) from E.coli (PDB ID – 3ITA).PDB ID 3ITA. |
The compound HP14 showing very good interactions in the active binding site of Penicillin binding protein 6 (PBP6) from E.coli (PDB ID – 3ITA). The conventional hydrogen bond interaction of HP14 with ARG196 and carbon hydrogen bond with GLU249, ASN246. Pi-alkyl interaction with PHE245, ARG242, LEU197, ARG196. These interactions results due to triazole tethered imidazole moiety as shown in 3D docked pose of HP14 compound.
The compound HP4 also exhibited good interactions in the active binding site of Penicillin binding protein 6 (PBP6) from E.coli (PDB ID – 3ITA). The carbon hydrogen bond interaction with THR185 and ALA8. Pi-alkyl interaction with ARG190 and PHE186. The benzimidazole tethered imidazole moiety of HP14 compound exhibited good interaction as shown in 3D docked pose of HP4 compound.
Conclusion
In conclusion, we have synthesized some new tetra-substituted imidazole derivatives containing various azole moieties. The clay obtained from brick is used as catalyst in this work with ultrasonic irradiation as the energy source. This protocol enhanced the greenness thereby favoring the environment. The high yield of product clearly reveals the efficiency of this protocol. Use of ultrasonic irradiation as energy source significantly minimized the byproduct formation and the reaction time from hours to few minutes. The screening of synthesized compounds for antibacterial and antifungal activity also provided some interesting results which were confirmed by the molecular docking studies. The docking score of HP4 and HP14 compounds matches the docking score of standard drug used against the selected pathogen. Therefore, these compounds can provide lead for development of more potent future drugs.
Acknowledgment
HP, KK, NM are thankful to SIC, Dr Harisingh Gour Vishwavidyalaya Sagar and MNIT for providing the research facility.
Conflict of Interest
The authors have no conflict of interest.
Funding Sources
There is no funding sources.
References
- N. Rani, N.; A. Sharma, A.; R. Singh, R. Mini-Reviews Org. Chem. 2015, 12, 34–65.
CrossRef - Laufer, S.A.; Zimmermann, W.; Ruff, K.J. J. Med. Chem. 2004, 47, 6311–6325.
CrossRef - Chary, M.V.; Keerthysri, N.C.; Vupallapati, S.V.; Lingaiah, N.; Kantevari, S. Catal. Commun. 2008, 9, 2013–2017.
CrossRef - Takle, A.K.; Brown, M.J.; Davies, S.; Dean, D.K.; Francis, G.; Gaiba, A.; Hird, A.W.; King, F.D.; Lovell, P.J.; Naylor, A. Bioorg. Med. Chem. Lett. 2006, 16, 378–381.
CrossRef - Lee, J.C.; Laydon, J.T.; McDonnell, P.C.; Gallagher, T.F.; Kumar, S.; Green, D.; McNulty, D.; Blumenthal, M.J.; Keys, J.R.; Strickler, J.E. Nature 1994, 372, 739–746.
CrossRef - Schmierer, R.; Mildenberger, H.; Buerstell, H. German Patent. 1987, 361464, 186936–186951.
- de Laszlo, S.E.; Hacker, C.; Li, B.; Kim, D.; MacCoss, M.; Mantlo, N.; Pivnichny, J.V.; Colwell, L.; Koch, G.E.; Cascieri, M.A. Bioorg. Med. Chem. Lett. 1999, 9, 641–646.
CrossRef - Khan, M.S.; Siddiqui, S.A.; Siddiqui, M.S.R.A.; Goswami, U.; Srinivasan, K.V.; Khan, M.I. Chem. Biol. Drug Des. 2008, 72, 197–204.
CrossRef - Wang, L.; Woods, K.W.; Li, Q.; Barr, K.J.; McCroskey, R.W.; Hannick, S.M.; Gherke, L.; Credo, R.B.; Hui, Y.H.; Marsh, K.; J. Med. Chem. 2002, 45, 1697–1711.
CrossRef - Pandey, H.; Shrivastava, S.P. Orient. J. Chem., 2021, 37(3), 583-588.
CrossRef - Zang, H.; Wang, M.; Cheng, B.W.; Song, J. Ultrason. Sonochem. 2009, 16, 301
CrossRef - Li, J.T.; Li, Y.W.; Song, Y.L.; Chen, G.F., Ultrason Sonochem. 2012, 19, 1-4.
CrossRef - Kowsari, E.; Mallakmohammadi, M. Ultrason Sonochem. 2011 18, 447–454.
CrossRef - Ghahremanzadeh, R.; Fereshtehnejad, F.; Mirzaei, P.; Bazgir, A. Ultrason Sonochem. 2011, 18, 415–418.
CrossRef - Li, J.T.; Sun, M.X.; Yin, Y. Ultrason Sonochem. 2010 17 359–362.
CrossRef - Bazgir, A.; Ahadi, S.; Ghahremanzadeh, R.; Khavasi, H.R.; Mirzaei, P. Ultrason Sonochem. 2010, 17, 447–452.
CrossRef - Khosropour, A.R. Ultrason Sonochem. 2008, 15, 659–664.
CrossRef - Safari, J.; Banitab, S.H.; Khalili, S.H. Ultrason Sonochem. 2012, 19, 1061–1069.
CrossRef - Li, J.T.; Yin, Y.; Sun, M.X. Ultrason Sonochem. 2010, 17, 363-366.
CrossRef - Banerjee, B. Ultrason.Sonochem., 2017, 35, 15–35.
CrossRef - Mason, T.J.; Sonochemistry. 1999, 92.
- Suslick, K.S.; Hammerton, D.A.; Cline, D.E.; Sonochemical hot spot. J. Am. Chem. Soc. 1986, 108, 5641–5645.
CrossRef - Mason, T.J., Ultrason. Sonochem. 2003, 10: 175–179.
CrossRef - Shrivastava, S. D.; Shukla, D. K. J. Ind. Chem. Soc., 2008, 85, 306; Vincent, J. G.; Vincent, H. W. Proc. Soc. Exptl. Siol. Med. 1955, 55, 112.
- Marrow, G.; Berry, G P. J. Bact., 1988, 38, 290.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









