The reaction of (2-oxo-2-phenyl-ethyl sulfanyl)-Acetic Acid with Chalcones: Double Michael Addition-Aldol Reaction Formation of Unexpected (5-Benzoyl-2-hydroxy-2,4-6-triphenyl-cyclohexyl) -phenyl-methanone
Department of Chemistry, Cardamom Planters’ Association College, Bodinayakanur – 625513. Madurai Kamaraj University, Madurai. Tamil Nadu. India.
Corresponding Author E-mail: gururguru77@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/380521
Article Received on : 05 Sep 2022
Article Accepted on : 07 Oct 2022
Article Published : 24 Oct 2022
Reviewed by: Dr. M.A Shah
Second Review by: Dr. Voltaire Organo
Final Approval by: Dr. Ashutosh Pal
A greener approach one-pot reaction of (2-oxo-2-phenyl-ethyl sulfanyl)-acetic acid with chalcones in presence of a base to afford an unexpected (5-Benzoyl-2-hydroxy-2,4-6-triphenyl-cyclohexyl) – phenyl-methanone product. The reaction proceeds via sequential double Michael addition-aldol reaction mediated three consecutive C-C bonds are formed in one pot. The procedure has a short reaction time, easy workup, and moderate yield of products. The structure of all synthesized compounds was determined by using 1HNMR and 13CNMR Spectral analysis.
KEYWORDS:Aldol Reaction; Chalcone; 13CNMR; 1HNMR; Michael Addition; Solid-State
Download this article as:| Copy the following to cite this article: Suriyakala T, Ravindran G. The reaction of (2-oxo-2-phenyl-ethyl sulfanyl)-Acetic Acid with Chalcones: Double Michael Addition-Aldol Reaction Formation of Unexpected (5-Benzoyl-2-hydroxy-2,4-6-triphenyl-cyclohexyl) -phenyl-methanone. Orient J Chem 2022;38(5). |
| Copy the following to cite this URL: Suriyakala T, Ravindran G. The reaction of (2-oxo-2-phenyl-ethyl sulfanyl)-Acetic Acid with Chalcones: Double Michael Addition-Aldol Reaction Formation of Unexpected (5-Benzoyl-2-hydroxy-2,4-6-triphenyl-cyclohexyl) -phenyl-methanone. Orient J Chem 2022;38(5). Available from: https://bit.ly/3DqqZyD |
Introduction
A traditional and essential carbon-carbon bond formation reaction is the Michael addition of carbon nucleophiles to olefins with a deficiency in electrons 1. The synthesis of organic materials has made considerable use of this reaction and its near relatives 2. Michael additions are typically carried out in a suitable solvent with a strong base present, either at room temperature or at high temperatures 3. The Michael reaction has recently been found to be facilitated by unconventional techniques like carrying out the reaction on the surface of a dry medium 4 or while being exposed to microwave radiation 5. It is a “green Chemistry” reaction that is accomplished without the use of solvents and produces little or no by-products 6.
Chalcone is one of the good Michael acceptors. Chalcones are a member of a large group of substances that are found in practically all vascular plants, including their roots, flakes, and seeds in addition to their terrestrial components. Chalcones are precursors to flavonoids and are essential for the manufacture of those compounds 7. Different chalcones exhibit a wide spectrum of pharmacological activity depending on the pattern of substitution on the two aromatic rings 8. Among them include cytotoxic, antiprotozoal 9, antibacterial, antifungal 10, and anticancer 11 properties. Recent years have seen a rise in interest in chalcones and bis-chalcones’ antimalarial properties 12. All of these characteristics make for desirable synthetic targets, and even more significantly, they have been widely used as intermediates in the synthesis of heterocyclic and carbocyclic systems.
Michael additions have recently been used in several tandem reactions that help build carbon skeletons. Sabita Nayak and co-workers 13 reported chalcone products through the recent advances of organocatalytic enantioselective Michael addition to chalcone. One of the reactant molecules (2-oxo-2-phenyl-ethyl sulfanyl)-acetic acid is applied to the limited amount of research. Gamal Abdel-Nasser Gohar co-workers 14 published the kinetic studies of the reaction of phenacyl bromide derivatives with sulfur nucleophiles.
Given the above data, the present investigation was undertaken, and an efficient and green approach synthesis of unexpected (5-Benzoyl-2-hydroxy-2,4-6-triphenyl-cyclohexyl) – phenyl-methanone was reported by using (2-oxo-2-phenyl-ethyl sulfanyl)-acetic acid 1 and chalcones 2.
Results and Discussion
As part of our ongoing research on the synthesis of novel heterocyclic molecules 15. We have investigated the interaction between, α,β-unsaturated carbonyl compounds and (2-oxo-2-phenyl-ethyl sulfanyl)-acetic acid 1. In the past, a similar reaction between chalcone and diphenacyl aniline produced a substantially substituted piperidine derivative 16. In the current instance, we anticipate that a Michael addition-aldol condensation sequence will result in such a domino reaction, producing 4,6-dibenzoyl-5-phenyl-dihydro-thiopyran-3-one 6. Interestingly, the reaction between (2-oxo-2-phenyl-ethyl sulfanyl)-acetic acid 1 with chalcones and a base to obtained the unexpected product 3. The resultant product is (5-Benzoyl-2-hydroxy-2,4-6-triphenyl-cyclohexyl) – phenyl-methanone 3 (Scheme: 1)
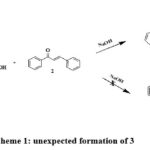 |
Scheme 1: unexpected formation of 3 |
In the first approach for the preparation of (2-oxo-2-phenyl-ethyl sulfanyl)-acetic acid 1. It is prepared by the addition of sodium hydroxide solution to one equivalent of thioglycolic acid and one equivalent of phenacyl bromide in solid-state thermal heating to yield the corresponding 1. Claisen Schmidt condensation, in which aromatic aldehydes and acetophenones are condensed, is the typical method for producing chalcones 17-19. Two aromatic rings are present in this compound class, joined by a three-carbon bridge, along with a keto carbonyl group and one, α, β – unsaturation 20.
The synthesis of cyclohexanol derivatives 3 was performed by reaction between an equimolar mixture of (2-oxo-2-phenyl-ethyl sulfanyl)-acetic acid 1 and various chalcones 2 in the presence of sodium hydroxide as a base under the solid-state thermal heating with moderate yield in short reaction time. The synthetic protocol for the formation of product 3 was presented in Scheme:2.
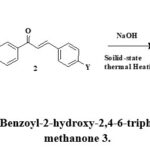 |
Scheme 2: Synthesis of (5-Benzoyl-2-hydroxy-2,4-6-triphenyl-cyclohexyl) – phenyl -methanone 3. |
Table 1: X, Y of (5-Benzoyl-2-hydroxy-2,4-6-triphenyl-cyclohexyl) – phenyl -methanone 3.
|
S.No |
X |
Y |
|
1 |
H |
H |
|
2 |
H |
p-Cl |
|
3 |
H |
p-CH3 |
|
4 |
p-CH3 |
p-CH3 |
|
5 |
H |
m-Cl |
Yield and Melting point of product 3.
We inserted several substituents at various chalcone locations using the established reaction technique, such as p-Chloro, m-Chloro, p-methyl, and p-methoxy groups which were reacted with (2-oxo-2-phenyl-ethyl sulfanyl)-acetic acid 1to generate cyclohexanol derivatives 3. The outcome demonstrated that the aromatic chalcone rings’ electron-withdrawing and electron-donor substituents both produced same product with reasonable yields. Chemical structures of synthesized compounds 3 were characterized by 1HNMR and 13CNMR spectroscopic analysis.
In the 1HNMR spectrum of 3c (Fig:1) assigned two singlet signals at 1.92 and 2.12 ppm assignable for two -CH3 proton, respectively, three of the doublets at 2.20-2.23, 4.01-4.04, and 4.44-4.47 ppm with J-value 9Hz for cyclohexyl ring protons and one triplet signal at 2.43-2.52 ppm and one multiple signals at 4.09-4.22 ppm also protons of cyclohexanol ring protons. The aromatic protons appear at three doublets in 6.61, 6.88, 7.52 ppm and multiplets in 7.04, 7.14, and 7,22 ppm respectively. The broad singlet appears at 5.35 indicating one OH group is present in the cyclohexanol ring. In 13CNMR spectrum of 3c (Fig:2) showed the characteristic signals at 204.0 and 207.0 ppm assigned for two carbonyl carbons. The cyclohexanol ring six carbons appear at 43.3, 46.4, 48.0, 57.3, 57.4, and 75.7 ppm. The five aromatic ring carbons appear between 125.2 to 146.2 ppm.
 |
Figure 1: 1HNMR Spectrum (5-Benzoyl-2-hydroxy-2-phenyl-4,6-di-p-tolyl-cyclohexyl)-phenyl-methanone (3c). |
 |
Figture 2: 13CNMR Spectrum (5-Benzoyl-2-hydroxy-2-phenyl-4,6-di-p-tolyl-cyclohexyl)-phenyl-methanone (3c) |
The plausible mechanism for the formation of (5-Benzoyl-2-hydroxy-2,4-6-triphenyl-cyclohexyl) – phenyl-methanone 3 was explained in Scheme:3. The First carbanion generated by the addition of base to sulfonylacetic acid 1, followed by the carbanion first Michael addition to chalcone to form an intermediate of symmetrical 1,5-diketones 4 and the thioglycolic acid group (-HSCH2COOH) is eliminated. The 1,5-diketone followed the second Michael addition with chalcone to afford another intermediate 5. Finally, the intermediate of 5 is followed by aldol reaction cyclization to form cyclohexyl derivatives of 3.
 |
Scheme 3: Proposed mechanism of formation of (5-Benzoyl-2-hydroxy-2,4-6-triphenyl-cyclohexyl) – phenyl-methanone 3. |
Experimental
Starting compound 1 and 2 preparation reagents and solvents were purchased from the suppliers and used directly in the experiment. Melting point was measured on a Melting point instrument. 1H and 13C NMR spectra were recorded on a Bruker 300 MHz Spectrometer. The solvent used for the NMR spectrum was CDCl3 and TMS was used as the internal standard. TLC was performed on silica gel-G
General Procedure for the synthesis of 3.
A mixture of (2-oxo-2-phenyl-ethyl sulfanyl)-acetic acid (1, 1 mmol), chalcone (2, 1mmol) and sodium hydroxide (2 mmol) was heated in 15 minutes at 80oC. After completion of the reaction (checked by Thin Layer Chromatography) the mixture was cooled to RT, the solid products were filtered and then recrystallized from ethanol to give pure compounds 3. Products have been characterized by melting points and NMR spectroscopy. Spectra data of all products are presented as follows.
(5-Benzoyl-2-hydroxy-2,4,6-triphenyl-cyclohexyl)-phenyl-methanone (3a)
White solid, 1HNMR: 2.23-2.27 (d, 1H, J=12Hz), 2.47-2.56 (t, 1H, J=12Hz ), 4.06-4.09(d, 1H, J=9Hz), 4.17-4.23(t, 2H, J=9Hz ), 4.48-4.51(d, 1H, J=9Hz), 5.40 (s, OH), 6.71-6.73 (d, 1H, J=6Hz), 6.80-6.83 (d, 2H), 7.04-7.44 (m, 19H), 7.54-7.56 (d, 3H) ppm. 13CNMR: 43.7, 46.2, 48.4, 57.1, 75.7, 125.2, 127.0, 127.2, 127.3, 127.7, 127.9, 128.0, 128.1, 128.3, 128.4, 128.5, 128.7, 129.0, 132.1, 133.0, 138.5, 139.0, 139.3, 142.4, 146.2, 203.9, 207.5 ppm.
[5-Benzoyl-4,6-bis-(4-chloro-phenyl)-2-hydroxy-2-phenyl-cyclohexyl]-phenyl-methanone (3b)
White solid, 1HNMR: 2.19-2.20 (d, 1H, J=3Hz), 2.24-2.45 (t, 1H), 4.09-4.16(m, 3H), 4.42-4.46(d, 1H, J=12Hz), 5.32 (s, OH), 6.79-6.82 (d, 2H, J=9Hz), 7.05-7.12 (m, 10H), 7.18-7.45 (m, 9H), 7.51-7.54 (d, 2H) ppm. 13CNMR: 30.0, 43.1, 46.1, 47.8, 56.9, 75.6, 125.1, 127.5, 127.7, 128.0, 128.0, 128.2, 1286, 128.9, 129.6, 132.7, 132.8, 133.1, 133.3, 137.5, 138.2, 138.8, 140.8, 145.8, 203.2, 207.0 ppm.
(5-Benzoyl-2-hydroxy-2-phenyl-4,6-di-p-tolyl-cyclohexyl)-phenyl-methanone (3c)
1HNMR: white solid, 1.92 (s, 3H), 2.12 (s, 3H), 2.20-2.23 (d, 1H, J=9Hz), 2.43-2.52 (t, 1H, J=12Hz), 4.01-4.04(d, 1H, J=9Hz), 4.09-4.22(m, 2H), 4.44-4.47(d, 1H, J=9Hz), 5.35 (s, OH), 6.61-6.63 (d, 2H, J=6Hz), 6.80-6.83 (d, 2H), 7.04-7.44 (m, 19H), 7.54-7.56 (d, 3H) ppm. 13CNMR: 43.3, 46.4, 48.0, 57.3, 57.4, 75.7, 125.2, 127.0, 127.2, 127.3, 127.7, 127.9, 128.0, 128.1, 128.3, 128.4, 128.5, 128.7, 129.0, 132.1, 133.0, 138.5, 139.0, 139.3, 142.4, 146.2, 204.0, 207.0 ppm.
[2-Hydroxy-5-(4-methyl-benzoyl)-2-phenyl-4,6-di-p-tolyl-cyclohexyl]-p-tolyl-methanone (3d).
1HNMR: white solid, 1.91(s, 3H), 2.12(s, 3H), 2.15(s, 3H), 2.20(s, 3H), 2.26-2.46 (m, 2H), 3.98-4.02 (d, 1H, J=12Hz ), 4.11-4.13 (d, 2H, J= 6Hz), 4.40-4.43 (d, 1H, J=9Hz), 5.43(s, OH), 6.60-6.62 (d, 2H, J=6Hz), 6.83-7.01 (m, 9H), 7.12-7.22 (m, 8H), 7.39-7.42 (d, 2H, J=9Hz) ppm. 13CNMR: 21.0, 21.2, 21.7, 21.8, 43.3, 46.8, 48.0, 56.9, 75.7, 128.4, 128.5, 128.7, 128.9, 129.1, 129.2, 136.2, 136.3, 136.5, 136.6, 136.8, 139.7, 143.7, 204.0, 207.0 pm.
[5-Benzoyl-4,6-bis-(3-chloro-phenyl)-2-hydroxy-2-phenyl-cyclohexyl]-phenyl-methanone: (3e)
1HNMR: white solid, 2.21-2.26(d, 1H, J=15Hz), 2.40-2.48 (t, 1H, J=12Hz), 3.56-4.15 (m, 3H), 4.44-4.48 (d, 1H, J=12Hz), 5.38 (s, OH), 6.70-6.76 (t, 4H, J=12Hz), 6.96-7.00 (d, 2H, J=12Hz), 7.09-7.27(m, 10H), 7.30-7.56 (m, 7H) ppm. 13CNMR: 43.1, 45.8, 47.7, 56.0, 56.2, 75.2, 124.6, 126.5, 127.0, 127.1, 127.3,127.7, 127.8, 128.0, 128.3, 129.5, 129.8, 132.4, 133.0, 134.0, 134.2, 136.7, 137.8, 138.4, 140.8, 144.0, 145.3, 148.5, 202.5, 206.5 ppm.
Conclusion
We have described a productive solid-phase interaction of (2-oxo-2-phenyl-ethyl sulfanyl)-acetic acid 1 with chalcones 2 in presence of a base to afford an unexpected (5-Benzoyl-2-hydroxy-2,4-6-triphenyl-cyclohexyl) – phenyl-methanone product. The reaction proceeds via a sequential double Michael addition-aldol reaction mediated by three successive C-C bond creations in one pot. The procedure has a short reaction time, easy workup, and moderate yield of products. Additionally, the straight forward work-up process and solvent-free surroundings offer an environmentally responsible method.
Acknowledgment
No specific grant was given to this research by funding organizations in the public, private, or not-for-profit sectors.
Conflict of Interest
According to the author, there is no conflict of interest.
References
- (a) Gawley. R.E. Synthesis, 1976, 777. (b) Jung, M.E. Tetrahedron,1976, 32,3. (c) Peters, J.A Synthesis, 1979, 321.
CrossRef - Jung, M.E. Comprehensive organic synthesis (eds), 1993. B.M. Trost and Fleming (Oxford: Pergamon Press) vol.4, p. 1-68.
- Bergmann. E.D,; Ginsburg, D,; Pappo, R, Organic React., 1959, 10, 179.
- (a) Ranu, B,C.; Bhar, S.; Tetrahedron,1992,48, 1342. (b).Ranu, B.C.;Bhar, S.; Sarkar, D.C. Tetrahedron Lett., 1991, 32, 2811.
CrossRef - Romanova, N.N.; Gravis, A.G.; Sharidullina, G.M.; Leshcheva, I.F.; Bundle, Y.G. Mendeleev Commun., 1997, 213.
- Nagendrappa, G. Resonance, 2002, 59.
CrossRef - Mojzisa, J.; Varinskaa, L.; Mojzisovab, G.; Kostova, I.; Mirossaya, L.; pharmacological Research, 2008, 57, 259-265.
CrossRef - (a). Nowakowska, Z.; Dzia, B.K.; Schroede, G.; Eur.J.Med.Chem. 2008, 43, 707-713. (b).Chimenti, F.; Fioravanti, R.; Bolasco, A.; Chimenti, P.; Secci, D.; Rossi, F.; Yanez, M.; Orallo, F.; Ortuso, F.; Alcaro, S.; J.Med.Chem. 2009, 52, 2818-2824.
CrossRef - Tsukiyama, R.I.; Katsura, H.; Tokuriki, N.; Kobayashi, M. Antimicrob.Agents Chemother, 2002, 46, 1226-1230.
CrossRef - Lahtchev, K.L.; Batovska, D.I.; Parushev, S.P.; Ubiyvovk, V.M.; Sibirny, A.A.; Eur.J.Med.Chem. 2008, 43, 2220-2228.
CrossRef - (a). Won, S.J.; Liu, C.T.; Tsao, L.T.; Weng, J.R.; Ko, H.H.; Wang, J.P.; Lin, C.N. Eur.J.Med.Chem, 2005, 40, 103-112. (b). Bandgar, B.P.; Gawande, S.S.; Bodade, R.G.; Totre, J.V.; Khobragade, C.N. Bioorg.Med.Chem. 2010, 18, 1364-1370.
CrossRef - Ram, V.J.; Saxena, A.S.; Srivastava, S.; Chandra, S. Bioorg.Med. Chem.Lett. 2000, 10, 2159-2161.
CrossRef - Sabita Nayak, Subhendu Chakroborty, SujitlalBhakta, Pravati panda, Seetaram Mohapatra, Res.Chem.Intermed., 2016, 42, 2731-2747. DOI: https://doi.org/10.1007/s11164-015-2193-0.
CrossRef - Gamal Abdel-Nasser Gohar, Sherine Nabil Khattab, Omaima Osman Farahat, Hosam Hassan Khalil, J.Phys.Org.Chem. 2012, 25, 343-350. DOI: 10.1002/poc.1921
CrossRef - (a). G.Ravindran, Nidhin Paul. S.Muthusubramanian, S. Perumal, J.Sulfur.Chemistry, 2008, 29, 575 (b). G. Ravindran, N.G. Renganathan, Org. Commun,2010,3, 76. (c). G.Rajmohan, R. Shanmugam, A. Elangovan, R.K. Sankaranarayanan, G. Ravindran, P.Dineshkumar, G. Arivazhagan, Journal of Molecular Structure, 2022, 1251, 132028.
CrossRef - Ravindran, G. Muthusubramanian, S.; Perumal, S. Arkivoc 2008, (xiii), 57.
CrossRef - Amanaganti, J.; Subhashini, N. Potential Biological Activity of Chalcones: A Review. Int.J.Chem.Sci. 2013, 11(3), 1335-1341.
- Wang, Z. Claisen-Schmidt condensation. Compr.Org. Name React. Reagents, 2010, 660-664.
- Dong, F.; Jian, C.; Zhenghao, F.; Kai, G.; Zuliang, L. Catal.Commun, 2008, 9, 1924-1927. DOI: doi.org/10.1016/j.catcom.2008.03.023.
CrossRef - Custodio, J.M.F.; Faria, E.C.M.; Sallum, L.O.; Duarte, V.S.; Vaz, W.F.; DeAquino, G.L.B.; Carvalho, P.S.; Napolitano, H.B. J.Braz.Chem.Soc, 2017, 28(11), 2180-2191. DOI: dx.doi.org/10.21577/01103.20170067.

This work is licensed under a Creative Commons Attribution 4.0 International License.










