Development of a Novel One-Pot Process for the Synthesis of Tolcapone
Arjun Bodkhe* , Vilas Sudrik
, Vilas Sudrik , Dnyaneshwar Karpe
, Dnyaneshwar Karpe and Shamrao Lawande
and Shamrao Lawande
Department of chemistry from Shri Chhatrapati Shivaji Mahavidyalaya, Shrigonda, Maharashtra, India.
Corresponding Author E-mail: arjunbodkhe@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/380631
Article Received on : 15 Nov 2022
Article Accepted on : 16 Dec 2022
Article Published : 23 Dec 2022
Reviewed by: Dr. R. Sekhar
Second Review by: Dr.Aman Deshpandey
Final Approval by: Dr. Tawkir Sheikh
Novel one-pot process for the preparation of tolcapone., 2-methoxy anisole compound 9 treated with 4-methyl benzoyl chloride compound 10 using aluminium chloride gives 4-hydroxy -3-methoxy-4-methyl benzophenone compound 11 Further on nitration using new nitrating agent i.e. melamine nitrate to get corresponding nitro benzophenone compound 6. After demethylation using 48% HBr-Acetic acid to get pure Tolcapone 1 by the cost-effective and commercially feasible process.
KEYWORDS:Industrially Feasible; Melamine nitrate; One-pot process; Parkinson's disease; Tolcapone
Download this article as:| Copy the following to cite this article: Bodkhe A, Sudrik V, Karpe D, Lawande S. Development of a Novel One-Pot Process for the Synthesis of Tolcapone. Orient J Chem 2022;38(6). |
| Copy the following to cite this URL: Bodkhe A, Sudrik V, Karpe D, Lawande S. Development of a Novel One-Pot Process for the Synthesis of Tolcapone. Orient J Chem 2022;38(6). Available from: https://bit.ly/3YHl2Ge |
Introduction
Tolcapone an FDA approved drug used for Parkinson’s disease. It inhibits the Enzyme Catechol-O methyltransferase (COMT). By inhibiting COMT, Tolcapone decreases the degradation of levodopa in the periphery, which allows more levodopa to reach the brain and potentially allows for a decrease in the dosage of levodopa.1, 2 Tolcapone is available in a tablet of 100 mg and 200 mg oral dose.3 Overdose of Tolcapone cause liver toxicity. 4
Patients with Parkinson’s disease will be identified in the society by their primary care physician, but further care should involve the efforts of an entire healthcare team. 5
Bernauer K. 6, 7 first disclosed tolcapone preparation in patents. 6, 7 (Scheme 1). Another method is described in the same patent as shown in Scheme 2.
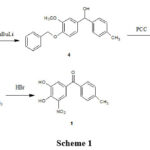 |
Scheme 1 |
 |
Scheme 2 |
Further Bruhin J. 8 prepared tolcapone from 1, 2- dimethoxybenzene and p-methyl benzoyl chloride as shown in Scheme 3 below.
 |
Scheme 3 Click here to View scheme |
Manikumar G. 9 reported another method for tolcapone synthesis from 4-benzyloxy-3-methoxy benzaldehyde which is schematically represented below (Scheme 4).
 |
Scheme 4 |
Reddy B.S. 10 discloses another route for synthesizing Tolcapone from 3-nitro 4, 5- dihydroxy benzoic acid as shown below (Scheme 5).
 |
Scheme 5 Click here to View scheme |
Irudayaraj V.P.R.11 prepared Tolcaone as per below (Scheme 6).
 |
Scheme 6 Click here to View scheme |
The above processes used hazardous chemicals, longer reaction times and more steps hence, not cost-effective and not commercially viable and the isolated yield was low.
Hence need to develop a less hazardous and cost-effective process of Tolcapone 1 which gives maximum conversion and purity.
We report here a one-pot process that is cost-effective and commercially viable.
Results and Discussion
Our reported one-pot process is cost-effective and commercially viable to get pure tolcapone 1 with maximum purity and conversion. (Scheme-7).
 |
Scheme 7 Click here to View scheme |
The prior art process involves purification processes during the preparation of Tolcapone, making it difficult and costlier on large-scale preparation. The impurities formed during the process, if not removed then it is undesirable in the active pharmaceutical product since they may harm the patient being treated. Hence, need to develop a less hazardous and cost-effective process of Tolcapone 1. In this article, we disclosed cost-effective and commercially viable one-pot synthesis of tolcapone 1 from commercially available 1, 2 dimethoxy benzene.
Initially, treatment of 1,2-dimethoxy benzene 9 and 4-methyl benzoyl chloride 10 with aluminum chloride to form benzophenone derivative 11 in situ.further on nitration using melamine nitrate nitro benzophenone derivative 6 in situ. By using 48% HBr-Acetic acid to produce pure Tolcapone 1.
Nitration of phenol gives a di-nitro compound by use of the nitrating mixture. To overcome this problem need to use a mild nitrating agent. For regioselective nitration of phenol, melamine nitrate is the best choice 12 This inspired us to think that melamine nitrate may be useful in Tolcapone synthesis. Before this nobody used melamine nitrate in tolcapone synthesis. So in this process, we used melamine nitrate for regioselective nitration.
 |
scheme 8 Click here to View scheme |
Table 1: Screening in different solvents by use of melamine nitrate (step ii) and yield of Tolcapone 1.
|
Entry |
Nitrating agent |
Solvent |
Tolcapone 1 Yield (%) |
|
1 |
Melamine nitrate |
DCM |
50 |
|
2 |
Melamine nitrate |
Chloroform |
62 |
|
3 |
Melamine nitrate |
CCl4 |
55 |
|
4 |
Melamine nitrate |
Acetone |
75 |
|
5 |
Melamine nitrate |
Methanol |
64 |
|
6 |
Melamine nitrate |
Ethanol |
68 |
|
7 |
Melamine nitrate |
IPA |
65 |
Reaction Condition: 4-hydroxy -3-methoxy-4-methyl benzophenone (in situ), melamine nitrate (10 mmoles), temp. at 40 °C and Reaction time 12 hrs 1isolated yield.
We study the effect of the use of different solvents at low temp. i.e. 40 °C and impact on the output of Tolcapone 1. In a variety of solvents and carried out Nitration using melamine nitrate (step ii) for our one-pot process and we got the following observations.
By use of DCM solvent, we got a 65 % yield.
By use of chloroform solvent, we got a 62 % yield.
By use of CCl4 solvent, we got a 55 % yield.
By use of acetone solvent, we got a maximum yield of 75 % yield.
By use of methanol solvent, we got a 64 % yield.
By use of ethanol solvent, we got a 68 % yield.
By use of IPA solvent, we got a 65 % yield. (Table 1)
Hence, acetone is an excellent solvent for nitration and we got a maximum yield of final Tolcapone 1 by comparing it with other solvents.
Table 2: Screening at different temp. in acetone solvent (step ii) and yield of Tolcapone 1.
|
Sr.No. |
Nitrating agent |
Maintaining Time (h) |
Temp (°C) |
Tolcapone 1 (%) |
|
1 |
Melamine nitrate |
12 |
30 |
60 |
|
2 |
Melamine nitrate |
12 |
35 |
65 |
|
3 |
Melamine nitrate |
12 |
40 |
75 |
|
4 |
Melamine nitrate |
12 |
45 |
70 |
|
5 |
Melamine nitrate |
12 |
50 |
60 |
|
6 |
Melamine nitrate |
12 |
55 |
55 |
|
7 |
Melamine nitrate |
12 |
60 |
50 |
Reaction Condition: 4-hydroxy -3-methoxy-4-methyl benzophenone (in situ), melamine nitrate (10 mmoles), TsOH (0.14 mmole), Solvent- Acetone, maintaining 12 hrs 1 isolated yield.
We study the effect of different temp. using acetone as a solvent for nitration using melamine nitrate (step-ii) and to check the maximum yield of Tolcapone 1 for our one-pot process. We carried out several experiments and got the following observations.
Nitration at 30 °C in acetone we got 75% yield.
Nitration at 35 °C in acetone we got 70% yield.
Nitration at 40 °C in acetone we got 75% yield.
Nitration at 45 °C in acetone we got 70% yield.
Nitration at 50 °C in acetone we got 60% yield.
Nitration at 55 °C in acetone we got 55% yield.
Nitration at 60 °C in acetone we got 50% of the yield.
Hence, by increasing temp. for the nitration step, the yield of tolcapone 1 decreases. Reaction favored at lowered temp. Hence, 40°C is the best temperature and got the maximum yield.
Table 3: Different Melamine nitrate concentration (step-ii) and yield of Tolcapone 1
|
Amount of melamine nitrate (mmoles) |
Reaction Time (h) |
Tolcapone 1 Yield (%) |
|
7.2 |
24 |
55 |
|
7.9 |
18 |
62 |
|
8.6 |
15 |
71 |
|
9.3 |
12 |
75 |
|
10.0 |
12 |
75 |
|
10.8 |
12 |
75 |
Reaction Condition: 4-hydroxy -3-methoxy-4-methyl benzophenone (in situ), TsOH (0.14 mmole), Solvent-Acetone 1 isolated yield at 40°C.
Nitration was studied at different Melamine nitrate concentrations i.e. 7.2, 7.9, 8.6 and 9.3 mmoles at different maintaining times and checked the effect on the yield of Tolcapone 1. We got 55%, 62%, 71%, and 75% yield, further increase of melamine concentration yield is the same.
Based on optimization results by the use of 9.3 mmoles of melamine nitrate in acetone solvent maintaining the reaction at 40°C for 12 hrs stirring, we got maximum yield i.e.75%.
Table 4: Optimised conditions
|
Entry |
Nitrating agent |
Solvent |
Reaction Time (h) |
Temp(°C) |
Tolcapone 1 Yield (%) |
|
1 |
Melamine nitrate (9.3 mmoles) |
Acetone |
12 |
40 |
75 |
Some advantages of the one-pot process are like in situ process and the use of less hazardous reagents
Experimental
Materials
All solvents and reagents used from analytical grade (A.R.) and purchased from Sigma Aldrich. 1,2-dimethoxy benzene, p-methyl benzoyl chloride, aluminum chloride, Melamine nitrate, 48% HBr-Acetic acid. Progress of reaction checked on Thin Layer Chromatography (TLC).
Instrumentation
The PMR spectra were recorded on 400 MHz and CMR spectra on 100 MHz Bruker Spectrophotometer in DMSO/CDCl3 using a Bruker instrument. The chemical shift values in the δ scale. Tetramethylsilane (TMS) was used as an internal standard.
Experimental procedure for Preparation of Tolcapone (1)
1, 2 dimethoxy benzene 9 (1.0 g, 7.2 mmol) dissolve in DCM (10 ml), cool it at 0-5°C then add dropwise p-methyl benzoyl chloride 10 (2.22 g, 1.4 mmol) in 10 min. Followed by the addition of aluminium chloride (4.32 g, 32.4 mmol) and DCM (10 ml) and stirred for 1 hour at 0-5°C temperature, monitoring reaction TLC. After completion of reaction raise temperature to 28°C and maintain for 8 hrs. Again monitor the reaction by TLC. After completion of the reaction give 10 ml water washing to the organic layer. Concentrate organic layer to form residue 11. Add acetone (10 ml), melamine nitrate (1.77 g, 9.3 mmol)and PTSA (0.024 g, 0.14 mmol) at 25-30°C, heat reaction mass to 40°C and stirred for 12 hours, monitor reaction by TLC. Concentrate acetone to get residue 6. To a concentratedreaction mass add Toluene (10 ml), 48% HBr-AcOH (2 ml) ad heat to 100°C for 3 hrs, cool to 25-30°C.
Distilled out toluene and add Chloroform (10 ml) and water (10 ml) stirred for 30 min. at 25-30°C. Distilled out chloroform layer under vacuum. Add Chloroform (10 ml) to the reaction mass and stirred for 30 min. at 0-5 °C. Filter product and dry at 40-45°C under vacuum to get pure tolcapone 1 (1.50 g; 75% yield) as a yellow solid.
Spectral data
HPLC purity= 99.17% (M.P.147 °C) (lit.11:146-148°C); ); FTIR (KBr cm-1 ): 3392 (-OH), 2924 (-CH3), 1629 (-CO),1541 and 1363 (-NO2); 1H NMR (DMSO-d6, δ ppm): 2.43 (s,3H), 7.38 (d, 2H), 7.48 (s, 1H), 7.65 (d, 2H), 7.67 (d 1H), 13C NMR, DMSO-d6): δ 21.61, 118.29, 119.45, 127.43, 129.60, 130, 134.66, 137.09, 143.39, 146.25, 148.19, 193.30; Mass Spectra (EI): M+ = 273, m/z = 119 (+OC-Ph-CH3), m/z= 91 (.Ph-CH3).
 |
Figure 1: FTIR Click here to View figure |
 |
Figure 2: 1H NMR |
 |
Figure 3: 13C NMR Click here to View figure |
 |
Figure 4: HPLC |
 |
Figure 5: Mass Spectra |
Conclusion
In conclusion, we have developed an in-situ novel cost-effective and commercially feasible one-pot process for the preparation of pure Tolcapone 1 without the isolation of any intermediate.
Acknowledgement
We thank the Principal and Management of Shri Chhatrapati Shivaji Mahavidyalaya, Shrigonda for providing infrastructure for this research work.
Conflict of Interest
There are no conflict of interest.
Funding Sources
There is no funding source for this article.
References
- Truong D.D. 𝘊𝘭𝘪𝘯 𝘐𝘯𝘵𝘦𝘳𝘷 𝘈𝘨𝘪𝘯𝘨. 2009; 4, 109-13.
CrossRef - Pinheiro SD, Serrão MP, Silva T, Borges F, Soares-da-Silva P., 𝘌𝘶𝘳. 𝘑. 𝘰𝘧 𝘗𝘩𝘢𝘳𝘮𝘢𝘤𝘰𝘭.2019. 15;847:53-60.
- Jorga K.M., Fotteler B, Heizmann P, Zürcher G. , 𝘌𝘶𝘳. 𝘑. 𝘰𝘧 𝘗𝘩𝘢𝘳𝘮𝘢𝘤𝘰𝘭 , 1998 ;54(5):443-7.
CrossRef - 𝘕𝘢𝘵𝘪𝘰𝘯𝘢𝘭 𝘐𝘯𝘴𝘵𝘪𝘵𝘶𝘵𝘦 𝘰𝘧 𝘋𝘪𝘢𝘣𝘦𝘵𝘦𝘴 𝘢𝘯𝘥 𝘋𝘪𝘨𝘦𝘴𝘵𝘪𝘷𝘦 𝘢𝘯𝘥 𝘒𝘪𝘥𝘯𝘦𝘺 𝘋𝘪𝘴𝘦𝘢𝘴𝘦𝘴-𝘉𝘦𝘵𝘩𝘦𝘴𝘥𝘢 (𝘔𝘋): , 2021.
- Patel T, Chang F., 𝘊𝘢𝘯.𝘗𝘩𝘢𝘳𝘮. 𝘑. (𝘖𝘵𝘵). 2014 May;147(3):161-70.
CrossRef - Bernauer K., Borgulya J., Bruderer H., DaPrada M., Zurcher G., 𝘌𝘗 𝘗𝘢𝘵𝘦𝘯𝘵 237929 1987.
- Bernauer K., Borgulya J., Bruderer H., DaPrada M., Zurcher G., 𝘜𝘚 𝘗𝘢𝘵𝘦𝘯𝘵 5236952, 1993.
- Bruhin J., Process for the preparation of benzophenone derivatives, 𝘜𝘚 𝘗𝘢𝘵𝘦𝘯𝘵 5877353, 1999.
- Manikumar G., Chunyang J., and Kenneth S.R., 𝘚𝘺𝘯𝘵𝘩𝘦𝘵𝘪𝘤 𝘊𝘰𝘮𝘮𝘶𝘯𝘪𝘤𝘢𝘵𝘪𝘰𝘯𝘴, 2008, 38, 810-815.
CrossRef - Reddy B.S., Suresh Babu S., Suri Babu G., 𝘗𝘢𝘵𝘦𝘯𝘵 𝘞𝘖 2014147464, 2014.
- Irudayaraj V.P.R., Govindaraj Rao V., , Hanumantha D., Gnanapragasam A., Jayaraman B.,; Thanukrishnan K. Tangirala V., Sengodan S., Ranganathan S., 𝘐𝘕201641042235𝘈, 2018.
- Chen Y. and Jiang H., 𝘏𝘪𝘯𝘥𝘢𝘸𝘪 𝘗𝘶𝘣.𝘊𝘰𝘳𝘱. 𝘖𝘳𝘨𝘢𝘯𝘪𝘤 𝘊𝘩𝘦𝘮𝘪𝘴𝘵𝘳𝘺 𝘐𝘯𝘵𝘦𝘳𝘯𝘢𝘵𝘪𝘰𝘯𝘢𝘭, 2011, Volume 4.

This work is licensed under a Creative Commons Attribution 4.0 International License.









